Performance of Ethane Dehydrogenation over PtSn Loaded onto a Calcined Mg(Al)O LDH with Three Mg:Al Molar Ratios Using a Novel Method
Abstract
1. Introduction
2. Results and Discussion
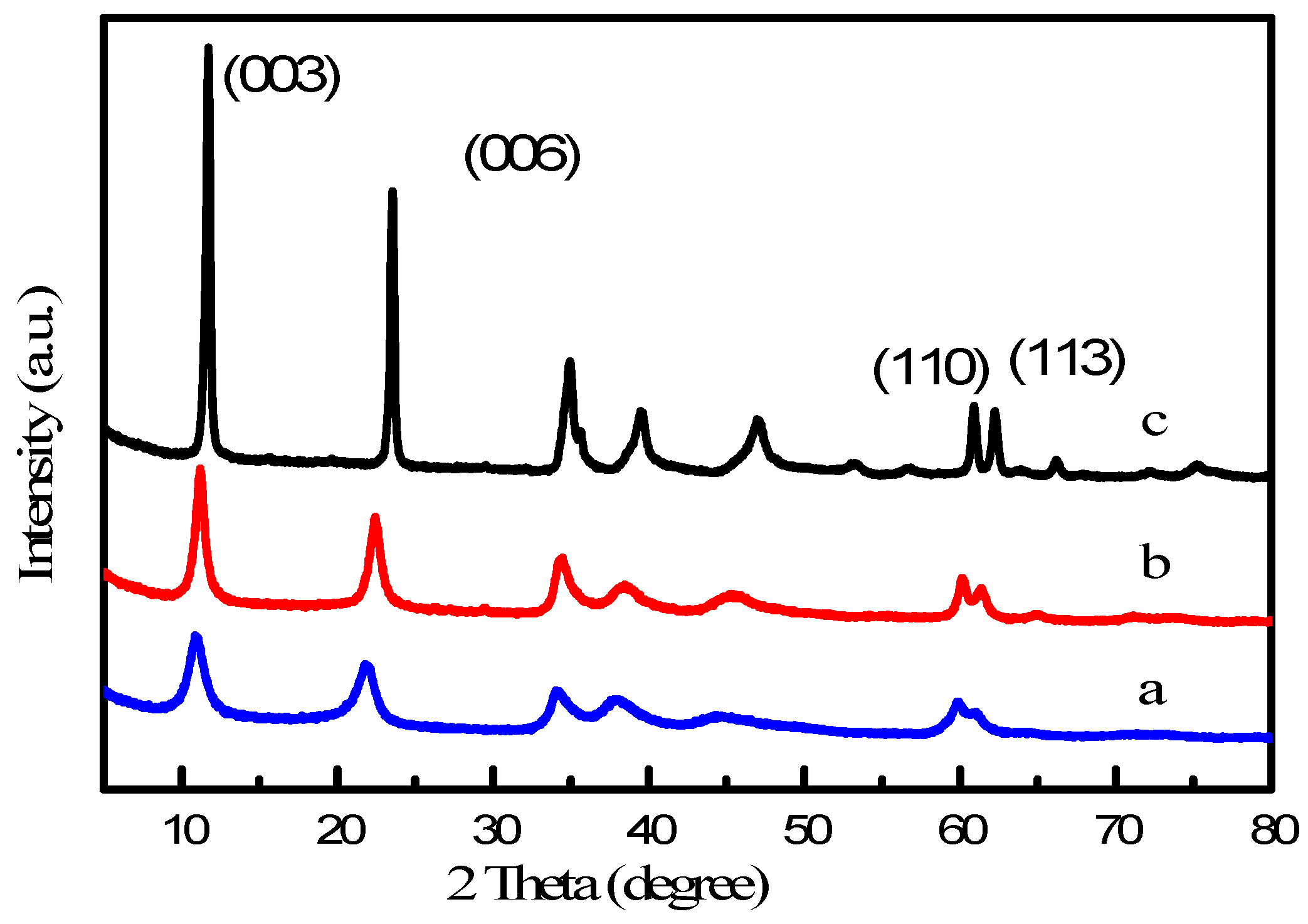
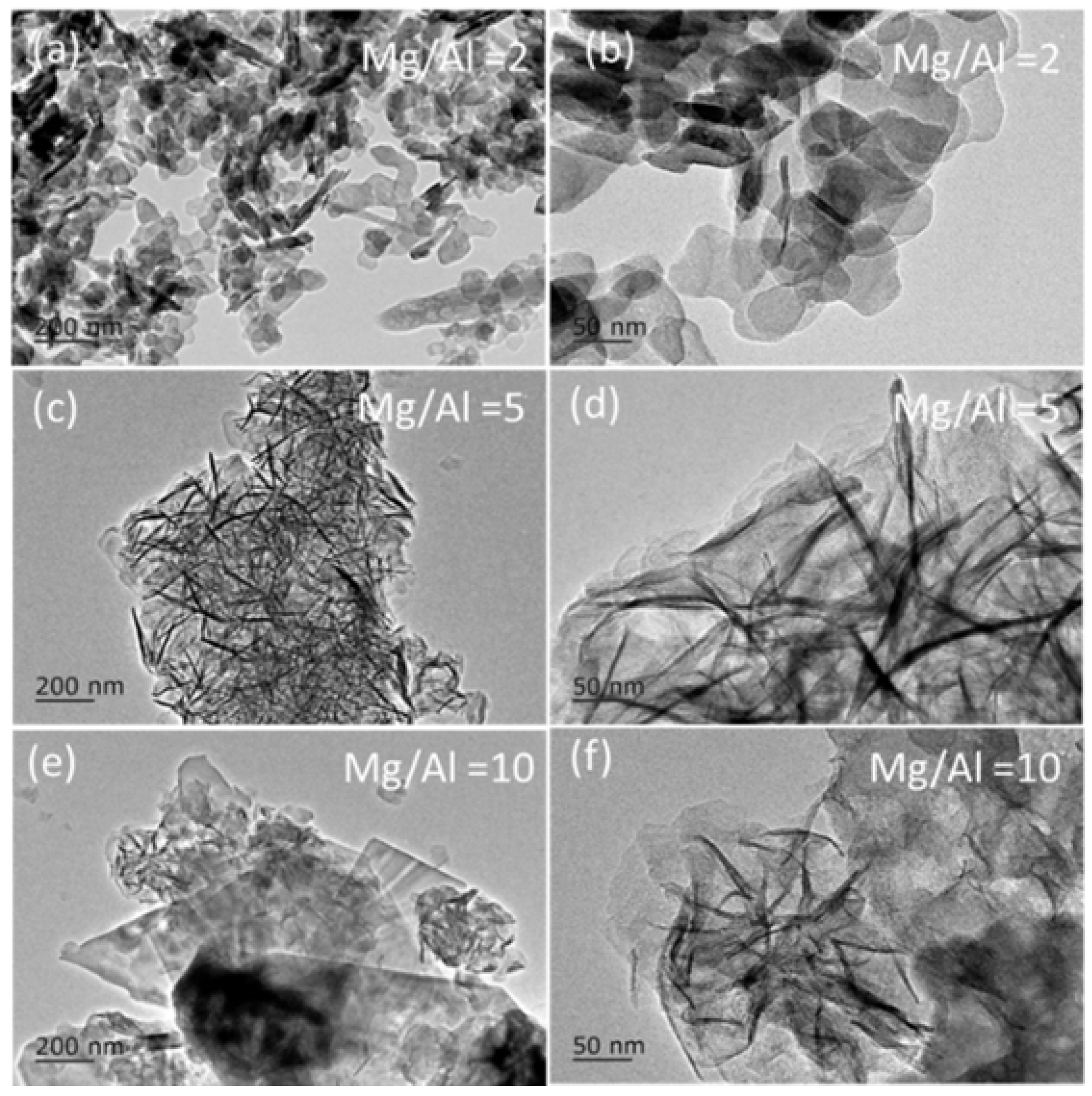
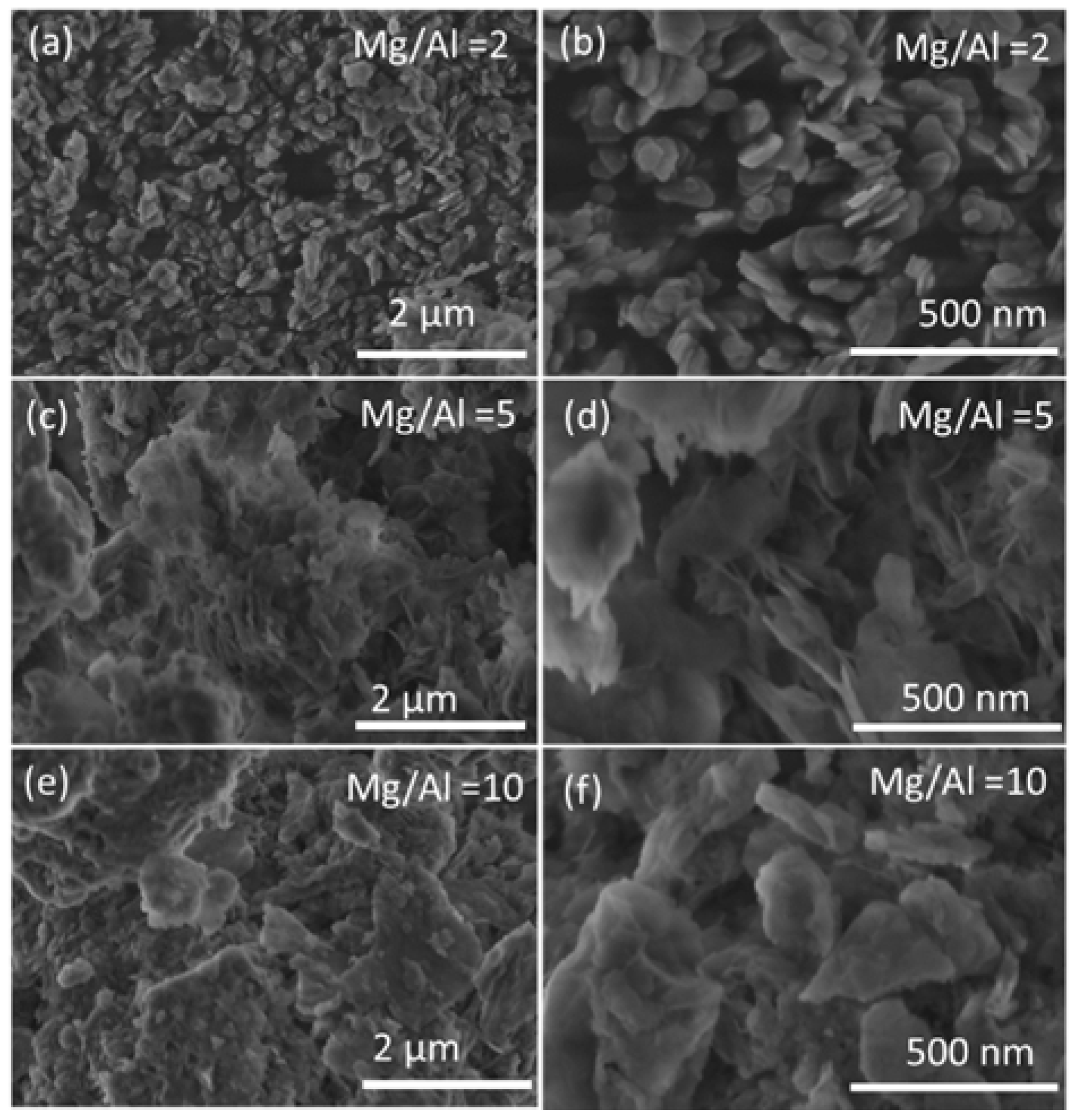
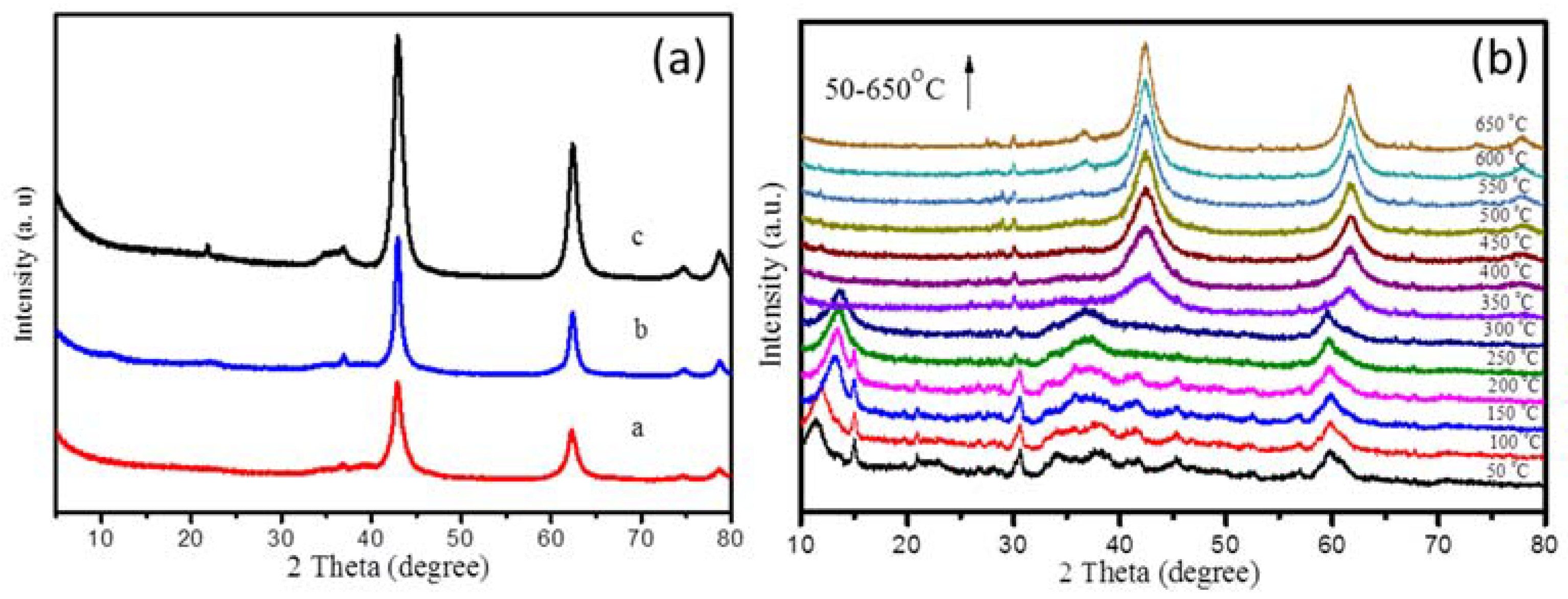
2.1. Synthesis of Mg(Al)O LDH with Different Mg:Al Ratios
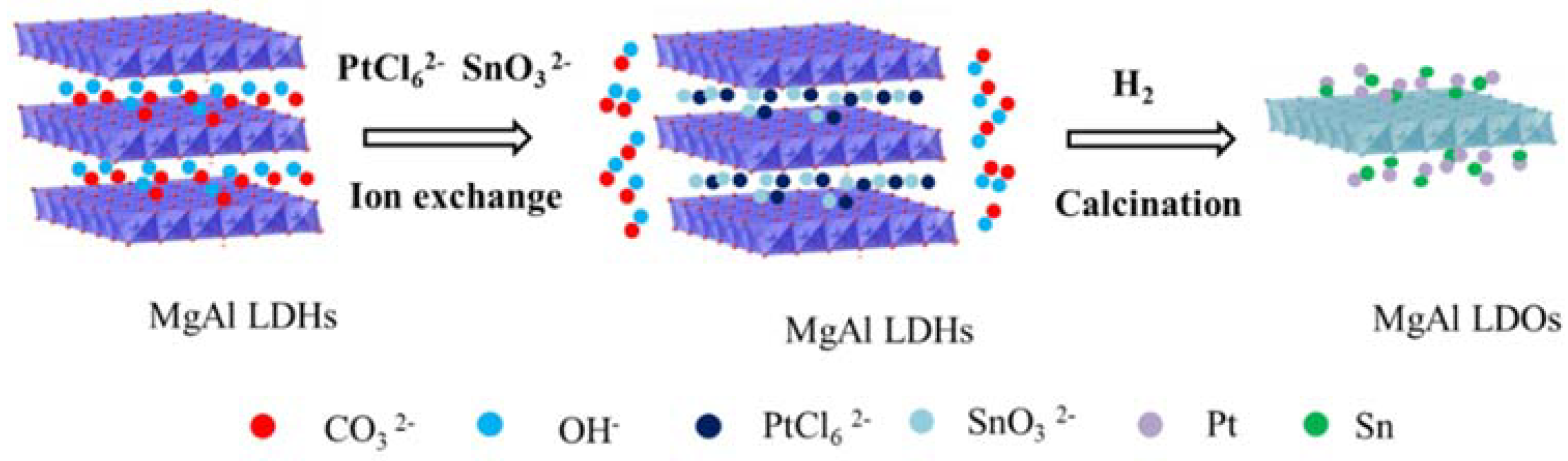
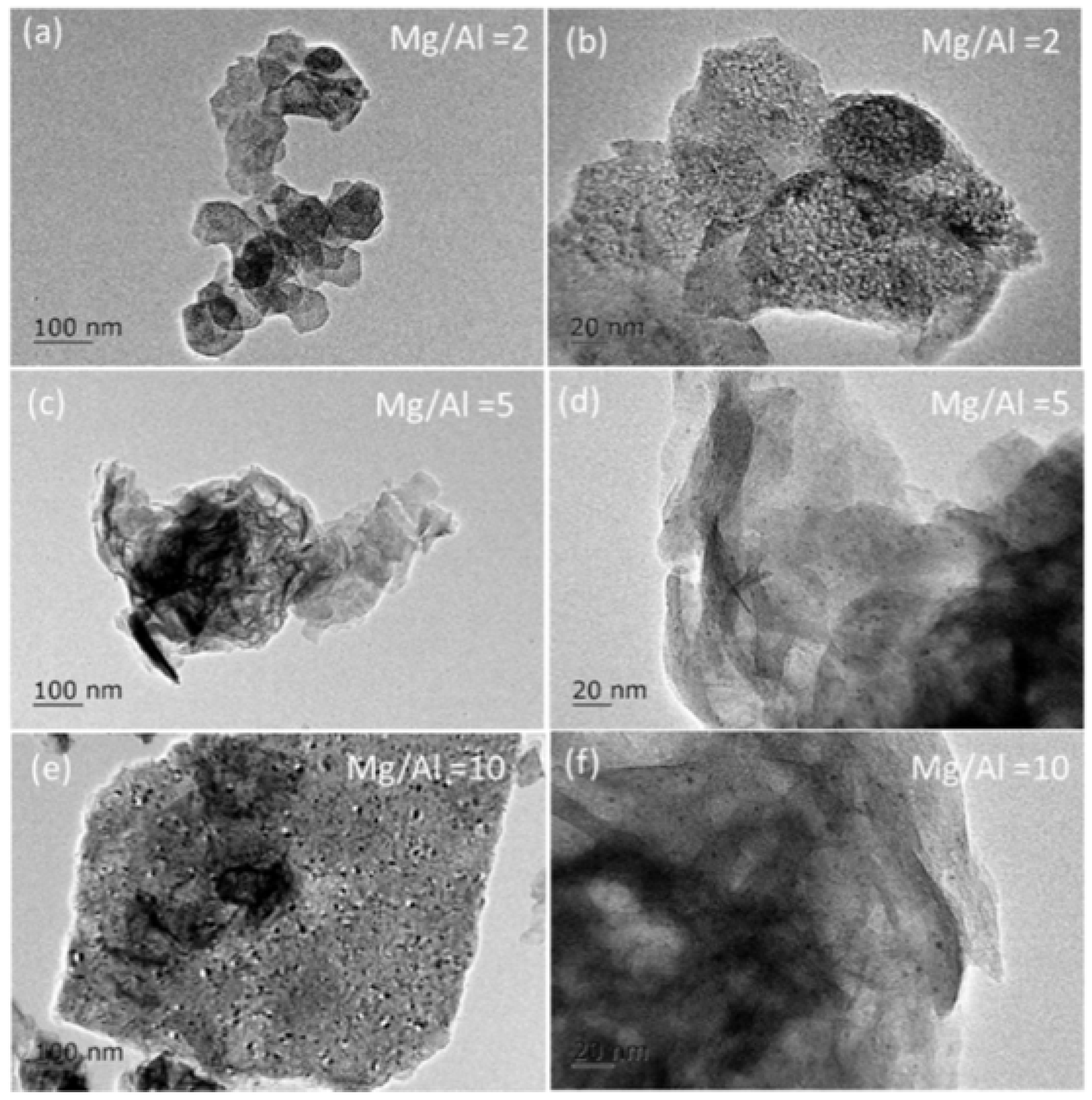
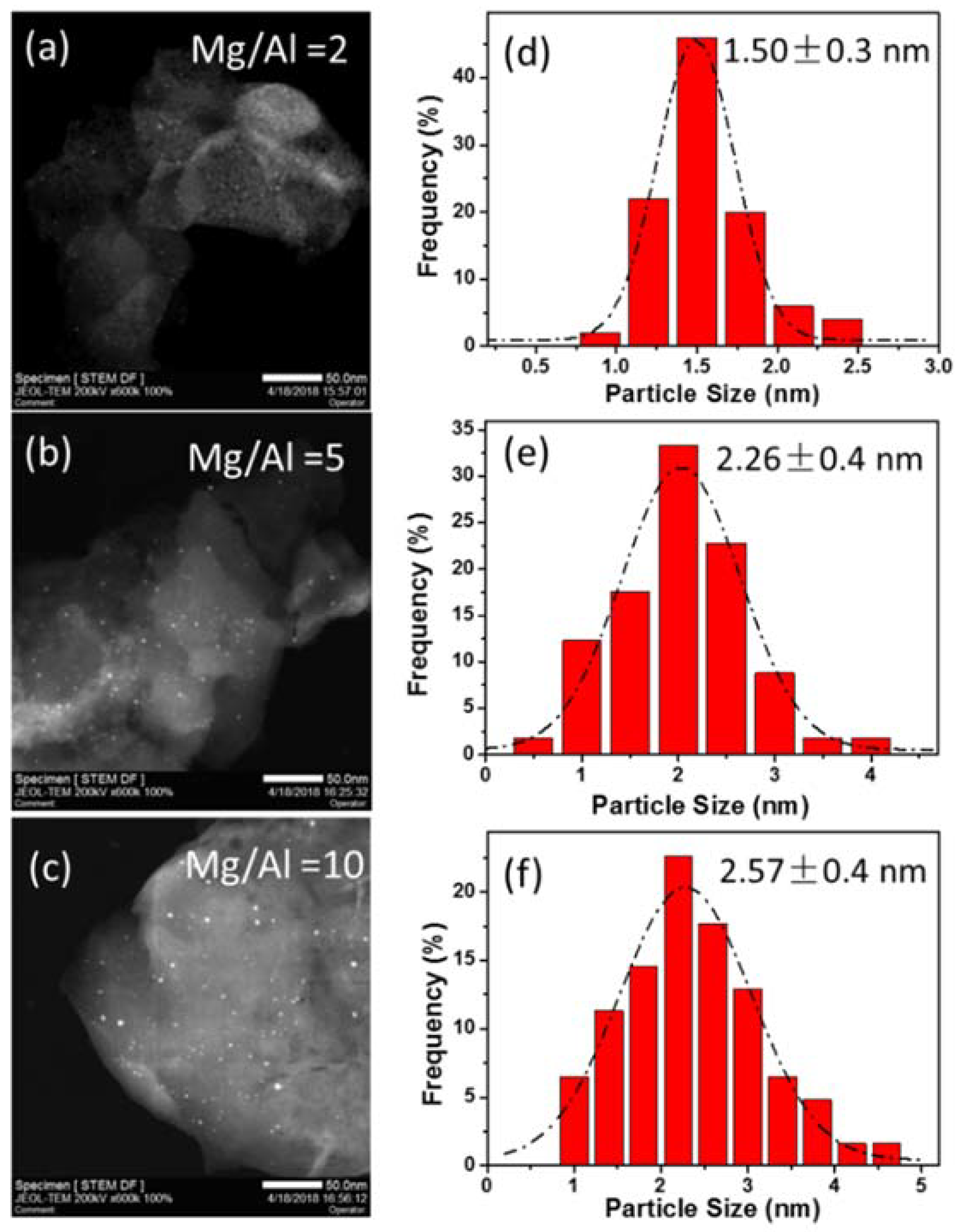
2.2. Synthesis of PtSn/Mg(Al)O via the Anion Exchange Method
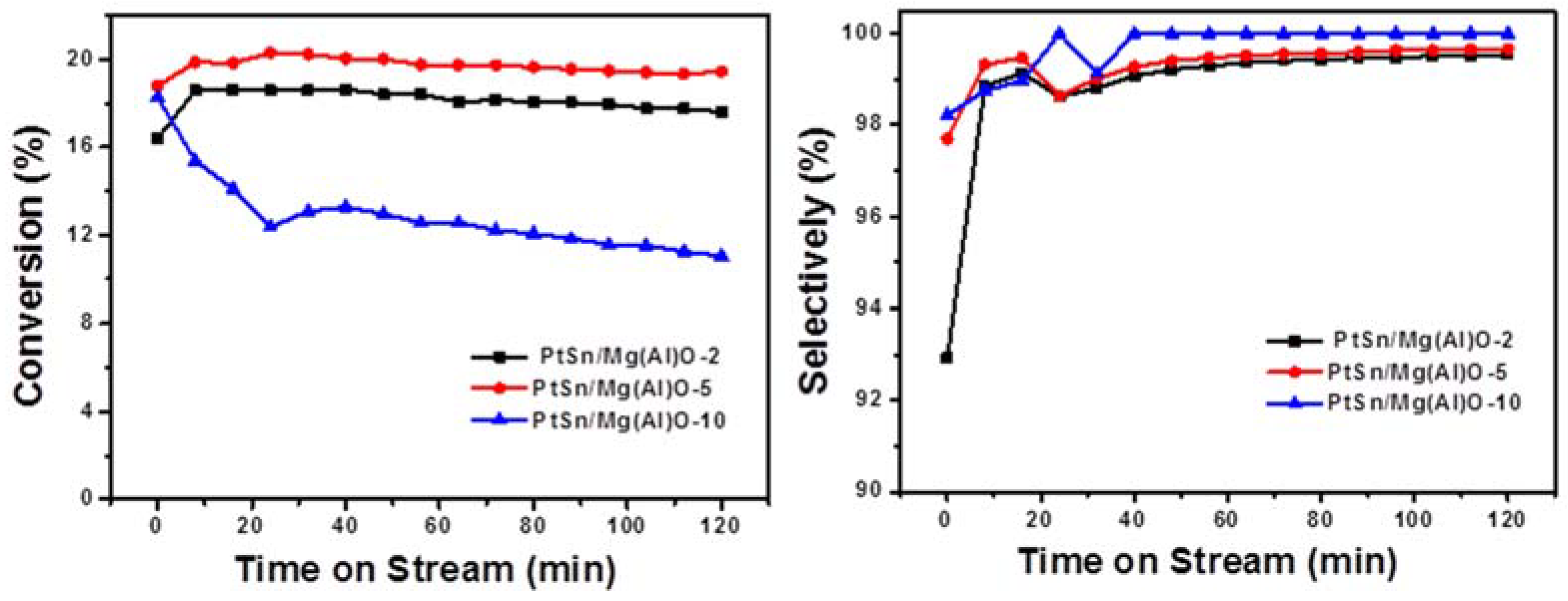
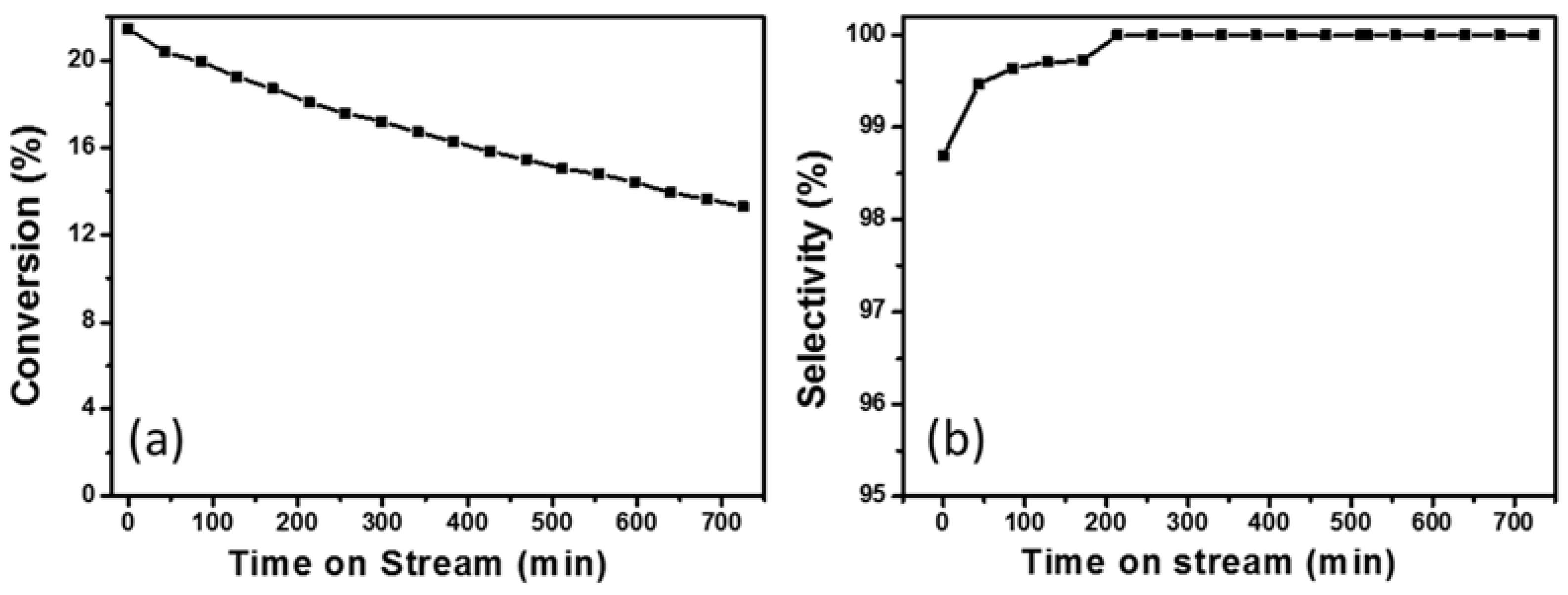
2.3. Catalytic Performances of PtSn/Mg(Al)O with Different Mg:Al Ratios in Ethane Dehydrogenation
2.4. Discussion of PtSn/Mg(Al)O with Different Mg:Al Ratios in Ethane Dehydrogenation
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kumar, A.; Bhatti, T.M.; Goldman, A.S. Dehydrogenation of Alkanes and Aliphatic Groups by Pincer-Ligated Metal Complexes. Chem. Rev. 2017, 117, 12357–12384. [Google Scholar] [CrossRef] [PubMed]
- Sattler, J.J.; Ruiz-Martinez, J.; Santillan-Jimenez, E.; Weckhuysen, B.M. Catalytic dehydrogenation of light alkanes on metals and metal oxides. Chem. Rev. 2014, 114, 10613–10653. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, Z. Light alkane dehydrogenation to light olefin technologies: A comprehensive review. Rev. Chem. Eng. 2015, 31, 413–436. [Google Scholar] [CrossRef]
- Mallikarjun Sharada, S.; Zimmerman, P.M.; Bell, A.T.; Head-Gordon, M. Insights into the kinetics of cracking and dehydrogenation reactions of light alkanes in H-MFI. J. Phys. Chem. C 2013, 117, 12600–12611. [Google Scholar] [CrossRef]
- Bhasin, M.; McCain, J.; Vora, B.; Imai, T.; Pujado, P. Dehydrogenation and oxydehydrogenation of paraffins to olefins. Appl. Catal. A 2001, 221, 397–419. [Google Scholar] [CrossRef]
- Saeedizad, M.; Sahebdelfar, S.; Mansourpour, Z. Deactivation kinetics of platinum-based catalysts in dehydrogenation of higher alkanes. Chem. Eng. J. 2009, 154, 76–81. [Google Scholar] [CrossRef]
- Iglesias-Juez, A.; Beale, A.M.; Maaijen, K.; Weng, T.C.; Glatzel, P.; Weckhuysen, B.M. A combined in situ time-resolved UV-Vis, Raman and high-energy resolution X-ray absorption spectroscopy study on the deactivation behavior of Pt and Pt Sn propane dehydrogenation catalysts under industrial reaction conditions. J. Catal. 2010, 276, 268–279. [Google Scholar] [CrossRef]
- Liu, G.; Zeng, L.; Zhao, Z.-J.; Tian, H.; Wu, T.; Gong, J. Platinum-Modified ZnO/Al2O3 for Propane Dehydrogenation: Minimized Platinum Usage and Improved Catalytic Stability. ACS Catal. 2016, 6, 2158–2162. [Google Scholar] [CrossRef]
- Hauser, A.W.; Horn, P.R.; Head-Gordon, M.; Bell, A.T. A systematic study on Pt based, subnanometer-sized alloy cluster catalysts for alkane dehydrogenation: effects of intermetallic interaction. Phys. Chem. Chem. Phys. 2016, 18, 10906–10917. [Google Scholar] [CrossRef] [PubMed]
- Llorca, J.; Homs, N.; Leon, J.; Sales, J.; Fierro, J.; De La Piscina, P.R. Supported Pt–Sn catalysts highly selective for isobutane dehydrogenation: preparation, characterization and catalytic behavior. Appl. Catal. A-Gen. 1999, 189, 77–86. [Google Scholar] [CrossRef]
- Pham, H.N.; Sattler, J.J.; Weckhuysen, B.M.; Datye, A.K. Role of Sn in the Regeneration of Pt/gamma-Al2O3 Light Alkane Dehydrogenation Catalysts. ACS Catal. 2016, 6, 2257–2264. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Lin, S.; Goetze, J.; Pletcher, P.D.; Kovarik, L.; Artyushkova, K.; Guo, H.; Weckhuysen, B.; Datye, A. Thermally Stable and Regenerable Pt-Sn Clusters for Propane Dehydrogenation Prepared via Atom Trapping on Ceria. Angew. Chem. Int. Ed. 2017, 131, 8986–8991. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Peng, Z.; Bell, A.T. Effects of composition and metal particle size on ethane dehydrogenation over PtxSn100-x/Mg(Al)O (70 ≤ x ≤ 100). J. Catal. 2014, 311, 161–168. [Google Scholar] [CrossRef]
- Siri, G.J.; Casella, M.L.; Santori, G.F.; Ferretti, O.A. Tin/platinum on alumina as catalyst for dehydrogenation of isobutane. Influence of the preparation procedure and of the addition of lithium on the catalytic properties. Ind. Eng. Chem. Res. 1997, 36, 4821–4826. [Google Scholar] [CrossRef]
- Vértes, C.; Tálas, E.; Czakó-Nagy, I.; Ryczkowski, J.; Göbölös, S.; Vértes, A.; Margitfalvi, J. Mössbauer spectroscopy studies of Sn-Pt/Al2O3 catalysts prepared by controlled surface reactions. Appl. Catal. 1991, 68, 149–159. [Google Scholar] [CrossRef]
- Baronetti, G.T.; de Miguel, S.R.; Scelza, O.A.; Castro, A.A. State of metallic phase in PtSn/Al2O3 catalysts prepared by different deposition techniques. Appl. Catal. 1986, 24, 109–116. [Google Scholar] [CrossRef]
- Siri, G.J.; Bertolini, G.R.; Casella, M.L.; Ferretti, O.A. PtSn/γ-Al2O3 isobutane dehydrogenation catalysts: the effect of alkaline metals addition. Mater. Lett. 2005, 59, 2319–2324. [Google Scholar] [CrossRef]
- Siddiqi, G.; Sun, P.; Galvita, V.; Bell, A.T. Catalyst performance of novel Pt/Mg(Ga)(Al)O catalysts for alkane dehydrogenation. J. Catal. 2010, 274, 200–206. [Google Scholar] [CrossRef]
- Di Cosimo, J.; Dıez, V.; Xu, M.; Iglesia, E.; Apesteguıa, C. Structure and surface and catalytic properties of Mg-Al basic oxides. J. Catal. 1998, 178, 499–510. [Google Scholar] [CrossRef]
- Fang, S.; Zhang, K.; Wang, C.; Ma, L.; Zhang, Q.; Liu, Q.; Chen, L.; Chen, L.; Zhang, Q.; Tian, Z. The properties and catalytic performance of PtSn/Mg (x-Ga) AlO catalysts for ethane dehydrogenation. RSC Adv. 2017, 7, 22836–22844. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Wharton, D.; Hickey, L.; Frost, R.L. Infrared and Raman study of interlayer anions CO32−, NO3−, SO42−, and ClO4− in Mg/Al hydrotalcite. Am. Miner. 2002, 87, 623–629. [Google Scholar] [CrossRef]
- Virnovskaia, A.; Morandi, S.; Rytter, E.; Ghiotti, G.; Olsbye, U. Characterization of Pt, Sn/Mg (Al) O catalysts for light alkane dehydrogenation by FT-IR spectroscopy and catalytic measurements. J. Phys. Chem. C 2007, 111, 14732–14742. [Google Scholar] [CrossRef]
- Miyata, S. Anion-Exchange Properties of Hydrotalcite-Like Compounds. Clay. Clay Miner. 1983, 31, 305–311. [Google Scholar] [CrossRef]
- Lee, J.H.; Rhee, S.W.; Jung, D.Y. Solvothermal anion exchange of aliphatic dicarboxylates into the gallery space of layered double hydroxides immobilized on Si substrates. Chem. Mater. 2004, 16, 3774–3779. [Google Scholar] [CrossRef]
- Wang, D.-Y.; Costa, F.R.; Vyalikh, A.; Leuteritz, A.; Scheler, U.; Jehnichen, D.; Wagenknecht, U.; Häussler, L.; Heinrich, G. One-Step Synthesis of Organic LDH and Its Comparison with Regeneration and Anion exchange Method. Chem. Mater. 2009, 21, 4490–4497. [Google Scholar] [CrossRef]
- Rodriguez, P.G.; de Ruiter, M.; Wijnands, T.; Ten Elshof, J. Porous layered double hydroxides synthesized using oxygen generated by decomposition of hydrogen peroxide. Sci. Rep-UK 2017, 7. [Google Scholar]
- Sideris, P.J.; Nielsen, U.G.; Gan, Z.; Grey, C.P. Mg/Al ordering in layered double hydroxides revealed by multinuclear NMR spectroscopy. Science 2008, 321, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, R.; Pires, L.H.; de Souza, R.C.; Zamian, J.; de Souza, A.; da Rocha Filho, G.; da Costa, C. Thermal characterization of hydrotalcite used in the transesterification of soybean oil. J. Therm. Anal. Calorim. 2009, 97, 163. [Google Scholar] [CrossRef]
- Ma, R.; Wang, Z.; Yan, L.; Chen, X.; Zhu, G. Novel Pt-loaded layered double hydroxide nanoparticles for efficient and cancer-cell specific delivery of a cisplatin prodrug. J. Mater. Chem. B 2014, 2, 4868. [Google Scholar] [CrossRef]
- Belskaya, O.B.; Gulyaeva, T.I.; Talsi, V.P.; Kazakov, M.O.; Nizovskii, A.I.; Kalinkin, A.V.; Bukhtiyarov, V.I.; Likholobov, V.A. Formation of platinum sites on layered double hydroxide type basic supports: III. Effect of the mechanism of [PtCl6]2− complex binding to aluminum-magnesium layered double hydroxides on the properties of supported platinum in Pt/MgAlO x catalysts. Kinet. Catal. 2014, 55, 786–792. [Google Scholar] [CrossRef]
- Wu, J.; Sharada, S.M.; Ho, C.; Hauser, A.W.; Head-Gordon, M.; Bell, A.T. Ethane and propane dehydrogenation over PtIr/Mg (Al) O. Appl. Catal. A 2015, 506, 25–32. [Google Scholar] [CrossRef]
- Millange, F.; Walton, R.I.; O’Hare, D. Time-resolved in situ X-ray diffraction study of the liquid-phase reconstruction of Mg-Al-carbonate hydrotalcite-like compounds. J. Mater. Chem. 2000, 10, 1713–1720. [Google Scholar] [CrossRef]
- Xia, K.; Lang, W.-Z.; Li, P.-P.; Long, L.-L.; Yan, X.; Guo, Y.-J. The influences of Mg/Al molar ratio on the properties of PtIn/Mg(Al)O-x catalysts for propane dehydrogenation reaction. Chem. Eng. J. 2016, 284, 1068–1079. [Google Scholar] [CrossRef]
- Xia, K.; Lang, W.-Z.; Li, P.-P.; Yan, X.; Guo, Y.-J. The properties and catalytic performance of PtIn/Mg(Al)O catalysts for the propane dehydrogenation reaction: Effects of pH value in preparing Mg(Al)O supports by the co-precipitation method. J. Catal. 2016, 338, 104–114. [Google Scholar] [CrossRef]
- Shen, L.-L.; Xia, K.; Lang, W.-Z.; Chu, L.-F.; Yan, X.; Guo, Y.-J. The effects of calcination temperature of support on PtIn/Mg (Al) O catalysts for propane dehydrogenation reaction. Chem. Eng. J. 2017, 324, 336–346. [Google Scholar] [CrossRef]
- Liu, X.; Lang, W.-Z.; Long, L.-L.; Hu, C.-L.; Chu, L.-F.; Guo, Y.-J. Improved catalytic performance in propane dehydrogenation of PtSn/γ-Al2O3 catalysts by doping indium. Chem. Eng. J. 2014, 247, 183–192. [Google Scholar] [CrossRef]
- Galvita, V.; Siddiqi, G.; Sun, P.; Bell, A.T. Ethane dehydrogenation on Pt/Mg(Al)O and PtSn/Mg(Al)O catalysts. J. Catal. 2010, 271, 209–219. [Google Scholar] [CrossRef]
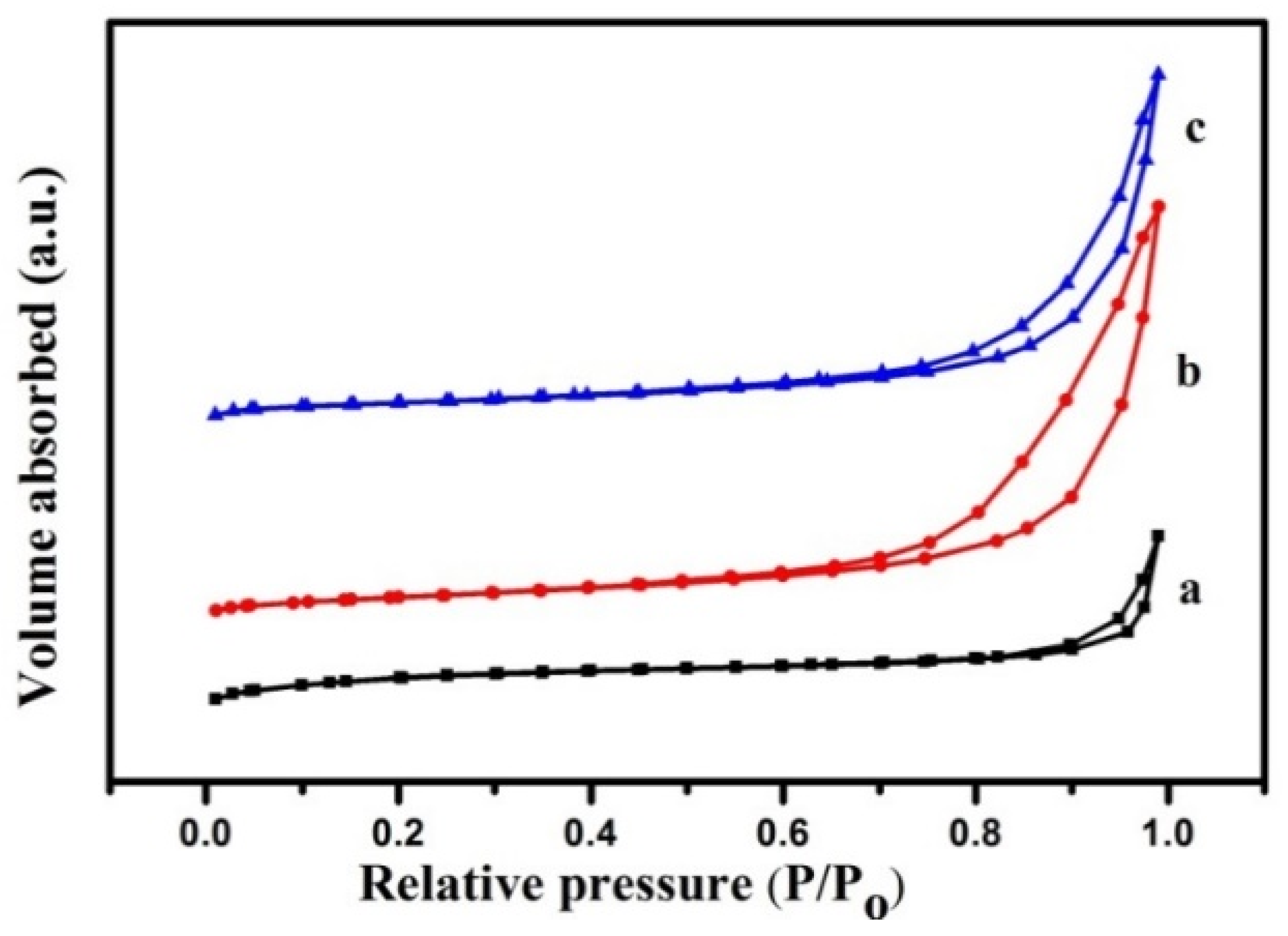
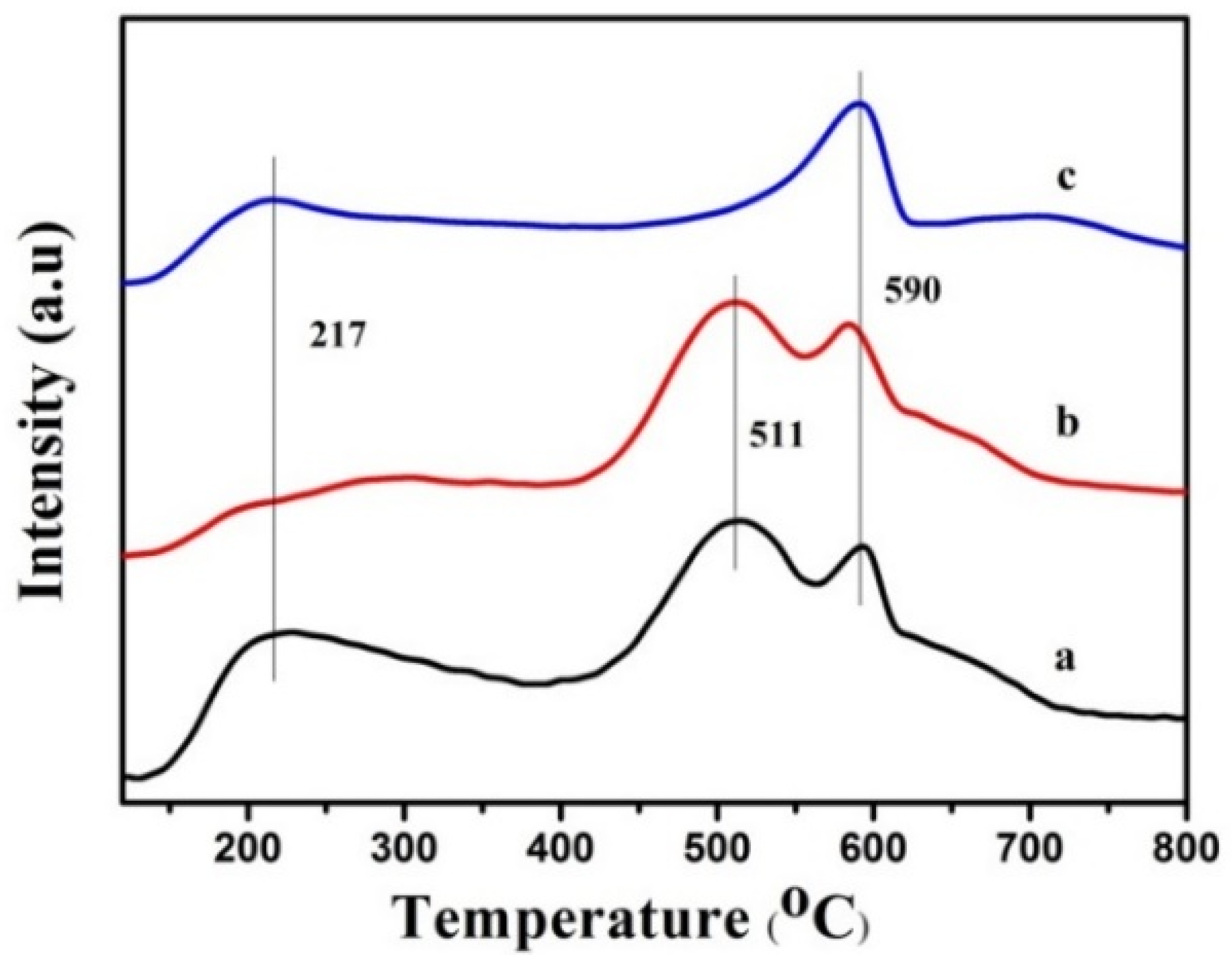


| Mg:Al Molar Ratio | Sample Mass (g) | Pt (mg/L) | Percentage of Pt (wt%) | Sn (mg/L) | Percentage of Sn (wt%) |
|---|---|---|---|---|---|
| 2:1 | 0.1006 | 3.426 | 0.34 | 0.836 | 0.08 |
| 5:1 | 0.1083 | 3.780 | 0.35 | 1.074 | 0.09 |
| 10:1 | 0.1006 | 3.288 | 0.33 | 0.842 | 0.08 |
| Sample | Mg:Al Molar Ratio | Surface Area (m2/g) | Pore Volume (cm3/g) |
|---|---|---|---|
| Mg(Al)O-2 | 2:1 | 188.0 | 0.30 |
| Mg(Al)O-5 | 5:1 | 113.0 | 0.65 |
| Mg(Al)O-10 | 10:1 | 84.8 | 0.54 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, S.; Bi, K.; Zhang, Q.; Chen, L.; Sun, Y.; Huang, H.; Ma, L.; Wang, C. Performance of Ethane Dehydrogenation over PtSn Loaded onto a Calcined Mg(Al)O LDH with Three Mg:Al Molar Ratios Using a Novel Method. Catalysts 2018, 8, 296. https://doi.org/10.3390/catal8080296
Fang S, Bi K, Zhang Q, Chen L, Sun Y, Huang H, Ma L, Wang C. Performance of Ethane Dehydrogenation over PtSn Loaded onto a Calcined Mg(Al)O LDH with Three Mg:Al Molar Ratios Using a Novel Method. Catalysts. 2018; 8(8):296. https://doi.org/10.3390/catal8080296
Chicago/Turabian StyleFang, Shuqi, Kang Bi, Qiao Zhang, Lingpeng Chen, Yongming Sun, Hongyu Huang, Longlong Ma, and Chenguang Wang. 2018. "Performance of Ethane Dehydrogenation over PtSn Loaded onto a Calcined Mg(Al)O LDH with Three Mg:Al Molar Ratios Using a Novel Method" Catalysts 8, no. 8: 296. https://doi.org/10.3390/catal8080296
APA StyleFang, S., Bi, K., Zhang, Q., Chen, L., Sun, Y., Huang, H., Ma, L., & Wang, C. (2018). Performance of Ethane Dehydrogenation over PtSn Loaded onto a Calcined Mg(Al)O LDH with Three Mg:Al Molar Ratios Using a Novel Method. Catalysts, 8(8), 296. https://doi.org/10.3390/catal8080296




