DRIFT Study on Promotion Effect of the Keggin Structure over V2O5-MoO3/TiO2 Catalysts for Low Temperature NH3-SCR Reaction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalytic Activity
2.2. Adsorption Behaviors of Reactants on the Surface of the Catalysts (In Situ DRIFT)
2.2.1. NH3 Adsorption on the Surface of the Catalysts
2.2.2. Co-Adsorption of NO + O2 on the Surface of the Catalysts
2.2.3. NO + O2 Adsorption on the Surface of the Catalysts after NH3 Pre-Adsorption
2.2.4. NH3 Adsorption on the Surface of the Catalysts after NO + O2 Pre-Adsorption
2.3. Effect of NH4+ in (NH3)4PMo12O40 for NH3-SCR
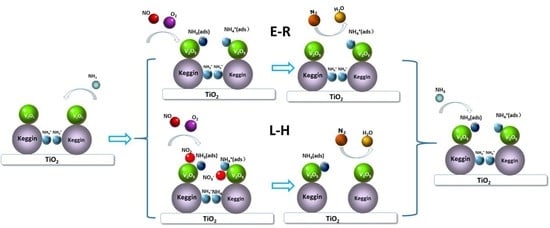
2.4. Reaction Mechanism
3. Experimental
3.1. Catalyst Preparation
3.2. Catalytic Activity Test
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Amiridis, M.D.; Duevel, R.V.; Wachs, I.E. The effect of metal oxide additives on the activity of V2O5/TiO2 catalysts for the selective catalytic reduction of nitric oxide by ammonia. Appl. Catal. B Environ. 1999, 20, 111–122. [Google Scholar] [CrossRef]
- Busca, G.; Lietti, L.; Ramis, G.; Berti, F. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: A review. Appl. Catal. B Environ. 1998, 18, 1–36. [Google Scholar] [CrossRef]
- Li, P.; Liu, Q.; Liu, Z. Behaviors of NH4HSO4 in SCR of NO by NH3 over different cokes. Chem. Eng. J. 2012, 181–182, 169–173. [Google Scholar] [CrossRef]
- Kamata, H.; Takahashi, K.; Odenbrand, C.I. Surface acid property and its relation to SCR activity of phosphorus added to commercial V2O5 (WO3)/TiO2 catalyst. Catal. Lett. 1998, 53, 65–71. [Google Scholar] [CrossRef]
- Putluru, S.S.R.; Schill, L.; Godiksen, A.; Poreddy, R.; Mossin, S.; Jensen, A.D.; Fehrmann, R. Promoted V2O5/TiO2 catalysts for selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. B Environ. 2016, 183, 282–290. [Google Scholar] [CrossRef] [Green Version]
- Hill, C.L. Foreword: Polyoxometalates in catalysis. J. Mol. Catal. A Chem. 1996, 114, 1. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Lu, J.; Wu, C. The viscosity reduction of nano-keggin-K3PMo12O40 in catalytic aquathermolysis of heavy oil. Fuel 2009, 88, 1426–1434. [Google Scholar] [CrossRef]
- Mizuno, N.; Misono, M. Heterogeneous catalysis. Chem. Rev. 1998, 98, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Bélanger, R.; Moffat, J.B. The sorption and reduction of nitrogen oxides by 12-tungstophosphoric acid and its ammonium salt. Catal. Today 1998, 40, 297–306. [Google Scholar] [CrossRef]
- Yang, R.T.; Chen, N. A new approach to decomposition of nitric oxide using sorbent/catalyst without reducing gas: Use of heteropoly compounds. Ind. Eng. Chem. Res. 1994, 33, 825–831. [Google Scholar] [CrossRef]
- Vaezzadeh, K.; Petit, C.; Pitchon, V. The removal of NOx from a lean exhaust gas using storage and reduction on H3PW12O40·6H2O. Catal. Today 2002, 73, 297–305. [Google Scholar] [CrossRef]
- Yoshimoto, R.; Ninomiya, T.; Okumura, K.; Niwa, M. Cooperative effect induced by the mixing of Na-ZSM-5 and Pd/H3PW12O40/SiO2 in the selective catalytic reduction of NO with aromatic hydrocarbons. Appl. Catal. B Environ. 2007, 75, 175–181. [Google Scholar] [CrossRef]
- Putluru, S.S.R.; Jensen, A.D.; Riisager, A.; Fehrmann, R. Heteropoly acid promoted V2O5/TiO2 catalysts for NO abatement with ammonia in alkali containing flue gases. Catal. Sci. Technol. 2011, 1, 631–637. [Google Scholar] [CrossRef]
- Weng, X.; Dai, X.; Zeng, Q.; Liu, Y.; Wu, Z. DRIFT studies on promotion mechanism of H3PW12O40 in selective catalytic reduction of NO with NH3. J. Colloid Interface Sci. 2016, 461, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Lietti, L.; Nova, I.; Ramis, G.; Dall’Acqua, L.; Busca, G.; Giamello, E.; Forzatti, P.; Bregani, F. Characterization and Reactivity of V2O5–MoO3/TiO2 De-NOx SCR Catalysts. J. Catal. 1999, 187, 419–435. [Google Scholar] [CrossRef]
- Ettireddy, P.R.; Ettireddy, N.; Boningari, T.; Pardemann, R.; Smirniotis, P.G. Investigation of the selective catalytic reduction of nitric oxide with ammonia over Mn/TiO2 catalysts through transient isotopic labeling and in situ FT-IR studies. J. Catal. 2012, 292, 53–63. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, Q.; Ma, Y.; Liu, Q.; Ning, P.; Liu, X.; Wang, J.; Zhao, B.; Huang, J.; Huang, Z. Mechanism-dependent on the different CeO2 supports of phosphotungstic acid modification CeO2 catalysts for the selective catalytic reduction of NO with NH3. J. Taiwan Inst. Chem. E 2017, 71, 277–284. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, L.; Li, L.; Liu, L.; Cao, Y.; Dong, X.; Gao, F.; Deng, Y.; Tang, C.; Chen, Z.; et al. Investigation of the structure, acidity, and catalytic performance of CuO/Ti0.95Ce0.05O2 catalyst for the selective catalytic reduction of NO by NH3 at low temperature. Appl. Catal. B Environ. 2014, 150–151, 315–329. [Google Scholar] [CrossRef]
- Thirupathi, B.; Smirniotis, P.G. Co-doping a metal (Cr, Fe, Co, Ni, Cu, Zn, Ce, and Zr) on Mn/TiO2 catalyst and its effect on the selective reduction of NO with NH3 at low-temperatures. Appl. Catal. B Environ. 2011, 110, 195–206. [Google Scholar] [CrossRef]
- You, Y.; Chang, H.; Zhu, T.; Zhang, T.; Li, X.; Li, J. The poisoning effects of phosphorus on CeO2-MoO3/TiO2 DeNOx catalysts: NH3-SCR activity and the formation of N2O. Mol. Catal. 2017, 439, 15–24. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, S.; Wang, Y.; Zhong, Q. CeO2 supported on reduced TiO2 for selective catalytic reduction of NO by NH3. J. Colloid Interface Sci. 2017, 496, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Oshihara, K.; Ueda, W. Catalytic performance for propane selective oxidation and surface properties of 12-molybdophosphoric acid treated with pyridine. Appl. Catal. A Gen. 1999, 182, 357–363. [Google Scholar] [CrossRef]
- Boningari, T.; Ettireddy, P.R.; Somogyvari, A.; Liu, Y.; Vorontsov, A.; McDonald, C.A.; Smirniotis, P.G. Influence of elevated surface texture hydrated titania on Ce-doped Mn/TiO2 catalysts for the low-temperature SCR of NOx under oxygen-rich conditions. J. Catal. 2015, 325, 145–155. [Google Scholar] [CrossRef]
- Pappas, D.K.; Boningari, T.; Boolchand, P.; Smirniotis, P.G. Novel manganese oxide confined interweaved titania nanotubes for the low-temperature Selective Catalytic Reduction (SCR) of NOx by NH3. J. Catal. 2016, 334, 1–13. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, S.; Li, J.; Ma, L. Promoting effect of MoO3 on the NOx reduction by NH3 over CeO2/TiO2 catalyst studied with in situ DRIFTS. Appl. Catal. B Environ. 2014, 144, 90–95. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Zhao, Q.; Ke, J.; Xiao, H.; Lv, X.; Liu, S.; Tadé, M.; Wang, S. Mechanistic investigation of the enhanced NH3-SCR on cobalt-decorated Ce-Ti mixed oxide: In situ FTIR analysis for structure-activity correlation. Appl. Catal. B Environ. 2017, 200, 297–308. [Google Scholar] [CrossRef]
- Valyon, J.; Hall, W. Surface Species Formed from NO on Copper Zeolites. ChemInform 1993, 24. [Google Scholar] [CrossRef]
- Herring, A.M.; McCormick, R.L. In situ infrared study of the absorption of nitric oxide by 12-tungstophosphoric acid. J. Phys. Chem. B 1998, 102, 3175–3184. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Ge, M. DRIFT Study on Cerium−Tungsten/Titiania Catalyst for Selective Catalytic Reduction of NOx with NH3. Environ. Sci. Technol. 2010, 44, 9590–9596. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, J.; Liu, S.; Ren, X.; Ma, J.; Su, W.; Peng, Y. Ultra hydrothermal stability of CeO2-WO3/TiO2 for NH3-SCR of NO compared to traditional V2O5-WO3/TiO2 catalyst. Catal. Today 2015, 258, 11–16. [Google Scholar] [CrossRef]
- Toops, T.J.; Smith, D.B.; Epling, W.S.; Parks, J.E.; Partridge, W.P. Quantified NOx adsorption on Pt/K/gamma-Al2O3 and the effects of CO2 and H2O. Appl. Catal. B Environ. 2005, 58, 255–264. [Google Scholar] [CrossRef]
- Büchel, R.; Strobel, R.; Baiker, A.; Pratsinis, S.E. Flame-Made Pt/K/Al2O3 for NOx Storage–Reduction (NSR) Catalysts. Top. Catal. 2009, 52, 1799–1802. [Google Scholar] [CrossRef]
- Kamasamudram, K.; Currier, N.W.; Chen, X.; Yezerets, A. Overview of the practically important behaviors of zeolite-based urea-SCR catalysts, using compact experimental protocol. Catal. Today 2010, 151, 212–222. [Google Scholar] [CrossRef]
- Schmieg, S.J.; Oh, S.H.; Kim, C.H.; Brown, D.B.; Lee, J.H.; Peden, C.H.F.; Kim, D.H. Thermal durability of Cu-CHA NH3-SCR catalysts for diesel NOx reduction. Catal. Today 2012, 184, 252–261. [Google Scholar] [CrossRef]
- Ramis, G.; Bregani, F.; Forzatti, P. Fourier transform-infrared study of the adsorption and coadsorption of nitric oxide, nitrogen dioxide and ammonia on vanadia-titania and mechanism of selective catalytic reduction. Appl. Catal. 1990, 64, 259–278. [Google Scholar] [CrossRef]
- Topsøe, N.-Y. Characterization of the nature of surface sites on vanadia-titania catalysts by FTIR. J. Catal. 1991, 128, 499–511. [Google Scholar] [CrossRef]
- Lietti, L.; Svachula, J.; Forzatti, P.; Ramis, G.; Bregani, P. Surface and catalytic properties of Vanadia-Titania and Tungsta-Titania systems in the Selective Catalytic Reduction of nitrogen oxides. Catal. Today 1993, 17, 131–139. [Google Scholar] [CrossRef]
- Topsøe, N.-Y. Mechanism of the selective catalytic reduction of nitric oxide by ammonia elucidated by in situ on-line Fourier transform infrared spectroscopy. Science 1994, 265, 1217–1219. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, K.; Li, J. Identification of the active sites on CeO2–WO3 catalysts for SCR of NOx with NH3: An in situ IR and Raman spectroscopy study. Appl. Catal. B Environ. 2013, 140, 483–492. [Google Scholar] [CrossRef]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, R.; Zhang, N.; Li, L.; He, H.; Song, L.; Qiu, W. DRIFT Study on Promotion Effect of the Keggin Structure over V2O5-MoO3/TiO2 Catalysts for Low Temperature NH3-SCR Reaction. Catalysts 2018, 8, 143. https://doi.org/10.3390/catal8040143
Wu R, Zhang N, Li L, He H, Song L, Qiu W. DRIFT Study on Promotion Effect of the Keggin Structure over V2O5-MoO3/TiO2 Catalysts for Low Temperature NH3-SCR Reaction. Catalysts. 2018; 8(4):143. https://doi.org/10.3390/catal8040143
Chicago/Turabian StyleWu, Rui, Ningqiang Zhang, Lingcong Li, Hong He, Liyun Song, and Wenge Qiu. 2018. "DRIFT Study on Promotion Effect of the Keggin Structure over V2O5-MoO3/TiO2 Catalysts for Low Temperature NH3-SCR Reaction" Catalysts 8, no. 4: 143. https://doi.org/10.3390/catal8040143