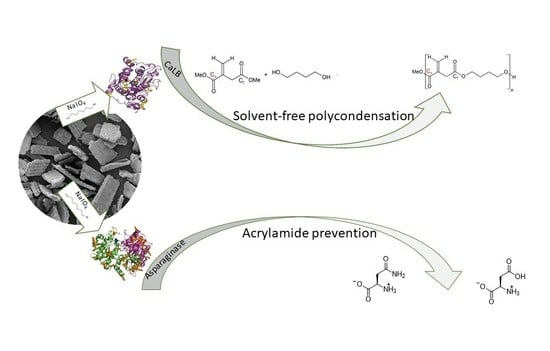
Rice Husk as an Inexpensive Renewable Immobilization Carrier for Biocatalysts Employed in the Food, Cosmetic and Polymer Sectors
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphological Characterization of the Rice Husk
2.2. Functionalization of RH with Amine Groups and Immobilization of CaLB
2.3. Covalent Immobilization of Asparaginases on Functionalized Rice Husk
3. Materials and Methods
3.1. Materials
3.2. Grinding and Sieving
3.3. SEM Microscopy
3.4. Light and Fluorescence Microscopy
3.5. 1H-NMR Spectra Related to Polycondensation of DMA and BDO
3.6. Thin Layer Chromatography (TLC)
3.7. Electrospray Ionization Mass Spectrometry (ESI-MS)
3.8. Moisture Determination
3.9. Titrations
3.10. Lipase Hydrolytic Activity (Tributyrin Assay)
3.11. Asparaginase Assay
3.12. FT-IR
3.13. UV-Vis
3.14. Determination of the Content of Carbonyl Groups
3.15. Determination of the Content of Carboxylic Groups with the Conductimetric Method
3.16. Oxidation of Rice Husk with Sodium Periodate
3.17. Functionalization of Oxidized Rice Husk with HMDA Diamine Spacer
3.18. Activation of Amine Functionalized Rice Husk with Glutaraldehyde Prior to Immobilization
3.19. Determination of the Leaching of the Enzymes after Covalent Immobilization
3.20. Immobilization of the CaLB on the Rice Husks Oxidized with Metaperiodate and Functionalized with Diamine Spacer
3.21. Immobilization of the CaLB on EC-EP/S Epoxy Methacrylic Resins
3.22. Lipase Catalyzed Synthesis of Propyl Laurate
3.23. Solvent-Free Polycondensation of Dimethyl Itaconate with 1,4-butandiol Catalyzed by CaLB Immobilized on Epoxy Methacrylic Resins
3.24. Solvent-Free Polycondensation of Dimethyl Itaconate with 1,4-butandiol Catalyzed by CaLB Immobilized on Functionalized Rice Husk
3.25. Lipase Hydrolytic Activity Assay for Determining Tributyrin Units (TBU)
3.26. Immobilization of Acrylaway L on Activated Rice Husk
3.27. Immobilization of Acrylaway High-T on Activated Rice Husk
3.28. Immobilization of Acrylaway L on EC-EP/S Resins
3.29. Immobilization of Acrylaway High-T on EC-EP/S Resins
3.30. Determination of Water Content in Enzymatic Preparations
3.31. Computational Construction of 3D Models and Analysis of the Surface of Asparaginases
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cantone, S.; Spizzo, P.; Fattor, D.; Ferrario, V.; Ebert, C.; Gardossi, L. Lipases for bio-based chemistry-Efficient immobilised biocatalysts for competitive biocatalysed processes. Chem. Today 2012, 30, 10–14. [Google Scholar] [CrossRef] [Green Version]
- Di Cosimo, R.; McAuliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef] [PubMed]
- Tufvesson, P.; Lima-Ramos, J.; Nordblad, M.; Woodley, J.M. Guidelines and Cost Analysis for Catalyst Production in Biocatalytic Processes. Org. Process Res. Dev. 2011, 15, 266–274. [Google Scholar] [CrossRef]
- Liu, Y.; Andryszkiewicz, M.; Peña, R. Outcome of a public consultation on the draft Statement on Exposure Assessment of Food Enzymes. EFSA Support. Publ. 2016, 13, 1106E. [Google Scholar] [CrossRef]
- Pellis, A.; Cantone, S.; Ebert, C.; Gardossi, L. Evolving biocatalysis to meet bioeconomy challenges and opportunities. New Biotechnol. 2017, 40, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Cantone, S.; Ferrario, V.; Corici, L.; Ebert, C.; Fattor, D.; Spizzo, P.; Gardossi, L. Efficient immobilisation of industrial biocatalysts: Criteria and constraints for the selection of organic polymeric carriers and immobilisation methods. Chem. Soc. Rev. 2013, 42, 6262–6276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravindran, R.; Jaiswal, A.K. Exploitation of food industry waste for high-value products. Trends Biotechnol. 2016, 34, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Enzyme Immobilization: The Quest for Optimum Performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Pellis, A.; Ferrario, V.; Cespugli, M.; Corici, L.; Guarneri, A.; Zartl, B.; Herrero Acero, E.; Ebert, C.; Guebitz, G.M.; Gardossi, L. Fully renewable polyesters via polycondensation catalyzed by Thermobifida cellulosilytica cutinase 1: An integrated approach. Green Chem. 2017, 19, 490–502. [Google Scholar] [CrossRef]
- Corici, L.; Ferrario, V.; Pellis, A.; Ebert, C.; Lotteria, S.; Cantone, S.; Voinovich, D.; Gardossi, L. Large scale applications of immobilized enzymes call for sustainable and inexpensive solutions: Rice husks as renewable alternatives to fossil-based organic resins. RSC Adv. 2016, 6, 63256–63270. [Google Scholar] [CrossRef]
- Contreras, L.M.; Schelle, H.; Sebrango, C.R.; Pereda, I. Methane potential and biodegradability of rice straw, rice husk and rice residues from the drying. Water Sci. Technol. 2012, 65, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://echa.europa.eu/regulations/reach (accessed on 23 March 2018).
- Wartelle, L.H.; Marshall, W.E. Quaternized agricultural by-products as anion exchange resins. J. Environ. Manag. 2005, 78, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Prado, H.J.; Matulewicz, M.C. Cationization of polysaccharides: A path to greener derivatives with many industrial applications. Eur. Polym. J. 2014, 52, 53–75. [Google Scholar] [CrossRef]
- Sud, D.; Mahajan, G.; Kaur, M.P. Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions—A review. Bioresour. Technol. 2008, 14, 6017–6602. [Google Scholar] [CrossRef] [PubMed]
- Tantrakulsiri, J.; Jeyashoke, N.; Krisanangkura, K.J. Utilization of rice hull ash as a support material for immobilization of Candida cylindracea lipase. Am. Oil Chem. Soc. 1997, 74, 173–175. [Google Scholar] [CrossRef]
- Park, B.; Wi, G.S.; Lee, H.K.; Singh, P.A.; Yoon, T.; Kim, S.Y. Characterization of anatomical features and silica distribution in rice husk using microscopic and micro-analytical techniques. Biomass Bioenergy 2003, 25, 319–327. [Google Scholar] [CrossRef]
- Coletta, C.V.; Rezende, V.C.; da Conceicao, F.R.; Polikarpov, I.; Giumaraes, G.E.F. Mapping the lignin distribution in pretreated sugarcane bagasse by confocal and fluorescence lifetime imaging microscopy. Biotechnol. Biofuels 2013, 6, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, B.; Hu, J.; Miller, M.E.; Xie, W.; Cai, M.; Gross, A.R. Candida antarctica lipase B chemically immobilized on epoxy-activated micro- and nanobeads: Catalysts for polyester synthesis. Biomacromolecules 2008, 9, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Kodama, Y. Time Gating of Chloroplast Autofluorescence Allows Clearer Fluorescence Imaging in Planta. PLoS ONE 2016, 11, e0152484. [Google Scholar] [CrossRef] [PubMed]
- Schoevaart, R.; Siebum, A.; Van Rantwijk, F.; Sheldon, R.; Kieboom, T. Glutaraldehyde Cross-link Analogues from Carbohydrates. Starch 2005, 57, 161–165. [Google Scholar] [CrossRef]
- Kobayashi, M.; Takatsu, K. Cross-linked stabilization of trypsin with dextran-dialdehyde. Biosci. Biotech. Biochem. 1994, 58, 275–278. [Google Scholar] [CrossRef]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques. 2014, 37, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Tashima, T.; Imai, M.; Kuroda, Y.; Yagi, S.; Nakagawa, T. Structure of a New Oligomer of Glutaraldehyde Produced by Aldol Condensation Reaction. J. Org. Chem. 1991, 2, 694–697. [Google Scholar] [CrossRef]
- Guigo, N.; Mazeau, K.; Putaux, J.L.; Heux, L. Surface modification of cellulose microfibrils by periodate oxidation and subsequent reductive amination with benzylamine: A topochemical study. Cellulose 2014, 21, 4119–4133. [Google Scholar] [CrossRef]
- Zhao, H.; Heindel, D.N. Determination of degree of substitution of formyl groups in polyaldehyde dextran by the hydroxylamine hydrochloride method. Pharm. Res. 1991, 8, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y.; Chanzy, H.; Vignon, M.R. TEMPO-mediated surface oxidation of cellulose whiskers. Cellulose 2006, 13, 679–687. [Google Scholar] [CrossRef] [Green Version]
- Perez, S.D.; Montanari, S.; Vignon, M.R. TEMPO-mediated oxidation of cellulose III. Biomacromolecules 2003, 4, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Monsan, P. Enzymes Immobilized on a Solid Support Containing Cellulose and Lignin. U.S. Patent 4405715, 20 September 1983. [Google Scholar]
- Pellis, A.; Corici, L.; Sinigoi, L.; D’Amelio, N.; Fattor, D.; Ferrario, V.; Ebert, C.; Gardossi, L. Towards feasible and scalable solvent-free enzymatic polycondensations: Integrating robust biocatalysts with thin film reactions. Green Chem. 2015, 17, 1756–1766. [Google Scholar] [CrossRef] [Green Version]
- Corici, L.; Pellis, A.; Ferrario, V.; Ebert, E.; Cantone, S.; Gardossi, L. Understanding Potentials and Restrictions of Solvent-Free Enzymatic Polycondensation of Itaconic Acid: An Experimental and Computational Analysis. Adv. Synth. Catal. 2015, 357, 1763–1774. [Google Scholar] [CrossRef] [Green Version]
- Ansorge-Schumacher, M.B.; Thum, O. Immobilised lipases in the cosmetics industry. Chem. Soc. Rev. 2013, 42, 6475–6490. [Google Scholar] [CrossRef] [PubMed]
- Pellis, A.; Acero, E.H.; Gardossi, L.; Ferrario, V.; Guebitz, G.M. Renewable building blocks for sustainable polyesters: New biotechnological routes for greener plastics. Polym. Int. 2016, 65, 861–871. [Google Scholar] [CrossRef]
- Pellis, A.; Guarneri, A.; Brandauer, M.; Herrero Acero, E.; Peerlings, H.; Gardossi, L.; Guebitz, G.M. Exploring mild enzymatic sustainable routes for the synthesis of bio-degradable aromatic-aliphatic oligoesters. Biotechnol. J. 2016, 11, 642–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robert, T.; Friebel, S. Itaconic acid—A versatile building block for renewable polyesters with enhanced functionality. Green Chem. 2016, 18, 2922–2934. [Google Scholar] [CrossRef]
- Miller, L.M.; Mei, Y.; Gao, W.; Gross, R.A. Imaging the distribution of immobilized enzymes using infrared micro-spectroscopy. Biomacromolecules 2003, 4, 70–74. [Google Scholar] [CrossRef]
- Hendriksen, H.V.; Kornbrust, B.A.; Ostergaard, P.R.; Stringer, M.A. Evaluating the Potential for Enzymatic Acrylamide Mitigation in a Range of Food Products Using an Asparaginase from Aspergillus oryzae. J. Agric. Food Chem. 2009, 57, 4168–4176. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.M.; Rimm, E.B.; Thompson, K.M.; Mucci, L.A. Dietary acrylamide and cancer risk in humans: A review. J. Verbrauch. Lebensm. 2006, 1, 19–27. [Google Scholar] [CrossRef]
- Teodor, E.; Litescu, S.C.; Lazar, V.; Somoghi, R. Hydrogel-magnetic nanoparticles with immobilized l-asparaginase for biomedical applications. J. Mater. Sci. Mater. Med. 2009, 20, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Kotzia, G.A.; Labrou, N.E. L-Asparaginase from Erwinia Chrysanthemi 3937: Cloning, expression and characterization. J. Biotechnol. 2006, 127, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Tao, M.L.; Shen, W.D.; Zhou, Y.Z.; Ding, Y.; Ma, Y.; Zhou, W.L. Immobilization of l-asparaginase on the microparticles of the natural silk sericin protein and its characters. Biomaterials 2004, 25, 3751–3759. [Google Scholar] [CrossRef] [PubMed]
- Aung, H.P.; Bocola, M.; Schleper, S.; Röhm, K.H. Dynamics of a mobile loop at the active site of Escherichia coli asparaginase. Biochim. Biophys. Acta 2000, 1481, 349–359. [Google Scholar] [CrossRef]
- Yao, M.; Yasutake, Y.; Morita, H.; Tanaka, I. Structure of the type I l-asparaginase from the hyperthermophilic archaeon Pyrococcus horikoshii at 2.16 Å resolution. Acta Crystallogr. D 2005, 61, 294–301. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Technical Report of EFSA: Explanatory Note on the Guidance of the Scientific Panel of Food Contact Material, Enzymes, Flavourings and Processing Aids (CEF) on the Submission of a Dossier on Food Enzymes; EFSA Supporting Publication: Parma, Italy, 2014; EN-689; Available online: www.efsa.europa.eu (accessed on 28 July 2018).
- Aghaiypour, K.; Wlodawer, A.; Lubkowski, J. Structural basis for the activity and substrate specificity of Erwinia chrysanthemi l-asparaginase. Biochemistry 2001, 40, 5655–5664. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, V.; Ebert, C.; Knapic, L.; Fattor, D.; Basso, A.; Spizzo, P.; Gardossi, L. Conformational changes of lipases in aqueous media: A comparative computational study and experimental implications. Adv. Synth. Catal. 2011, 353, 2466–2480. [Google Scholar] [CrossRef]
- Basso, A.; Braiuca, P.; Cantone, S.; Ebert, C.; Linda, P.; Spizzo, P.; Caimi, P.; Hanefeld, U.; Degrassi, G.; Gardossi, L. In silico analysis of enzyme surface and glycosylation effect as a tool for efficient covalent immobilization of CalB and PGA on Sepabeads®. Adv. Synth. Catal. 2007, 349, 877–886. [Google Scholar] [CrossRef]











| Material | Total Volume Intrusion (mL/g) | Total Pores Volume (m2/g) | Average Pore Diameter (µm) | Porosity (%) |
|---|---|---|---|---|
| RH | 0.2829 | 26.0 | 0.044 | 30.0 |
| Milled RH (200–400 µm) | 0.3889 | 17.3 | 0.090 | 37.9 |
| Carrier | Function Group | Immobilization Time (h) | Enzymatic U Loaded a (U gcarrier−1) | Immobilized Protein b (%) | Hydrolytic Activity a (U g−1) |
|---|---|---|---|---|---|
| Oxidized Rice Husk | Amine (HMDA + GA) | 24 | 25,000 c | 35 | 317 |
| Oxidized Rice Husk | Amine (HMDA + GA) | 24 | 10,000 | 33 | 178 |
| Oxidized Rice Husk | Amine (HMDA + GA) | 48 | 10,000 | 72 | 316 |
| Methacrylic EC-EP/S | Epoxy | 24 | 10,000 | 95 | 709 |
| Biocatalyst | U gmonomers−1 | Reaction Time (h) | Conversion (%) |
|---|---|---|---|
| CaLB RH | 158 | 24 | 69 |
| 48 | 87 | ||
| 72 | 92 | ||
| CaLB EC-EP/S | 297 | 24 | 70 |
| 48 | 87 | ||
| 72 | 88 |
| Carrier | Asparaginase | Loaded Protein (mg·gcarrier−1) | Actual Loaded Protein % | Actual Loaded Protein (mg·gcarrier−1) | Hydrolyzed Asparagine (%) | Hydrolyzed Asparagine after 10 Cycles (%) |
|---|---|---|---|---|---|---|
| EC-EP/S | Acrylaway L | 26.8 | 72 | 12.3 | 100 | 100 |
| Acrylaway High-T | 26.8 | 93 | 24.9 | 100 | 98.5 | |
| RH | Acrylaway L | 17.9 | 44 | 7.9 | 100 | 100 |
| Acrylaway High-T | 17.9 | 38 | 6.8 | 93 | 86.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cespugli, M.; Lotteria, S.; Navarini, L.; Lonzarich, V.; Del Terra, L.; Vita, F.; Zweyer, M.; Baldini, G.; Ferrario, V.; Ebert, C.; et al. Rice Husk as an Inexpensive Renewable Immobilization Carrier for Biocatalysts Employed in the Food, Cosmetic and Polymer Sectors. Catalysts 2018, 8, 471. https://doi.org/10.3390/catal8100471
Cespugli M, Lotteria S, Navarini L, Lonzarich V, Del Terra L, Vita F, Zweyer M, Baldini G, Ferrario V, Ebert C, et al. Rice Husk as an Inexpensive Renewable Immobilization Carrier for Biocatalysts Employed in the Food, Cosmetic and Polymer Sectors. Catalysts. 2018; 8(10):471. https://doi.org/10.3390/catal8100471
Chicago/Turabian StyleCespugli, Marco, Simone Lotteria, Luciano Navarini, Valentina Lonzarich, Lorenzo Del Terra, Francesca Vita, Marina Zweyer, Giovanna Baldini, Valerio Ferrario, Cynthia Ebert, and et al. 2018. "Rice Husk as an Inexpensive Renewable Immobilization Carrier for Biocatalysts Employed in the Food, Cosmetic and Polymer Sectors" Catalysts 8, no. 10: 471. https://doi.org/10.3390/catal8100471