Development of Active and Stable Low Nickel Content Catalysts for Dry Reforming of Methane
Abstract
:1. Introduction
2. Results
2.1. Characterization of the Catalysts
2.2. Catalyst Performance in the DRM Reaction
3. Discussion
3.1. Activity of Mg.Al Supported Catalysts
3.2. Coking Resistance and Stability of Mg.Al Supported Catalysts
4. Materials and Methods
4.1. Catalyst Preparation
4.2. Catalyst Characterization
4.3. Catalytic Tests
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Havran, V.; Duduković, M.P.; Lo, C.S. Conversion of Methane and Carbon Dioxide to Higher Value Products. Ind. Eng. Chem. Res. 2011, 50, 7089–7100. [Google Scholar] [CrossRef]
- Rostrupnielsen, J.R.; Hansen, J.H.B. CO2-Reforming of Methane over Transition Metals. J. Catal. 1993, 144, 38–49. [Google Scholar] [CrossRef]
- Fan, M.-S.; Abdullah, A.Z.; Bhatia, S. Catalytic Technology for Carbon Dioxide Reforming of Methane to Synthesis Gas. Chemcatchem 2009, 1, 192–208. [Google Scholar] [CrossRef]
- Bradford, M.C.J.; Vannice, M.A. CO2 Reforming of CH4. Catal. Rev. 1999, 41, 1–42. [Google Scholar] [CrossRef]
- Wurzel, T.; Malcus, S.; Mleczko, L. Reaction engineering investigations of CO2 reforming in a fluidized-bed reactor. Chem. Eng. Sci. 2000, 55, 3955–3966. [Google Scholar] [CrossRef]
- Oyama, S.T.; Hacarlioglu, P.; Gu, Y.; Lee, D. Dry reforming of methane has no future for hydrogen production: Comparison with steam reforming at high pressure in standard and membrane reactors. Int. J. Hydrog. Energy 2012, 37, 10444–10450. [Google Scholar] [CrossRef]
- Albarazi, A.; Gálvez, M.E.; Da Costa, P. Synthesis strategies of ceria–zirconia doped Ni/SBA-15 catalysts for methane dry reforming. Catal. Commun. 2015, 59, 108–112. [Google Scholar] [CrossRef]
- Usman, M.; Wan Daud, W.M.A.; Abbas, H.F. Dry reforming of methane: Influence of process parameters—A review. Renew. Sustain. Energy Rev. 2015, 45, 710–744. [Google Scholar] [CrossRef]
- Paksoy, A.I.; Caglayan, B.S.; Aksoylu, A.E. A study on characterization and methane dry reforming performance of Co–Ce/ZrO2 catalyst. Appl. Catal. B Environ. 2015, 168–169, 164–174. [Google Scholar] [CrossRef]
- Yu, M.; Zhu, Y.-A.; Lu, Y.; Tong, G.; Zhu, K.; Zhou, X. The promoting role of Ag in Ni-CeO2 catalyzed CH4-CO2 dry reforming reaction. Appl. Catal. B Environ. 2015, 165, 43–56. [Google Scholar] [CrossRef]
- Ma, J.; Sun, N.; Zhang, X.; Zhao, N.; Xiao, F.; Wei, W.; Sun, Y. A short review of catalysis for CO2 conversion. Catal. Today 2009, 148, 221–231. [Google Scholar] [CrossRef]
- Liu, C.-J.; Ye, J.; Jiang, J.; Pan, Y. Progresses in the Preparation of Coke Resistant Ni-based Catalyst for Steam and CO2 Reforming of Methane. ChemCatChem 2011, 3, 529–541. [Google Scholar] [CrossRef]
- Gao, J.; Hou, Z.; Lou, H.; Zheng, X. Chapter 7—Dry (CO2) Reforming. In Fuel Cells: Technologies for Fuel Processing; Elsevier: Amsterdam, The Netherlands, 2011; pp. 191–221. [Google Scholar]
- Edwards, J.H.; Maitra, A.M. The chemistry of methane reforming with carbon dioxide and its current and potential applications. Fuel Process. Technol. 1995, 42, 269–289. [Google Scholar] [CrossRef]
- Tang, S.B.; Qiu, F.L.; Lu, S.J. Effect of supports on the carbon deposition of nickel catalysts for methane reforming with CO2. Catal. Today 1995, 24, 253–255. [Google Scholar] [CrossRef]
- Roh, H.-S.; Jun, K.-W. Carbon Dioxide Reforming of Methane over Ni Catalysts Supported on Al2O3 Modified with La2O3, MgO, and CaO. Catal. Surv. Asia 2008, 12, 239–252. [Google Scholar] [CrossRef]
- Guo, J.; Lou, H.; Zhao, H.; Chai, D.; Zheng, X. Dry reforming of methane over nickel catalysts supported on magnesium aluminate spinels. Appl. Catal. A Gen. 2004, 273, 75–82. [Google Scholar] [CrossRef]
- Habibi, N.; Arandiyan, H.; Rezaei, M. Mesoporous MgO·Al2O3 nanopowder-supported meso-macroporous nickel catalysts: A new path to high-performance biogas reforming for syngas. RSC Adv. 2016, 6, 29576–29585. [Google Scholar] [CrossRef]
- Abdollahifar, M.; Haghighi, M.; Babaluo, A.A.; Talkhoncheh, S.K. Sono-synthesis and characterization of bimetallic Ni–Co/Al2O3–MgO nanocatalyst: Effects of metal content on catalytic properties and activity for hydrogen production via CO2 reforming of CH4. Ultrason. Sonochem. 2016, 31, 173–183. [Google Scholar] [CrossRef] [PubMed]
- García-Diéguez, M.; Herrera, C.; Larrubia, M.Á.; Alemany, L.J. CO2-reforming of natural gas components over a highly stable and selective NiMg/Al2O3 nanocatalyst. Catal. Today 2012, 197, 50–57. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Dalai, A.K. Effects of metal content on activity and stability of Ni-Co bimetallic catalysts for CO2 reforming of CH4. Appl. Catal. A Gen. 2008, 339, 121–129. [Google Scholar] [CrossRef]
- Kim, J.-H.; Suh, D.J.; Park, T.-J.; Kim, K.-L. Effect of metal particle size on coking during CO2 reforming of CH4 over Ni–alumina aerogel catalysts. Appl. Catal. A Gen. 2000, 197, 191–200. [Google Scholar] [CrossRef]
- Suárez-Toriello, V.A.; Santolalla-Vargas, C.E.; de los Reyes, J.A.; Vázquez-Zavala, A.; Vrinat, M.; Geantet, C. Influence of the solution pH in impregnation with citric acid and activity of Ni/W/Al2O3 catalysts. J. Mol. Catal. Chem. 2015, 404–405, 36–46. [Google Scholar] [CrossRef]
- Van Dillen, A.J.; Terörde, R.J.A.M.; Lensveld, D.J.; Geus, J.W.; de Jong, K.P. Synthesis of supported catalysts by impregnation and drying using aqueous chelated metal complexes. J. Catal. 2003, 216, 257–264. [Google Scholar] [CrossRef]
- Prescott, H.A.; Li, Z.-J.; Kemnitz, E.; Trunschke, A.; Deutsch, J.; Lieske, H.; Auroux, A. Application of calcined Mg-Al hydrotalcites for Michael additions: An investigation of catalytic activity and acid-base properties. J. Catal. 2005, 234, 119–130. [Google Scholar] [CrossRef]
- Ono, Y.; Hattori, H. Preparation and Catalytic Properties of Solid Base Catalysts—II. Specific Materials for Solid Bases. In Solid Base Catalysis; Springer: Berlin/Heidelberg, Germany, 2011; pp. 157–218. [Google Scholar]
- Kumar, P.A.; Reddy, M.P.; Hyun-Sook, B.; Phil, H.H. Influence of Mg Addition on the Catalytic Activity of Alumina Supported Ag for C3H6-SCR of NO. Catal. Lett. 2009, 131, 85–97. [Google Scholar] [CrossRef]
- Stanimirova, T.; Vergilov, I.; Kirov, G.; Petrova, N. Thermal decomposition products of hydrotalcite-like compounds: Low-temperature metaphases. J. Mater. Sci. 1999, 34, 4153–4161. [Google Scholar] [CrossRef]
- Takehira, K.; Kawabata, T.; Shishido, T.; Murakami, K.; Ohi, T.; Shoro, D.; Honda, M.; Takaki, K. Mechanism of reconstitution of hydrotalcite leading to eggshell-type Ni loading on MgAl mixed oxide. J. Catal. 2005, 231, 92–104. [Google Scholar] [CrossRef]
- Escobar, J.; De Los Reyes, J.A.; Viveros, T. Nickel on TiO2-modified Al2O3 sol–gel oxides: Effect of synthesis parameters on the supported phase properties. Appl. Catal. A Gen. 2003, 253, 151–163. [Google Scholar] [CrossRef]
- Damyanova, S.; Pawelec, B.; Arishtirova, K.; Fierro, J.L.G. Ni-based catalysts for reforming of methane with CO2. Int. J. Hydrog. Energy 2012, 37, 15966–15975. [Google Scholar] [CrossRef]
- Jafarbegloo, M.; Tarlani, A.; Mesbah, A.W.; Muzart, J.; Sahebdelfar, S. NiO–MgO Solid Solution Prepared by Sol–Gel Method as Precursor for Ni/MgO Methane Dry Reforming Catalyst: Effect of Calcination Temperature on Catalytic Performance. Catal. Lett. 2016, 146, 238–248. [Google Scholar] [CrossRef]
- Li, C.; Chen, Y.-W. Temperature-programmed-reduction studies of nickel oxide/alumina catalysts: Effects of the preparation method. Thermochim. Acta 1995, 256, 457–465. [Google Scholar] [CrossRef]
- Rogers, J.L.; Mangarella, M.C.; D’Amico, A.D.; Gallagher, J.R.; Dutzer, M.R.; Stavitski, E.; Miller, J.T.; Sievers, C. Differences in the Nature of Active Sites for Methane Dry Reforming and Methane Steam Reforming over Nickel Aluminate Catalysts. ACS Catal. 2016, 6, 5873–5886. [Google Scholar] [CrossRef]
- Lucrédio, A.F.; Bellido, J.D.A.; Assaf, E.M. Effects of adding La and Ce to hydrotalcite-type Ni/Mg/Al catalyst precursors on ethanol steam reforming reactions. Appl. Catal. A Gen. 2010, 388, 77–85. [Google Scholar] [CrossRef]
- Jiang, Z.; Su, J.; Jones, M.O.; Shi, H.; Xiao, T.; Edwards, P.P. Catalytic Partial Oxidation of Methane over Ni-Based Catalysts Derived from Ni-Mg/Al Ternary Hydrotalcites. Energy Fuels 2009, 23, 1634–1639. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, M.C.; Navarro, R.M.; Fierro, J.L.G. Ethanol steam reforming over Ni/MxOy-Al2O3 (M = Ce, La, Zr and Mg) catalysts: Influence of support on the hydrogen production. Int. J. Hydrog. Energy 2007, 32, 1462–1471. [Google Scholar] [CrossRef]
- Özdemir, H.; Öksüzömer, M.A.F.; Gürkaynak, M.A. Effect of the calcination temperature on Ni/MgAl2O4 catalyst structure and catalytic properties for partial oxidation of methane. Fuel 2014, 116, 63–70. [Google Scholar] [CrossRef]
- NIST X-ray Photoelectron Spectroscopy Database: Version 3.4 (Web Version). Available online: http://srdata.nist.gov/xps/ (accessed on 6 February 2017).
- Cimino, A.; Gazzoli, D.; Indovina, V.; Moretti, G.; Occhiuzzi, M.; Pepe, F. High and low surface area NiO–MgO and CoO–MgO solid solutions: A study of XPS surface composition and CO oxidation activity. Top. Catal. 1999, 8, 171–178. [Google Scholar] [CrossRef]
- Ruckenstein, E.; Hu, Y.H. Methane partial oxidation over NiO/MgO solid solution catalysts. Appl. Catal. A Gen. 1999, 183, 85–92. [Google Scholar] [CrossRef]
- Scheffer, B.; Heijeinga, J.J.; Moulijn, J.A. An electron spectroscopy and X-ray diffraction study of nickel oxide/alumina and nickel-oxide-tungsten trioxide/alumina catalysts. J. Phys. Chem. 1987, 91, 4752–4759. [Google Scholar] [CrossRef]
- Boukha, Z.; Jiménez-González, C.; de Rivas, B.; González-Velasco, J.R.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Synthesis, characterisation and performance evaluation of spinel-derived Ni/Al2O3 catalysts for various methane reforming reactions. Appl. Catal. B Environ. 2014, 158–159, 190–201. [Google Scholar] [CrossRef]
- Heracleous, E.; Lee, A.F.; Wilson, K.; Lemonidou, A.A. Investigation of Ni-based alumina-supported catalysts for the oxidative dehydrogenation of ethane to ethylene: Structural characterization and reactivity studies. J. Catal. 2005, 231, 159–171. [Google Scholar] [CrossRef]
- Jiménez-González, C.; Boukha, Z.; de Rivas, B.; González-Velasco, J.R.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Behavior of Coprecipitated NiAl2O4/Al2O3 Catalysts for Low-Temperature Methane Steam Reforming. Energy Fuels 2014, 28, 7109–7121. [Google Scholar] [CrossRef]
- Skoufa, Z.; Xantri, G.; Heracleous, E.; Lemonidou, A.A. A study of Ni–Al–O mixed oxides as catalysts for the oxidative conversion of ethane to ethylene. Appl. Catal. A Gen. 2014, 471, 107–117. [Google Scholar] [CrossRef]
- Zecchina, A.; Spoto, G.; Coluccia, S.; Guglielminotti, E. Spectroscopic study of the adsorption of carbon monoxide on solid solutions of nickel oxide and magnesium oxide. Part 1—Standard samples. J. Chem. Soc. Faraday Trans. 1984, 80, 1875–1889. [Google Scholar] [CrossRef]
- Lucrédio, A.F.; Jerkiewickz, G.; Assaf, E.M. Nickel catalysts promoted with cerium and lanthanum to reduce carbon formation in partial oxidation of methane reactions. Appl. Catal. A Gen. 2007, 333, 90–95. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, N.H.; Roberts, N.R.M.; Joseph, B.; Kuhn, J.N. Low temperature dry reforming of methane over Pt–Ni–Mg/ceria–zirconia catalysts. Appl. Catal. B Environ. 2015, 179, 213–219. [Google Scholar] [CrossRef]
- Sokolov, S.; Kondratenko, E.V.; Pohl, M.-M.; Barkschat, A.; Rodemerck, U. Stable low-temperature dry reforming of methane over mesoporous La2O3-ZrO2 supported Ni catalyst. Appl. Catal. B Environ. 2012, 113–114, 19–30. [Google Scholar] [CrossRef]
- Lavoie, J.-M. Review on dry reforming of methane, a potentially more environmentally-friendly approach to the increasing natural gas exploitation. Front. Chem. 2014, 2, 81. [Google Scholar] [CrossRef] [PubMed]
- Newson, E. Catalyst Deactivation Due to Pore-Plugging by Reaction Products. Ind. Eng. Chem. Proc. Des. Dev. 1975, 14, 27–33. [Google Scholar] [CrossRef]
- Muraza, O.; Galadima, A. A review on coke management during dry reforming of methane. Int. J. Energy Res. 2015, 39, 1196–1216. [Google Scholar] [CrossRef]
- Alipour, Z.; Rezaei, M.; Meshkani, F. Effect of alkaline earth promoters (MgO, CaO, and BaO) on the activity and coke formation of Ni catalysts supported on nanocrystalline Al2O3 in dry reforming of methane. J. Korean Ind. Eng. Chem. 2014, 20, 2858–2863. [Google Scholar] [CrossRef]
- York, A.P.E.; Xiao, T.-C.; Green, M.L.H.; Claridge, J.B. Methane Oxyforming for Synthesis Gas Production. Catal. Rev. 2007, 49, 511–560. [Google Scholar] [CrossRef]

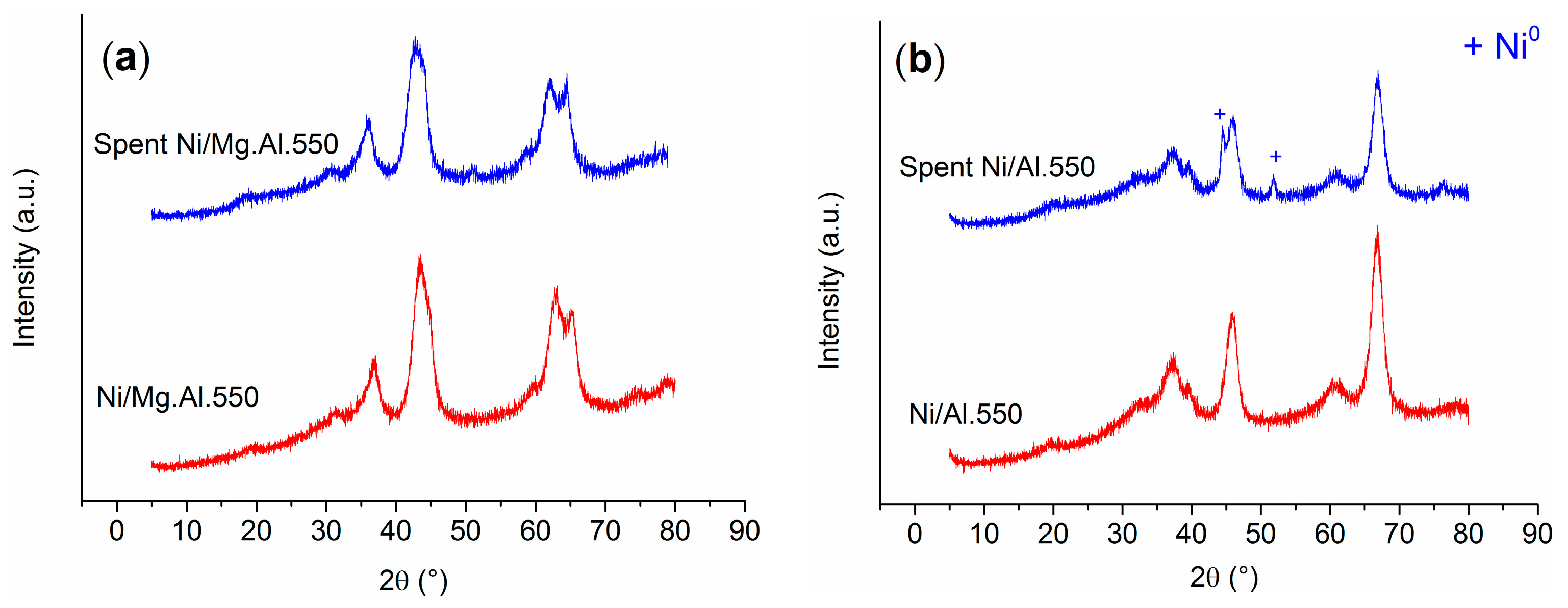
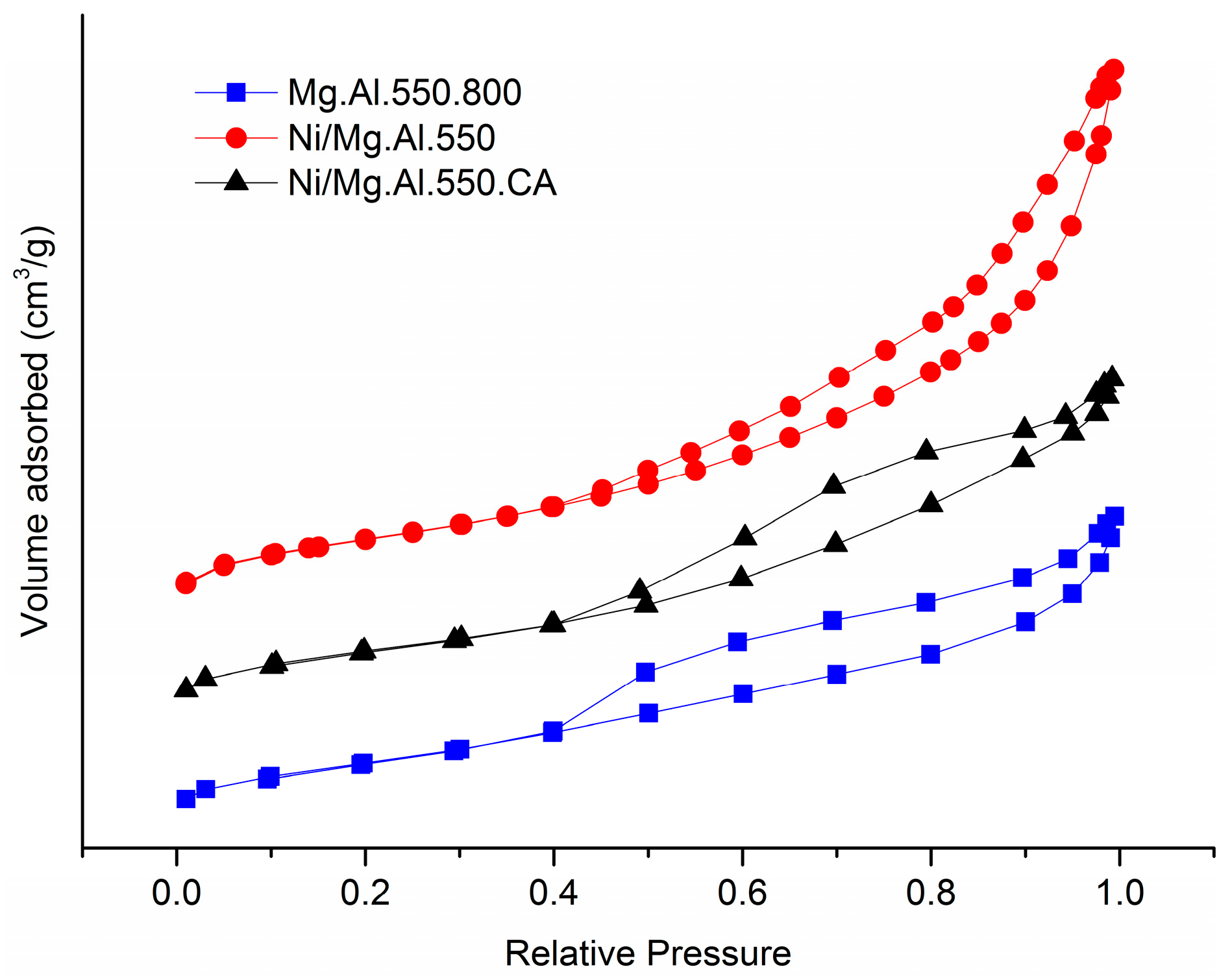
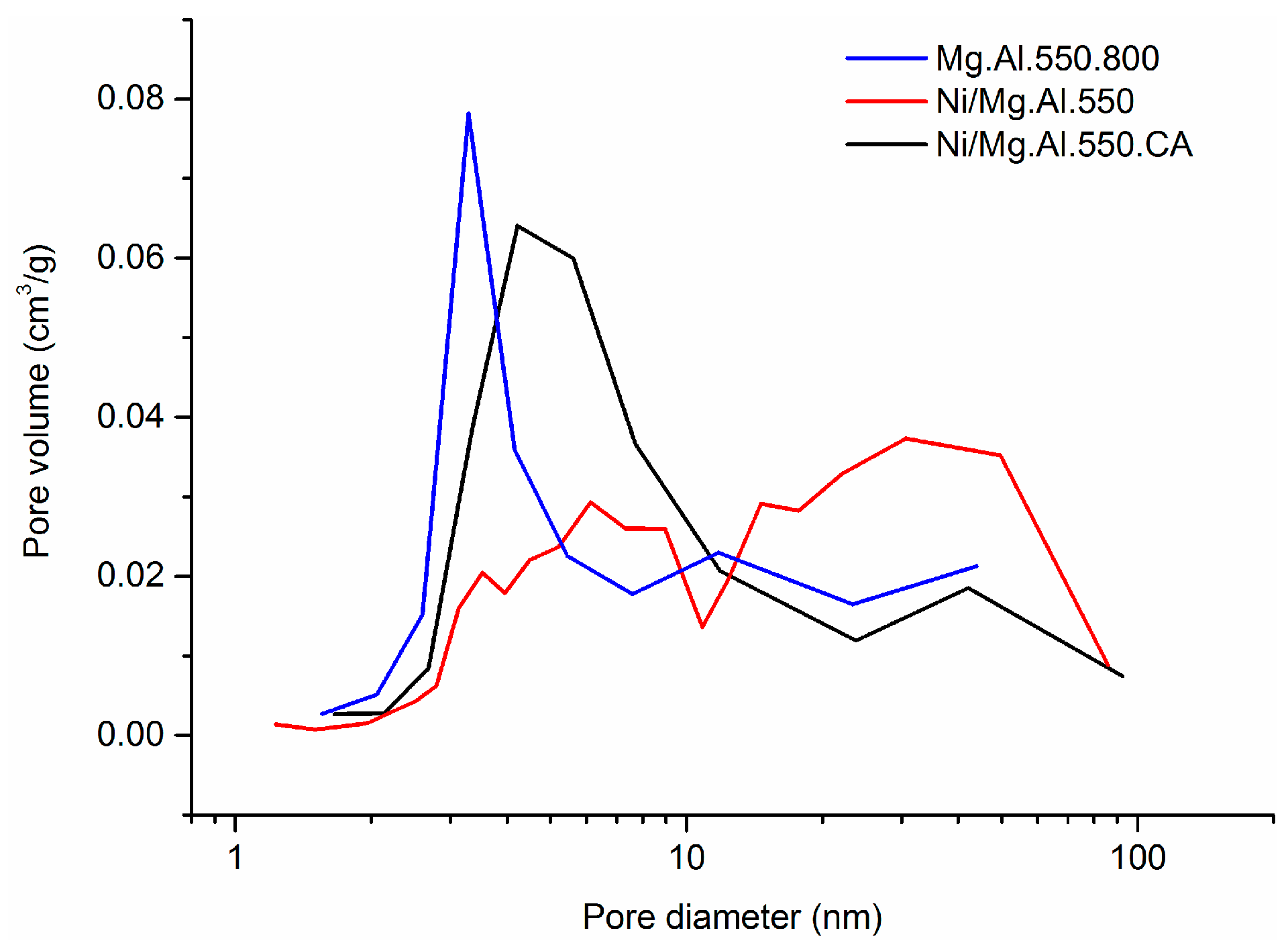
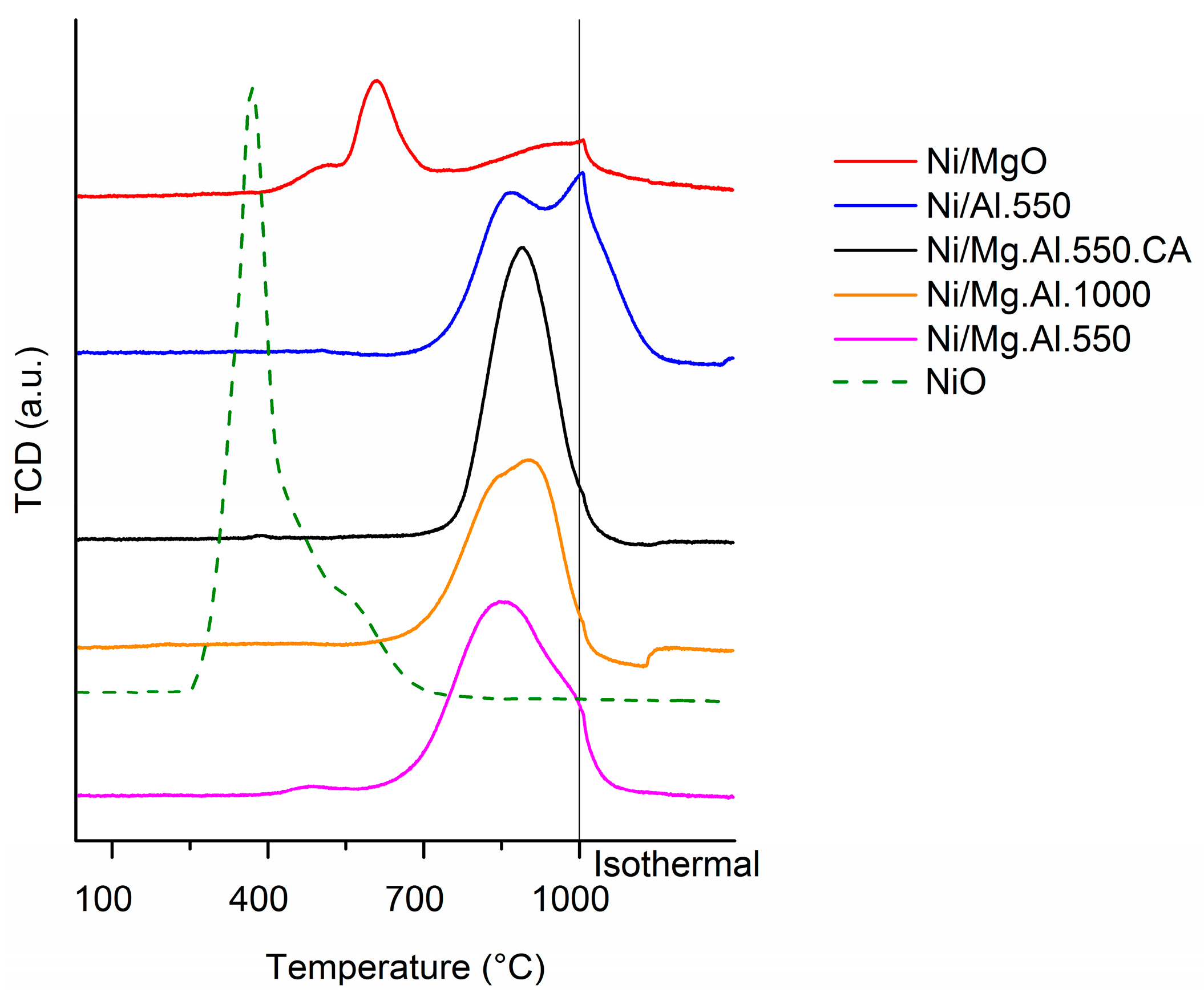

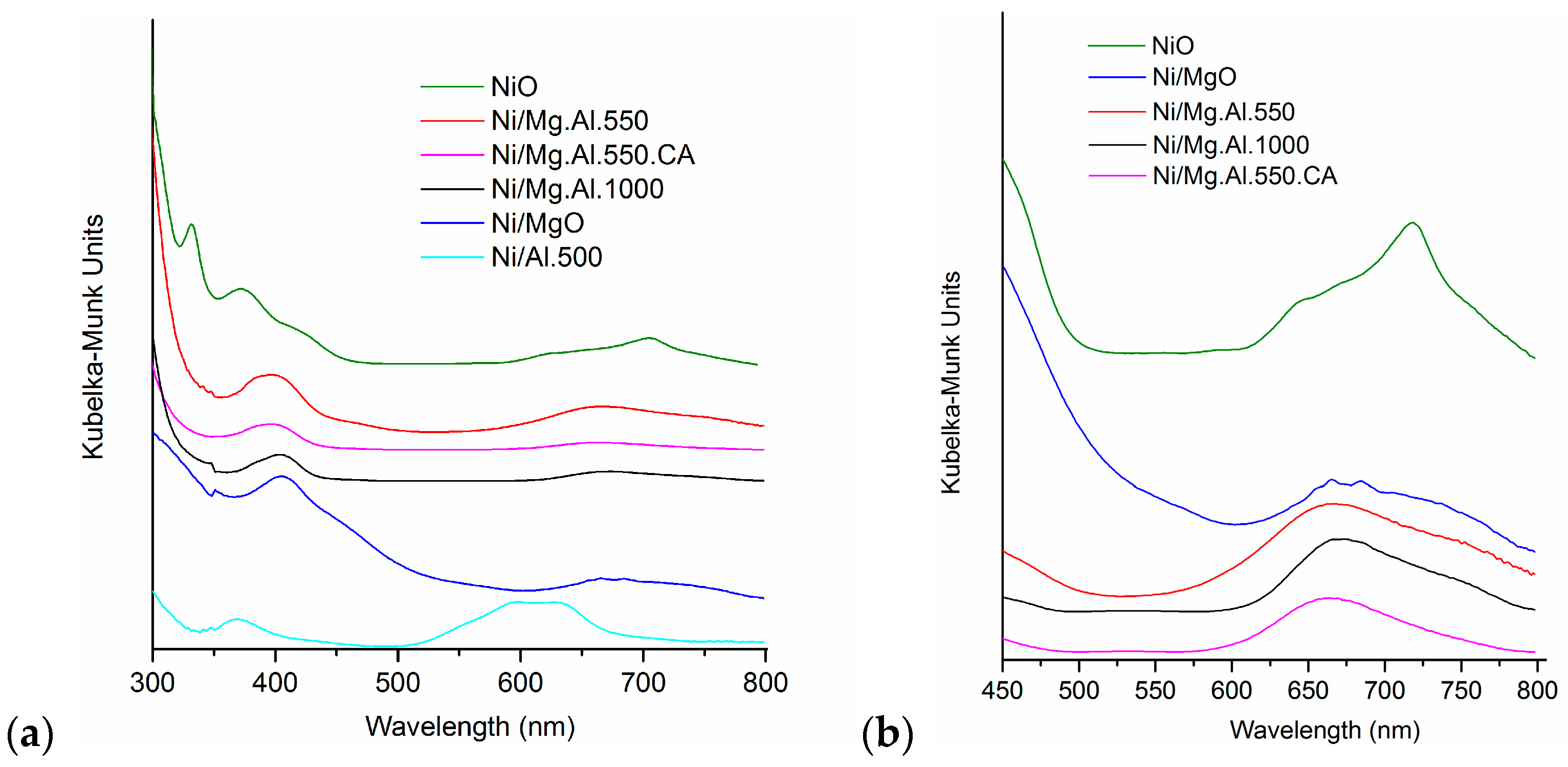
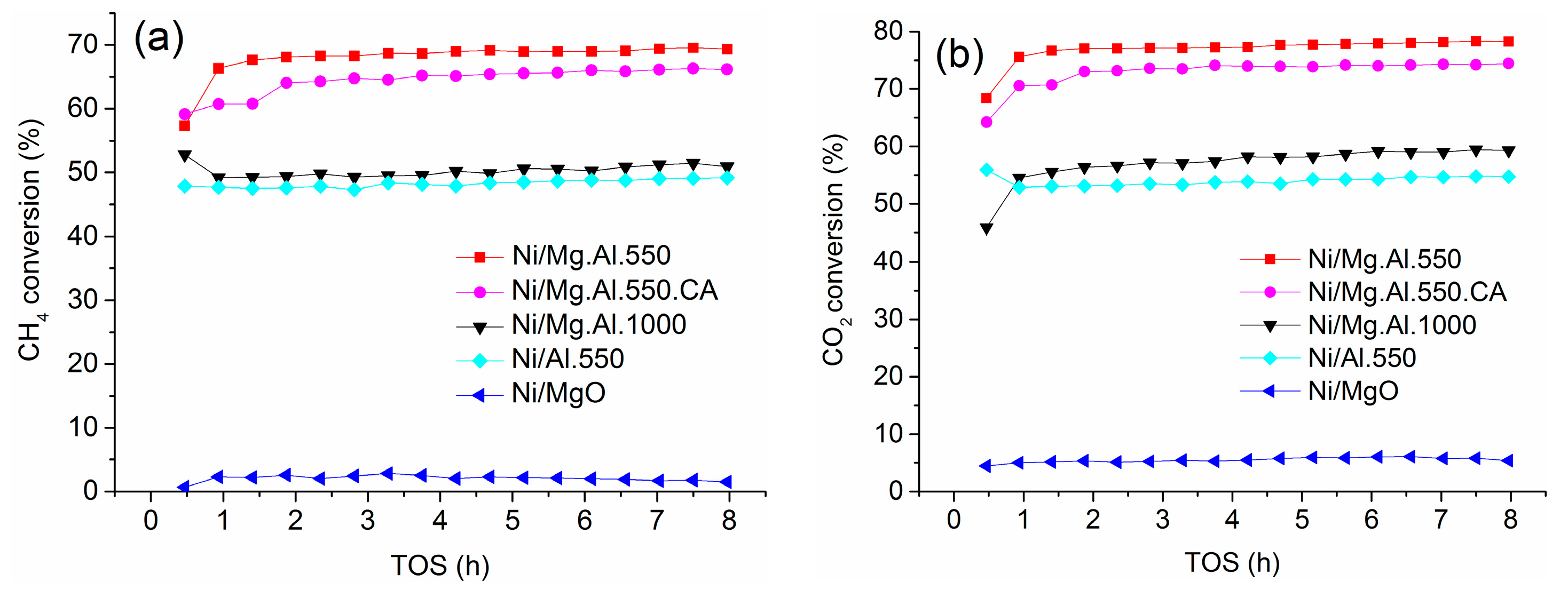
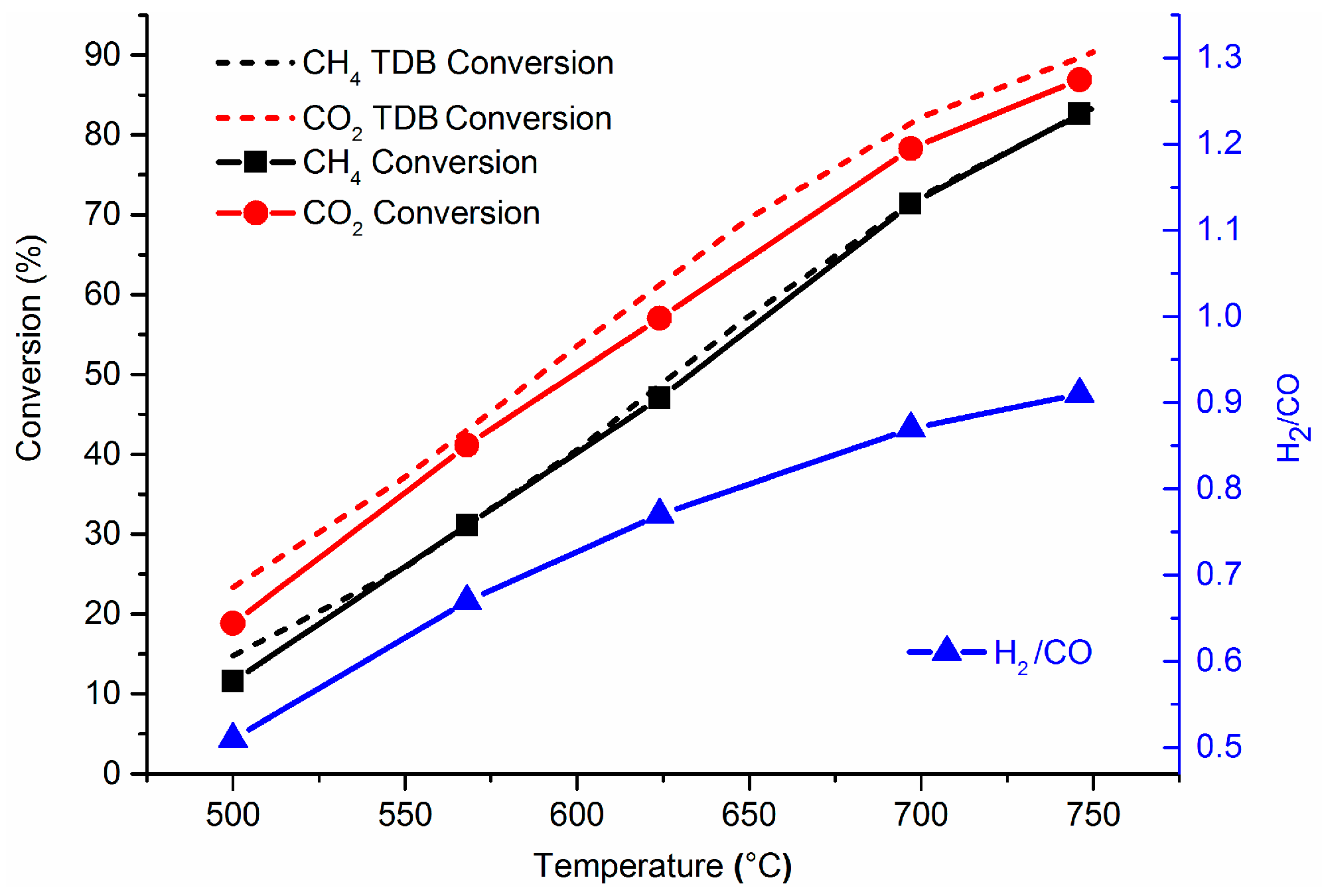



| Catalyst | SBET (m2/g) | Total Pore Volume (cm3/g) |
|---|---|---|
| Mg.Al.550 | 180 | 0.24 |
| Mg.Al.550.800 1 | 147 | 0.24 |
| Mg.Al.1000 | 85 | 0.37 |
| Al.550 | 234 | 0.54 |
| MgO | 50 | 0.35 |
| Ni/Mg.Al.550 | 174 | 0.40 |
| Ni/Mg.Al.550.CA 2 | 156 | 0.27 |
| Ni/Mg.Al.1000 | 82 | 0.36 |
| Ni/Al.550 | 178 | 0.47 |
| Ni/MgO | 45 | 0.33 |
| Sample | H2 Consumption (µmol/g) |
|---|---|
| Ni/Al.550 | 397 |
| Ni/MgO | 181 |
| Ni/Mg.Al.550 | 480 |
| Ni/Mg.Al.1000 | 404 |
| Ni/Mg.Al.550.CA | 437 |
| Sample | Binding Energy (eV) | Surface Molar Ratio 1 | Bulk Molar Ratio 2 | ||
|---|---|---|---|---|---|
| Ni 2p3/2 | Ni/(Mg + Al) | Mg/Al | Ni/(Mg + Al) | Mg/Al | |
| Ni/Al.550 | 855.5 | 0.029 | - | 0.022 | 0 |
| Ni/MgO | - 3 | - 3 | - 3 | 0.017 | - |
| Ni/Mg.Al.550 | 856.9 | 0.063 | 0.4 | 0.019 | 1.3 |
| Ni/Mg.Al.1000 | 856.1 | 0.057 | 0.3 | 0.019 | 1.3 |
| Ni/Mg.Al.550.CA | 855.2 | 0.071 | 1.6 | 0.019 | 1.3 |
| Mg.Al.550 | - | - | 1.2 | - | 1.3 |
| Mg.Al.550.800 | - | - | 1.1 | - | 1.3 |
| Mg.Al.1000 | - | - | 1.5 | - | 1.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, Q.L.M.; Armbruster, U.; Atia, H.; Schneider, M.; Lund, H.; Agostini, G.; Radnik, J.; Vuong, H.T.; Martin, A. Development of Active and Stable Low Nickel Content Catalysts for Dry Reforming of Methane. Catalysts 2017, 7, 157. https://doi.org/10.3390/catal7050157
Ha QLM, Armbruster U, Atia H, Schneider M, Lund H, Agostini G, Radnik J, Vuong HT, Martin A. Development of Active and Stable Low Nickel Content Catalysts for Dry Reforming of Methane. Catalysts. 2017; 7(5):157. https://doi.org/10.3390/catal7050157
Chicago/Turabian StyleHa, Quan Luu Manh, Udo Armbruster, Hanan Atia, Matthias Schneider, Henrik Lund, Giovanni Agostini, Jörg Radnik, Huyen Thanh Vuong, and Andreas Martin. 2017. "Development of Active and Stable Low Nickel Content Catalysts for Dry Reforming of Methane" Catalysts 7, no. 5: 157. https://doi.org/10.3390/catal7050157







