Factors Controlling the Redox Activity of Oxygen in Perovskites: From Theory to Application for Catalytic Reactions
Abstract
:1. Introduction
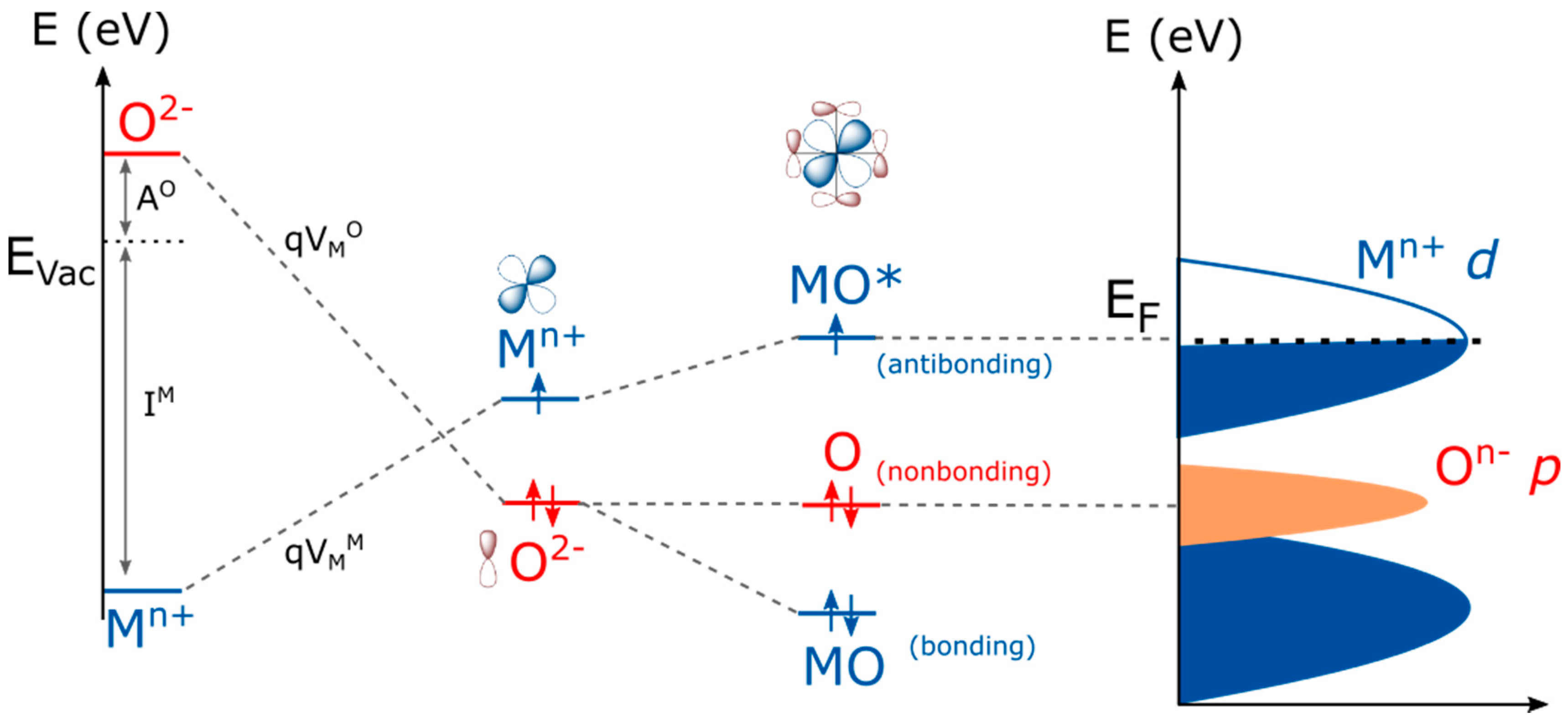
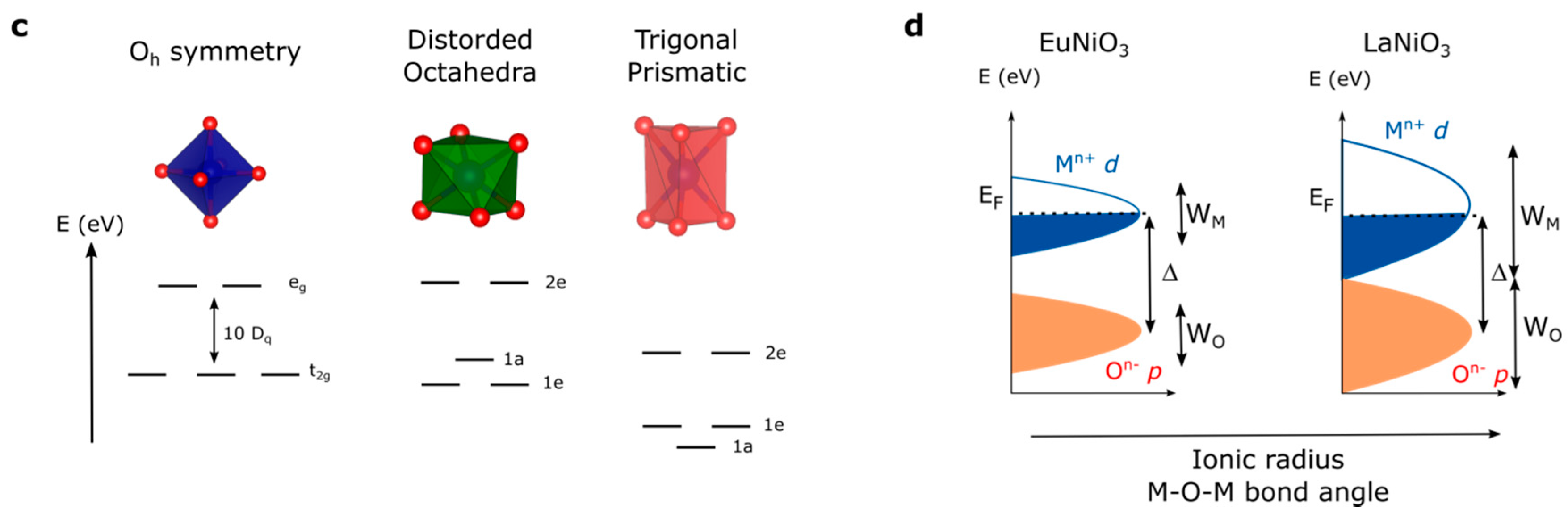
2. Perovskites’ Electronic Structure
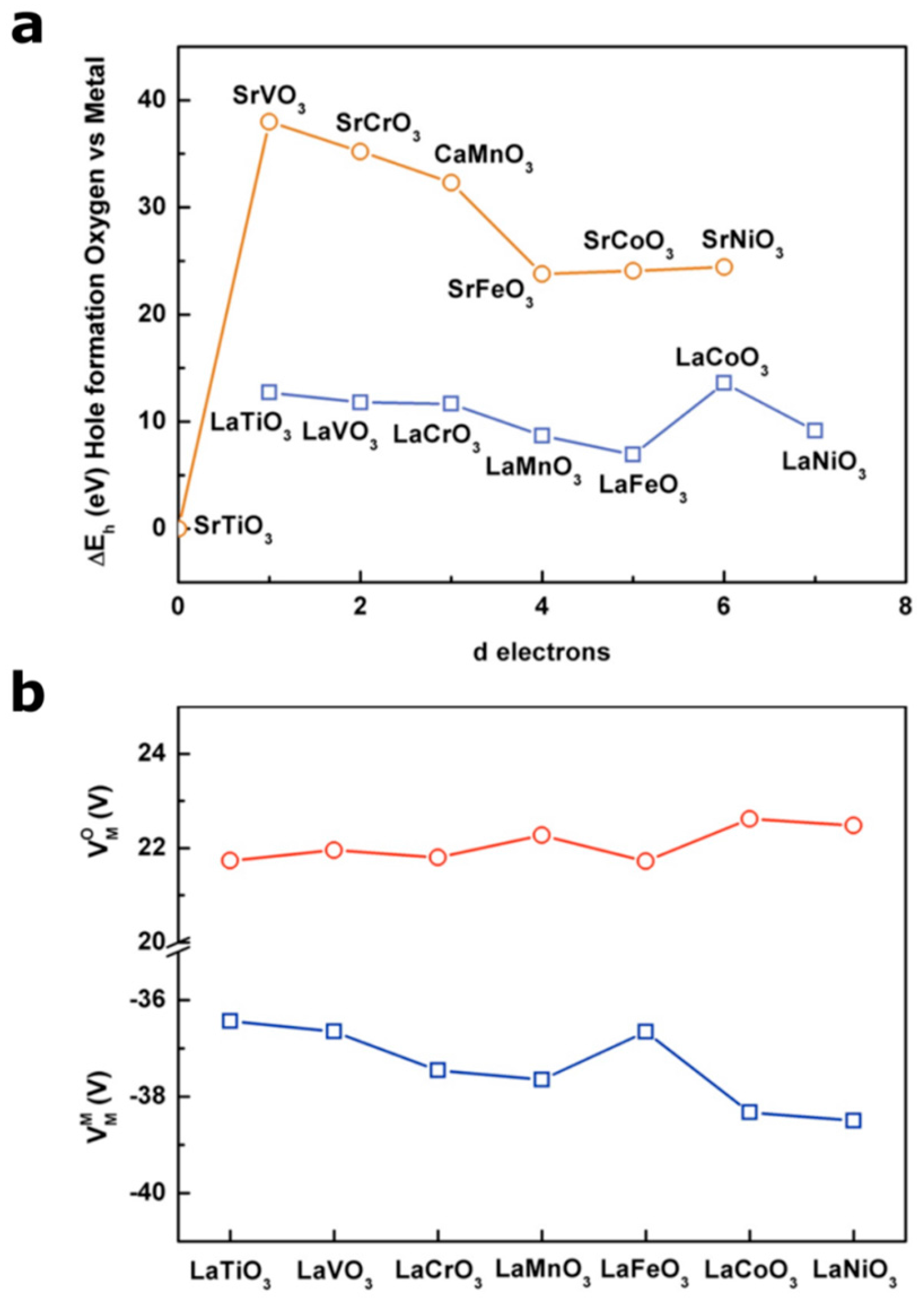
3. Use of Simple Pictures to Predict the Formation of Holes in Purely Oxygen States
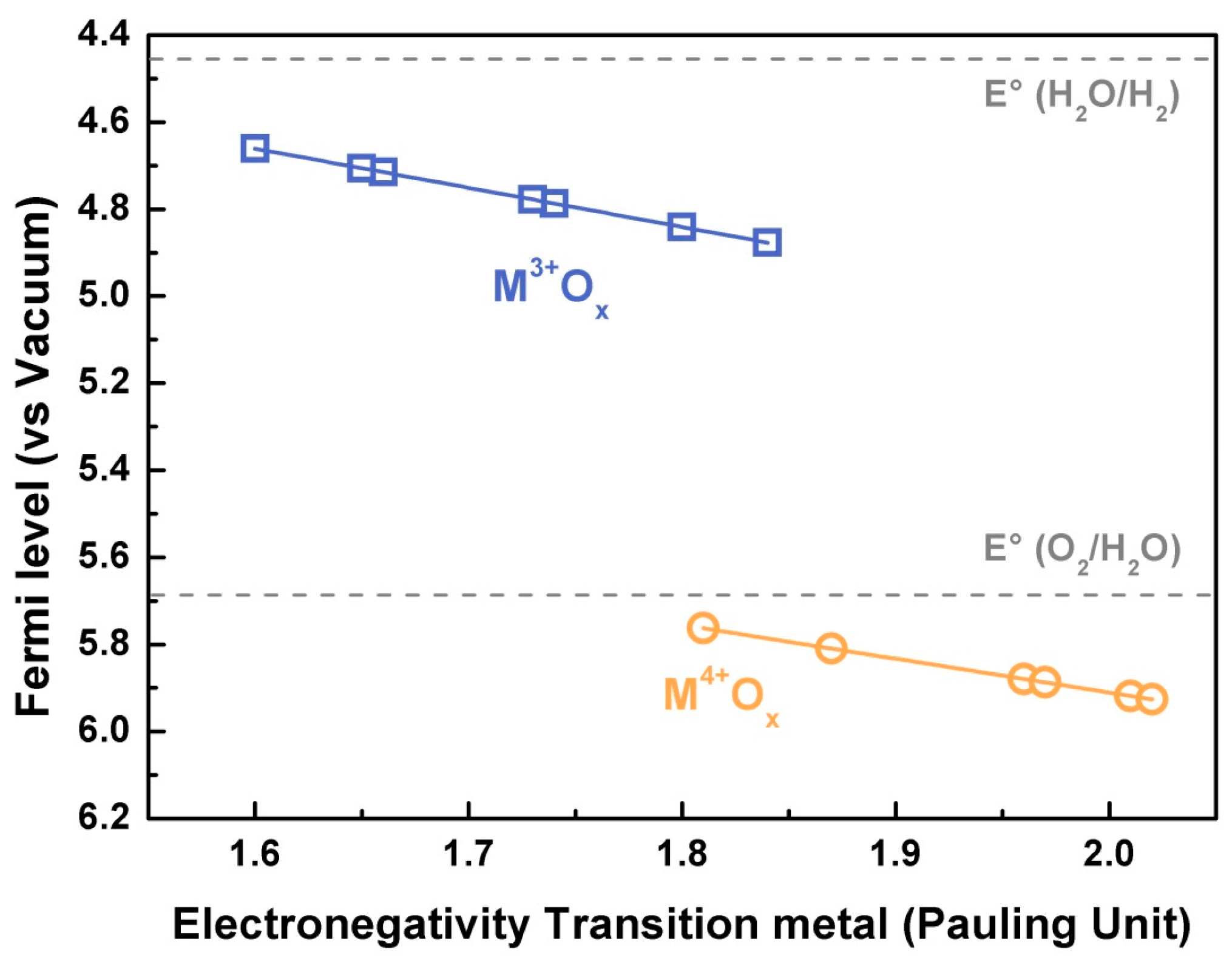
4. Tuning the Perovskite Electronic Structure
5. Reactivity of the Oxygen Species Formed Upon Oxidation
6. Examples of Perovskite Oxygen Oxidation for Catalytic Reactions
6.1. Low Temperature Oxygen Evolution Reaction
6.2. Gas Phase Catalytic Reaction
- reaction of the reactant with oxygen from the transition metal oxide:
- followed by the reoxidation of the reduced catalyst by gaseous oxygen:
6.3. Concluding Remarks on Using Redox Reactions of Oxygen Ions for Catalysis
7. Latest Development of Analytical Tools for Analyzing the Oxidation Reaction of Oxygen Ions
8. Conclusions
Acknowledgments
Conflicts of Interest
References
- Goodenough, J.B. Perspective on Engineering Transition-Metal Oxides. Chem. Mater. 2014, 26, 820–829. [Google Scholar] [CrossRef]
- Goodenough, J.B. Theory of the Role of Covalence in the Perovskite-Type Manganites [La,M(II)]MnO3. Phys. Rev. 1955, 100, 564–573. [Google Scholar] [CrossRef]
- Rondinelli, J.M.; May, S.J.; Freeland, J.W. Control of octahedral connectivity in perovskite oxide heterostructures: An emerging route to multifunctional materials discovery. MRS Bull. 2012, 37, 261–270. [Google Scholar] [CrossRef]
- Bednorz, J.G.; Müller, K.A. Condensed Matt Possible High Tc Superconductivity in the Ba-La-Cu-O System. Z. Phys. B Condens. Matter 1986, 64, 189–193. [Google Scholar] [CrossRef]
- Mefford, J.T.; Rong, X.; Abakumov, A.M.; Hardin, W.G.; Dai, S.; Kolpak, A.M.; Johnston, K.P.; Stevenson, K.J. Water electrolysis on La1−xSrxCoO3−δ perovskite electrocatalysts. Nat. Commun. 2016, 7, 11053. [Google Scholar] [CrossRef] [PubMed]
- Grimaud, A.; Diaz-Morales, O.; Han, B.; Hong, W.T.; Lee, Y.-L.; Giordano, L.; Stoerzinger, K.A.; Koper, M.T.M.; Shao-Horn, Y. Activating lattice oxygen redox reactions in metal oxides to catalyse oxygen evolution. Nat. Chem. 2017, 9, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Rong, X.; Parolin, J.; Kolpak, A.M. A Fundamental Relationship between Reaction Mechanism and Stability in Metal Oxide Catalysts for Oxygen Evolution. ACS Catal. 2016, 6, 1153–1158. [Google Scholar] [CrossRef]
- Lee, Y.-L.; Kleis, J.; Rossmeisl, J.; Morgan, D. Ab initioenergetics of LaBO3(001) (B = Mn, Fe, Co, and Ni) for solid oxide fuel cell cathodes. Phys. Rev. B 2009, 80, 224101. [Google Scholar] [CrossRef]
- Lee, Y.-L.; Kleis, J.; Rossmeisl, J.; Shao-Horn, Y.; Morgan, D. Prediction of solid oxide fuel cell cathode activity with first-principles descriptors. Energy Environ. Sci. 2011, 4, 3966. [Google Scholar] [CrossRef]
- Kuklja, M.M.; Kotomin, E.A.; Merkle, R.; Mastrikov, Y.A.; Maier, J. Combined theoretical and experimental analysis of processes determining cathode performance in solid oxide fuel cells. Phys. Chem. Chem. Phys. 2013, 15, 5443. [Google Scholar] [CrossRef] [PubMed]
- Kuklja, M.M.; Mastrikov, Y.A.; Jansang, B.; Kotomin, E.A. The Intrinsic Defects, Disordering, and Structural Stability of BaxSr1–xCoyFe1–yO3−δ Perovskite Solid Solutions. J. Phys. Chem. C 2012, 116, 18605–18611. [Google Scholar] [CrossRef]
- Mastrikov, Y.A.; Merkle, R.; Kotomin, E.A.; Kuklja, M.M.; Maier, J. Formation and migration of oxygen vacancies in La1−xSrxCo1−yFeyO3−δ perovskites: Insight from ab initio calculations and comparison with Ba1−xSrxCo1−yFeyO3−δ. Phys. Chem. Chem. Phys. 2013, 15, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-M.; He, J.; Biegalski, M.D.; Ambaye, H.; Lauter, V.; Christen, H.M.; Pantelides, S.T.; Pennycook, S.J.; Kalinin, S.V.; Borisevich, A.Y. Probing oxygen vacancy concentration and homogeneity in solid-oxide fuel-cell cathode materials on the subunit-cell level. Nat. Mater. 2012, 11, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Chen, Y.; Bluhm, H.; Yildiz, B. Electronic Structure Evolution of SrCoOx during Electrochemically Driven Phase Transition Probed by in Situ X-ray Spectroscopy. J. Phys. Chem. C 2016, 120, 24148–24157. [Google Scholar] [CrossRef]
- Mueller, D.N.; Machala, M.L.; Bluhm, H.; Chueh, W.C. Redox activity of surface oxygen anions in oxygen-deficient perovskite oxides during electrochemical reactions. Nat. Commun. 2015, 6, 6097. [Google Scholar] [CrossRef] [PubMed]
- Saubanère, M.; McCalla, E.; Tarascon, J.M.; Doublet, M.L. The intriguing question of anionic redox in high-energy density cathodes for Li-ion batteries. Energy Environ. Sci. 2016, 9, 984–991. [Google Scholar] [CrossRef]
- Xie, Y.; Saubanère, M.; Doublet, M.L. Requirements for reversible extra-capacity in Li-rich layered oxides for Li-ion batteries. Energy Environ. Sci. 2017, 10, 266–274. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Nakayama, M.; Takeuchi, M.; Komaba, S.; Hashimoto, Y.; Mukai, T.; Shiiba, H.; Sato, K.; Kobayashi, Y.; Nakao, A.; et al. Origin of stabilization and destabilization in solid-state redox reaction of oxide ions for lithium-ion batteries. Nat. Commun. 2016, 7, 13814. [Google Scholar] [CrossRef] [PubMed]
- Pearce, P.E.; Perez, A.J.; Rousse, G.; Saubanère, M.; Batuk, D.; Foix, D.; McCalla, E.; Abakumov, A.M.; Van Tendeloo, G.; Doublet, M.-L.; et al. Evidence for anionic redox activity in a tridimensional-ordered Li-rich positive electrode β-Li2IrO3. Nat. Mater. 2017, 16, 580–586. [Google Scholar] [CrossRef] [PubMed]
- McCalla, E.; Abakumov, A.M.; Saubanere, M.; Foix, D.; Berg, E.J.; Rousse, G.; Doublet, M.L.; Gonbeau, D.; Novak, P.; Van Tendeloo, G.; et al. Visualization of O–O peroxo-like dimers in high-capacity layered oxides for Li-ion batteries. Science 2015, 350, 1516–1521. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Roberts, M.R.; Hao, R.; Guerrini, N.; Pickup, D.M.; Liu, Y.-S.; Edström, K.; Guo, J.; Chadwick, A.V.; Duda, L.C.; et al. Charge-compensation in 3d-transition-metal-oxide intercalation cathodes through the generation of localized electron holes on oxygen. Nat. Chem. 2016, 8, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.-H.; Lee, J.; Urban, A.; Malik, R.; Kang, S.; Ceder, G. The structural and chemical origin of the oxygen redox activity in layered and cation-disordered Li-excess cathode materials. Nat. Chem. 2016, 8, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Grimaud, A.; Demortiere, A.; Saubanere, M.; Dachraoui, W.; Duchamp, M.; Doublet, M.-l.; Tarascon, J.-M. Activation of surface oxygen sites on an iridium-based model catalyst for the oxygen evolution reaction. Nat. Energy 2016, 2, 16189. [Google Scholar] [CrossRef]
- Torrance, J.B.; Metzger, R.M. Role of the Madelung energy in hole conductivity in copper oxides: Difference between semiconductors and high-Tc superconductors. Phys. Rev. Lett. 1989, 63, 1515–1518. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Umezawa, N. Hole localization, migration, and the formation of peroxide anion in perovskite SrTiO3. Phys. Rev. B 2014, 90, 035202. [Google Scholar] [CrossRef]
- Herlihy, D.M.; Waegele, M.M.; Chen, X.; Pemmaraju, C.D.; Prendergast, D.; Cuk, T. Detecting the oxyl radical of photocatalytic water oxidation at an n-SrTiO3/aqueous interface through its subsurface vibration. Nat. Chem. 2016, 8, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Choing, S.N.; Aschaffenburg, D.J.; Pemmaraju, C.D.; Prendergast, D.; Cuk, T. The Formation Time of Ti–O(·) and Ti–O(·)-Ti Radicals at the n-SrTiO3/Aqueous Interface during Photocatalytic Water Oxidation. J. Am. Chem. Soc. 2017, 139, 1830–1841. [Google Scholar] [CrossRef] [PubMed]
- Campet, G.; Portier, J.; Subramanian, M.A. Electronegativity versus Fermi energy in oxides: The role of formal oxidation state. Mater. Lett. 2004, 58, 437–438. [Google Scholar] [CrossRef]
- Matar, S.F.; Campet, G.; Subramanian, M.A. Electronic properties of oxides: Chemical and theoretical approaches. Prog. Solid State Chem. 2011, 39, 70–95. [Google Scholar] [CrossRef]
- Mortier, W.J.; Gosh, S.K.; Shankar, S. Electronegativity Equalization Method for the Calculation of Atomic Charges in Molecules. J. Am. Chem. Soc. 1986, 108, 4315–4320. [Google Scholar] [CrossRef]
- Van Genechten, K.A.; Mortier, W.J.; Geerlings, P. Intrinsic framework electronegativity: A novel concept in solid state chemistry. J. Chem. Phys. 1987, 86, 5063–5071. [Google Scholar] [CrossRef]
- Saubanère, M.; Ben Yahia, M.; Lebègue, S.; Doublet, M.-L. An intuitive and efficient method for cell voltage prediction of lithium and sodium-ion batteries. Nat. Commun. 2014, 5, 5559. [Google Scholar] [CrossRef] [PubMed]
- Zaanen, J.; Sawatzky, G.; Allen, J. Band gaps and electronic structure of transition-metal compounds. Phys. Rev. Lett. 1985, 55, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, T.; Bocquet, A.E.; Mizokawa, T.; Fujimori, A. Systematic variation of the electronic structure of 3d transition-metal compounds. Phys. Rev. B 1995, 52, 7934–7938. [Google Scholar] [CrossRef]
- Hong, W.T.; Stoerzinger, K.A.; Moritz, B.; Devereaux, T.P.; Yang, W.; Shao-Horn, Y. Probing LaMO3 Metal and Oxygen Partial Density of States Using X-ray Emission, Absorption, and Photoelectron Spectroscopy. J. Phys. Chem. C 2015, 119, 2063–2072. [Google Scholar] [CrossRef]
- Bocquet, A.E.; Mizokawa, T.; Saitoh, T.; Namatame, H.; Fujimori, A. Electronic structure of 3d-transition-metal compounds by analysis of the 2p core-level photoemission spectra. Phys. Rev. B Condens. Matter 1992, 46, 3771–3784. [Google Scholar] [CrossRef] [PubMed]
- Suntivich, J.; Hong, W.T.; Lee, Y.-L.; Rondinelli, J.M.; Yang, W.; Goodenough, J.B.; Dabrowski, B.; Freeland, J.W.; Shao-horn, Y. Estimating Hybridization of Transition Metal and Oxygen States in Perovskites from O K-edge X-ray Absorption Spectroscopy. J. Phys. Chem. C 2014, 118, 1856–1863. [Google Scholar] [CrossRef]
- Bocquet, A.E.; Fujimori, A.; Mizokawa, T.; Saitoh, T.; Namatame, H.; Suga, S.; Kimizuka, N.; Takeda, Y.; Takano, M. Electronic structure of SrFe4+O3 and related Fe perovskite oxides. Phys. Rev. B 1992, 45, 1561–1570. [Google Scholar] [CrossRef]
- Potze, R.; Sawatzky, G.; Abbate, M. Possibility for an intermediate-spin ground state in the charge-transfer material SrCoO3. Phys. Rev. B Condens. Matter 1995, 51, 11501–11506. [Google Scholar] [CrossRef] [PubMed]
- Abbate, M.; Zampieri, G.; Okamoto, J.; Fujimori, A.; Kawasaki, S.; Takano, M. X-ray absorption of the negative charge-transfer material SrFe1−xCoxO3. Phys. Rev. B 2002, 65. [Google Scholar] [CrossRef]
- Bisogni, V.; Catalano, S.; Green, R.J.; Gibert, M.; Scherwitzl, R.; Huang, Y.; Strocov, V.N.; Zubko, P.; Balandeh, S.; Triscone, J.-M.; et al. Ground-state oxygen holes and the metal–insulator transition in the negative charge-transfer rare-earth nickelates. Nat. Commun. 2016, 7, 13017. [Google Scholar] [CrossRef] [PubMed]
- Grisolia, M.N.; Varignon, J.; Sanchez-Santolino, G.; Arora, A.; Valencia, S.; Varela, M.; Abrudan, R.; Weschke, E.; Schierle, E.; Rault, J.E.; et al. Hybridization-controlled charge transfer and induced magnetism at correlated oxide interfaces. Nat. Phys. 2016, 12, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Mizumaki, M.; Fujii, H.; Yoshii, K.; Hayashi, N.; Saito, T.; Shimakawa, Y.; Uozumi, T.; Takano, M. Electronic structure of BaFeO3 studied by X-ray spectroscopy. Phys. Status Solidi 2015, 12, 818–821. [Google Scholar] [CrossRef]
- Mizumaki, M.; Saito, T.; Uozumi, T.; Shimakawa, Y. X-ray Spectroscopic Studies of A-Site Ordered Perovskite LaMn3B4O12 (B = V, Cr). e-J. Surf. Sci. Nanotechnol. 2012, 10, 575–577. [Google Scholar] [CrossRef]
- Lankhorst, M.H.R.; Bouwmeester, H.J.M.; Verweij, H. Use of the rigid band formalism to interpret the relationship between O chemical potential and electron concentration in La1−xSrxCoO3−δ. Phys. Rev. Lett. 1996, 77, 2989–2992. [Google Scholar] [CrossRef] [PubMed]
- Merkle, R.; Mastrikov, Y.A.; Kotomin, E.A.; Kuklja, M.M.; Maier, J. First Principles Calculations of Oxygen Vacancy Formation and Migration in Ba1−xSrxCo1−yFeyO3−δ Perovskites. J. Electrochem. Soc. 2012, 159, B219. [Google Scholar] [CrossRef]
- Mastrikov, Y.A.; Merkle, R.; Heifets, E.; Kotomin, E.A.; Maier, J. Pathways for Oxygen Incorporation in Mixed Conducting Perovskites: A DFT-Based Mechanistic Analysis for (La, Sr)MnO3−δ. J. Phys. Chem. C 2010, 114, 3017–3027. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, J.S.; Noh, T.W.; Byun, D.Y.; Yoo, K.S.; Yamaura, K.; Takayama-Muromachi, E. Systematic trends in the electronic structure parameters of the 4d transition-metal oxides SrMO3 (M = Zr, Mo, Ru, and Rh). Phys. Rev. B 2003, 67. [Google Scholar] [CrossRef]
- Sathiya, M.; Rousse, G.; Ramesha, K.; Laisa, C.P.; Vezin, H.; Sougrati, M.T.; Doublet, M.-L.; Foix, D.; Gonbeau, D.; Walker, W.; et al. Reversible anionic redox chemistry in high-capacity layered-oxide electrodes. Nat. Mater. 2013, 12, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Yabuuchi, N.; Takeuchi, M.; Nakayama, M.; Shiiba, H.; Ogawa, M.; Nakayama, K.; Ohta, T.; Endo, D.; Ozaki, T.; Inamasu, T.; et al. High-capacity electrode materials for rechargeable lithium batteries: Li3NbO4-based system with cation-disordered rocksalt structure. Proc. Natl. Acad. Sci. USA 2015, 112, 7650–7655. [Google Scholar] [CrossRef] [PubMed]
- Thackeray, M.M.; Kang, S.-H.; Johnson, C.S.; Vaughey, J.T.; Benedek, R.; Hackney, S.A. Li2MnO3-stabilized LiMO2 (M = Mn, Ni, Co) electrodes for lithium-ion batteries. J. Mater. Chem. 2007, 17, 3112. [Google Scholar] [CrossRef]
- Lu, Z.; Beaulieu, L.Y.; Donaberger, R.A.; Thomas, C.L.; Dahn, J.R. Synthesis, Structure, and Electrochemical Behavior of Li[NixLi1/3−2x/3Mn2/3−x/3]O2. J. Electrochem. Soc. 2002, 149, A778. [Google Scholar] [CrossRef]
- Grimaud, A.; Carlton, C.E.; Risch, M.; Hong, W.T.; May, K.J.; Shao-horn, Y. Oxygen Evolution Activity and Stability of Ba6Mn5O16, Sr4Mn2CoO9, and Sr6Co5O15: The Influence of Transition Metal Coordination. J. Phys. Chem. C 2013, 117, 25926–25932. [Google Scholar] [CrossRef]
- Villesuzanne, A.; Whangbo, M.-H. Comparative electronic band structure study of the intrachain ferromagnetic versus antiferromagnetic coupling in the magnetic oxides Ca3Co2O6 and Ca3FeRhO6. Inorg. Chem. 2005, 44, 6339–6345. [Google Scholar] [CrossRef] [PubMed]
- Torrance, J.B.; Lacorre, P.; Nazzal, A.I.; Ansaldo, E.J.; Niedermayer, C. Systematic study of insulator-metal transitions in perovskites RNiO3. Phys. Rev. B Condens. Matter 1992, 45, 8209–8212. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomie Distances in Halides and Chaleogenides. Acta Cryst. 1976, A32, 751. [Google Scholar] [CrossRef]
- Nemudry, A.; Goldberg, E.L.; Aguirre, M.; Alario-Franco, M.A. Electrochemical topotactic oxidation of nonstoichiometric perovskites at ambient temperature. Solid State Sci. 2002, 4, 677–690. [Google Scholar] [CrossRef]
- Grenier, J.C.; Wattiaux, A.; Doumerc, J.P.; Dordor, P.; Fournes, L.; Chaminade, J.P.; Pouchard, M. Electrochemical Oxygen Intercalation into Oxide Networks. J. Solid State Chem. 1992, 96, 20–30. [Google Scholar] [CrossRef]
- Götzfried, T.; Reller, A.; Ebbinghaus, S.G. Structural and Magnetic Properties of Hexagonal Perovskites La1.2Sr2.7MO7.33 (M = Ru, Ir) Containing Peroxide Ions. Inorg. Chem. 2005, 44, 6550–6557. [Google Scholar] [CrossRef] [PubMed]
- Grasset, F.; Dussarrat, C.; Darriet, J.; Schweitzer, A.A. Preparation, thermal stability and crystal structure of a new ruthenium(V) oxide containing peroxide ions: Ba2Ru5O9(O2). Structural relationships to the hexagonal-type perovskite. J. Mater. Chem. 1997, 7, 1911–1915. [Google Scholar] [CrossRef]
- Doornkamp, C.; Ponec, V. The universal character of the Mars and Van Krevelen mechanism. J. Mol. Catal. A Chem. 2000, 162, 19–32. [Google Scholar] [CrossRef]
- Mars, P.; van Krevelen, D.W. Oxidations carried out by means of vanadium oxide catalysts. Chem. Eng. Sci. 1954, 3, 41–59. [Google Scholar] [CrossRef]
- Grimaud, A.; Hong, W.T.; Shao-Horn, Y.; Tarascon, J.M. Anionic redox processes for electrochemical devices. Nat. Mater. 2016, 15, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Bielanski, A.; Haber, J. Oxygen in Catalysis on Transition Metal Oxides. Catal. Rev. 1979, 19, 1–41. [Google Scholar] [CrossRef]
- Croué, K.; Jolivet, J.-P.; Larcher, D. Direct Determination of Oxide Surface Free Energy through Potentiometric Measurements. Electrochem. Solid-State Lett. 2012, 15, F8. [Google Scholar] [CrossRef]
- Grimaud, A.; May, K.J.; Carlton, C.E.; Lee, Y.L.; Risch, M.; Hong, W.T.; Zhou, J.; Shao-Horn, Y. Double perovskites as a family of highly active catalysts for oxygen evolution in alkaline solution. Nat. Commun. 2013, 4, 2439. [Google Scholar] [CrossRef] [PubMed]
- Bockris, J.O.M.; Otagawa, T. The electrocatalysis of oxygen evolution on perovskites. J. Electrochem. Soc. 1984, 131, 290–302. [Google Scholar] [CrossRef]
- Bockris, J.O.; Otagawa, T. Mechanism of oxygen evolution on perovskites. J. Phys. Chem. 1983, 87, 2960–2971. [Google Scholar] [CrossRef]
- Dowden, D.A. Crystal and Ligand Field Models of Solid Catalysts. Catal. Rev. 1972, 5, 1–32. [Google Scholar] [CrossRef]
- Calle-Vallejo, F.; Inoglu, N.G.; Su, H.-Y.; Martínez, J.I.; Man, I.C.; Koper, M.T.M.; Kitchin, J.R.; Rossmeisl, J. Number of outer electrons as descriptor for adsorption processes on transition metals and their oxides. Chem. Sci. 2013, 4, 1245. [Google Scholar] [CrossRef]
- Suntivich, J.; Gasteiger, H.A.; Yabuuchi, N.; Nakanishi, H.; Goodenough, J.B.; Shao-Horn, Y. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal–air batteries. Nat. Chem. 2011, 3, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shao-Horn, Y. A Perovskite Oxide Optimized for Oxygen Evolution Catalysis from Molecular Orbital Principles. Science 2011, 334, 1383–1385. [Google Scholar] [CrossRef] [PubMed]
- Meadowcroft, D.B. Low-cost Oxygen Electrode Material. Nature 1970, 226, 847–848. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Sato, E. Electrocatalytic properties of transition metal oxides for oxygen evolution reaction. Mater. Chem. Phys. 1986, 14, 397–426. [Google Scholar] [CrossRef]
- Han, B.; Stoerzinger, K.A.; Tileli, V.; Gamalski, A.D.; Stach, E.A.; Shao-Horn, Y. Nanoscale structural oscillations in perovskite oxides induced by oxygen evolution. Nat. Mater. 2016, 16, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Raabe, S.; Mierwaldt, D.; Ciston, J.; Uijttewaal, M.; Stein, H.; Hoffmann, J.; Zhu, Y.; Blöchl, P.; Jooss, C. In Situ Electrochemical Electron Microscopy Study of Oxygen Evolution Activity of Doped Manganite Perovskites. Adv. Funct. Mater. 2012, 22, 3378–3388. [Google Scholar] [CrossRef]
- Risch, M.; Grimaud, A.; May, K.J.; Stoerzinger, K.A.; Chen, T.J.; Mansour, A.N.; Shao-Horn, Y. Structural Changes of Cobalt-Based Perovskites upon Water Oxidation Investigated by EXAFS. J. Phys. Chem. C 2013, 117, 8628–8635. [Google Scholar] [CrossRef]
- Man, I.C.; Su, H.-Y.; Calle-Vallejo, F.; Hansen, H.A.; Martínez, J.I.; Inoglu, N.G.; Kitchin, J.; Jaramillo, T.F.; Nørskov, J.K.; Rossmeisl, J. Universality in Oxygen Evolution Electrocatalysis on Oxide Surfaces. ChemCatChem 2011, 3, 1159–1165. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Petrie, J.R.; Cooper, V.R.; Freeland, J.W.; Meyer, T.L.; Zhang, Z.; Lutterman, D.A.; Lee, H.N. Enhanced Bifunctional Oxygen Catalysis in Strained LaNiO3 Perovskites. J. Am. Chem. Soc. 2016, 138, 2488–2491. [Google Scholar] [CrossRef] [PubMed]
- Mavros, M.G.; Tsuchimochi, T.; Kowalczyk, T.; McIsaac, A.; Wang, L.-P.; Voorhis, T.V. What Can Density Functional Theory Tell Us about Artificial Catalytic Water Splitting? Inorg. Chem. 2014, 53, 6386–6397. [Google Scholar] [CrossRef] [PubMed]
- Betley, T.A.; Wu, Q.; Van Voorhis, T.; Nocera, D.G. Electronic Design Criteria for O−O Bond Formation via Metal−Oxo Complexes. Inorg. Chem. 2008, 47, 1849–1861. [Google Scholar] [CrossRef] [PubMed]
- Vasala, S.; Karppinen, M. A2B′B″O6 perovskites: A review. Prog. Solid State Chem. 2015, 43, 1–36. [Google Scholar] [CrossRef]
- Diaz-Morales, O.; Raaijman, S.; Kortlever, R.; Kooyman, P.J.; Wezendonk, T.; Gascon, J.; Fu, W.T.; Koper, M.T.M. Iridium-based double perovskites for efficient water oxidation in acid media. Nat. Commun. 2016, 7, 12363. [Google Scholar] [CrossRef] [PubMed]
- Seitz, L.C.; Dickens, C.F.; Nishio, K.; Hikita, Y.; Montoya, J.; Doyle, A.; Kirk, C.; Vojvodic, A.; Hwang, H.Y.; Norskov, J.K.; et al. A highly active and stable IrOx/SrIrO3 catalyst for the oxygen evolution reaction. Science 2016, 353, 1011–1014. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Danilovic, N.; Chang, K.-C.; Subbaraman, R.; Paulikas, A.P.; Fong, D.D.; Highland, M.J.; Baldo, P.M.; Stamenkovic, V.R.; Freeland, J.W.; et al. Functional links between stability and reactivity of strontium ruthenate single crystals during oxygen evolution. Nat. Commun. 2014, 5, 4191. [Google Scholar] [CrossRef] [PubMed]
- Binninger, T.; Mohamed, R.; Waltar, K.; Fabbri, E.; Levecque, P.; Kötz, R.; Schmidt, T.J. Thermodynamic explanation of the universal correlation between oxygen evolution activity and corrosion of oxide catalysts. Sci. Rep. 2015, 5, 12167. [Google Scholar] [CrossRef] [PubMed]
- Björneholm, O.; Hansen, M.H.; Hodgson, A.; Liu, L.-M.; Limmer, D.T.; Michaelides, A.; Pedevilla, P.; Rossmeisl, J.; Shen, H.; Tocci, G.; et al. Water at Interfaces. Chem. Rev. 2016, 116, 7698–7726. [Google Scholar] [CrossRef] [PubMed]
- Rong, X.; Kolpak, A.M. Ab Initio Approach for Prediction of Oxide Surface Structure, Stoichiometry, and Electrocatalytic Activity in Aqueous Solution. J. Phys. Chem. Lett. 2015, 6, 1785–1789. [Google Scholar] [CrossRef] [PubMed]
- Tascón, J.M.D.; González Tejuca, L. Catalytic activity of perovskite-type oxides LaMeO3. React. Kinet. Catal. Lett. 1980, 15, 185–191. [Google Scholar] [CrossRef]
- Demazeau, G.; Oh-Kim, E.O.; Choy, J.-H.; Hagenmuller, P. La stabilisation partielle du manganese(V) en coordinence octahedrique au sein d’un reseau oxygéné: Préparation et caractérisation physico-chimique du composé La2LiMnO6−x. J. Solid 1992, 101, 221–228. [Google Scholar]
- Demazeau, G.; Buffat, B.; Pouchard, M.; Hagenmuller, P. Recent developments in the field of high oxidation states of transition elements in oxides stabilization of Six-coordinated Iron(V). Z. Anorg. Allg. Chem. 1982, 491, 60–66. [Google Scholar] [CrossRef]
- Buffat, B.; Tljilier, M.H.; Dexpert, H.; Demazeau, G. X-ray absorption investigation of some high oxidation states of six-co-ordinated iron in oxides of perovskite or k2nif4 -type structures. J. Phys. Chem. Solids 1986, 47, 491–496. [Google Scholar] [CrossRef]
- Yagi, S.; Yamada, I.; Tsukasaki, H.; Seno, A.; Murakami, M.; Fujii, H.; Chen, H.; Umezawa, N.; Abe, H.; Nishiyama, N.; et al. Covalency-reinforced oxygen evolution reaction catalyst. Nat. Commun. 2015, 6, 8249. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Fabbri, E.; Nachtegaal, M.; Castelli, I.E.; El Kazzi, M.; Haumont, R.; Marzari, N.; Schmidt, T.J. Oxygen Evolution Reaction on La1–xSrxCoO3 Perovskites: A Combined Experimental and Theoretical Study of Their Structural, Electronic, and Electrochemical Properties. Chem. Mater. 2015, 27, 7662–7672. [Google Scholar] [CrossRef]
- May, K.J.; Carlton, C.E.; Stoerzinger, K.A.; Risch, M.; Suntivich, J.; Lee, Y.-L.; Grimaud, A.; Shao-Horn, Y. Influence of oxygen evolution during water oxidation on the surface of perovskite oxide catalysts. J. Phys. Chem. Lett. 2012, 3, 3264–3270. [Google Scholar] [CrossRef]
- Tahini, H.A.; Tan, X.; Schwingenschlögl, U.; Smith, S.C. In Operando Self-Healing of Perovskite Electrocatalysts: A Case Study of SrCoO3 for the Oxygen Evolution Reaction. Part. Part. Syst. Charact. 2017, 34, 1600280. [Google Scholar] [CrossRef]
- Tarancón, A.; Burriel, M.; Santiso, J.; Skinner, S.J.; Kilner, J.A. Advances in layered oxide cathodes for intermediate temperature solid oxide fuel cells. J. Mater. Chem. 2010, 20, 3799. [Google Scholar] [CrossRef]
- Voorhoeve, R.J.H.; Johnson, D.W.; Remeika, J.P.; Gallagher, P.K. Perovskite Oxides: Materials Science in Catalysis. Science 1977, 195, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Voorhoeve, R.J.H.; Remeika, J.P.; Trimble, L.E. Defect Chemistry and Catalysis in Oxidation and Reduction over Perovskite-type Oxides. Ann. N. Y. Acad. Sci. 1976, 272, 3–21. [Google Scholar] [CrossRef]
- Yamazoe, N.; Teraoka, Y. Oxidation Catalysis of Perovskites—Relationships to Bulk Structure and Composition (Valency, Defects, etc.). Catal. Today 1990, 8, 175–199. [Google Scholar] [CrossRef]
- Yamazoe, N.; Teraoka, Y.; Seiyama, T. TPD and XPS Thermal Behavior of Adsorbed Oxygen in La1−xSrxCoO3. Chem. Lett. 1981, 10, 1767–1770. [Google Scholar] [CrossRef]
- Panov, G.; Dubkov, K.; Starokon, E. Active oxygen in selective oxidation catalysis. Catal. Today 2006, 117, 148–155. [Google Scholar] [CrossRef]
- Meng, Q.; Wang, W.; Weng, X.; Liu, Y.; Wang, H.; Wu, Z. Active Oxygen Species in Lan+1NinO3n+1 Layered Perovskites for Catalytic Oxidation of Toluene and Methane. J. Phys. Chem. C 2016, 120, 3259–3266. [Google Scholar] [CrossRef]
- Pereñíguez, R.; Hueso, J.L.; Gaillard, F.; Holgado, J.P.; Caballero, A. Study of Oxygen Reactivity in La1−xSrxCoO3−δ Perovskites for Total Oxidation of Toluene. Catal. Lett. 2012, 142, 408–416. [Google Scholar] [CrossRef]
- Tejuca, L.G.; Fierro, J.L.G.; Tascon, J.M.D. Structure and Reactivity of Perovskite-Type Oxides. Adv. Catal. 1989, 36, 237–328. [Google Scholar]
- Rremenic, G.; Nieto, J.M.L.; Tascon, J.M.D.; Tejuca, L.G. Chemisorption and Catalysis on LaMO3 Oxides. J. Chem. Soc. Faraday Trans. 1985, 1, 939–949. [Google Scholar] [CrossRef]
- Gellings, P.J.; Bouwmeester, H.J.M. Solid state aspects of oxidation catalysis. Catal. Today 2000, 58, 1–53. [Google Scholar] [CrossRef]
- Mildner, S.; Beleggia, M.; Mierwaldt, D.; Hansen, T.W.; Wagner, J.B.; Yazdi, S.; Kasama, T.; Ciston, J.; Zhu, Y.; Jooss, C. Environmental TEM Study of Electron Beam Induced Electrochemistry of Pr0.64Ca0.36MnO3 Catalysts for Oxygen Evolution. J. Phys. Chem. C 2015, 119, 5301–5310. [Google Scholar] [CrossRef]
- Halat, D.M.; Dervişoğlu, R.; Kim, G.; Dunstan, M.T.; Blanc, F.; Middlemiss, D.S.; Grey, C.P. Probing Oxide-Ion Mobility in the Mixed Ionic-Electronic Conductor La2NiO4+δ by Solid-State (17)O MAS NMR Spectroscopy. J. Am. Chem. Soc. 2016, 138, 11958–11969. [Google Scholar] [CrossRef] [PubMed]
- Wohlfahrt-Mehrens, M.; Heitbaum, J. Oxygen evolution on Ru and RuO2 electrodes studied using isotope labelling and on-line mass spectrometry. J. Electroanal. Chem. Interfacial Electrochem. 1987, 237, 251–260. [Google Scholar] [CrossRef]
- Fierro, S.; Nagel, T.; Baltruschat, H.; Comninellis, C. Investigation of the oxygen evolution reaction on Ti/IrO2 electrodes using isotope labelling and on-line mass spectrometry. Electrochem. Commun. 2007, 9, 1969–1974. [Google Scholar] [CrossRef]
- Diaz-Morales, O.; Calle-Vallejo, F.; de Munck, C.; Koper, M.T.M. Electrochemical water splitting by gold: Evidence for an oxide decomposition mechanism. Chem. Sci. 2013, 4, 2334. [Google Scholar] [CrossRef]





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Grimaud, A. Factors Controlling the Redox Activity of Oxygen in Perovskites: From Theory to Application for Catalytic Reactions. Catalysts 2017, 7, 149. https://doi.org/10.3390/catal7050149
Yang C, Grimaud A. Factors Controlling the Redox Activity of Oxygen in Perovskites: From Theory to Application for Catalytic Reactions. Catalysts. 2017; 7(5):149. https://doi.org/10.3390/catal7050149
Chicago/Turabian StyleYang, Chunzhen, and Alexis Grimaud. 2017. "Factors Controlling the Redox Activity of Oxygen in Perovskites: From Theory to Application for Catalytic Reactions" Catalysts 7, no. 5: 149. https://doi.org/10.3390/catal7050149




