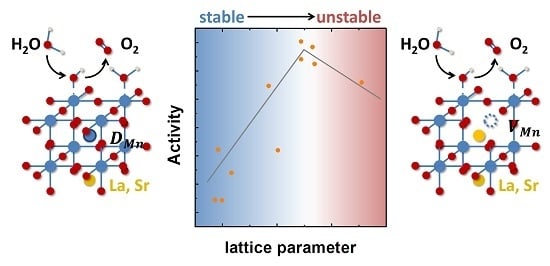
Tailoring the Oxygen Evolution Activity and Stability Using Defect Chemistry
Abstract
:1. Introduction
2. Results
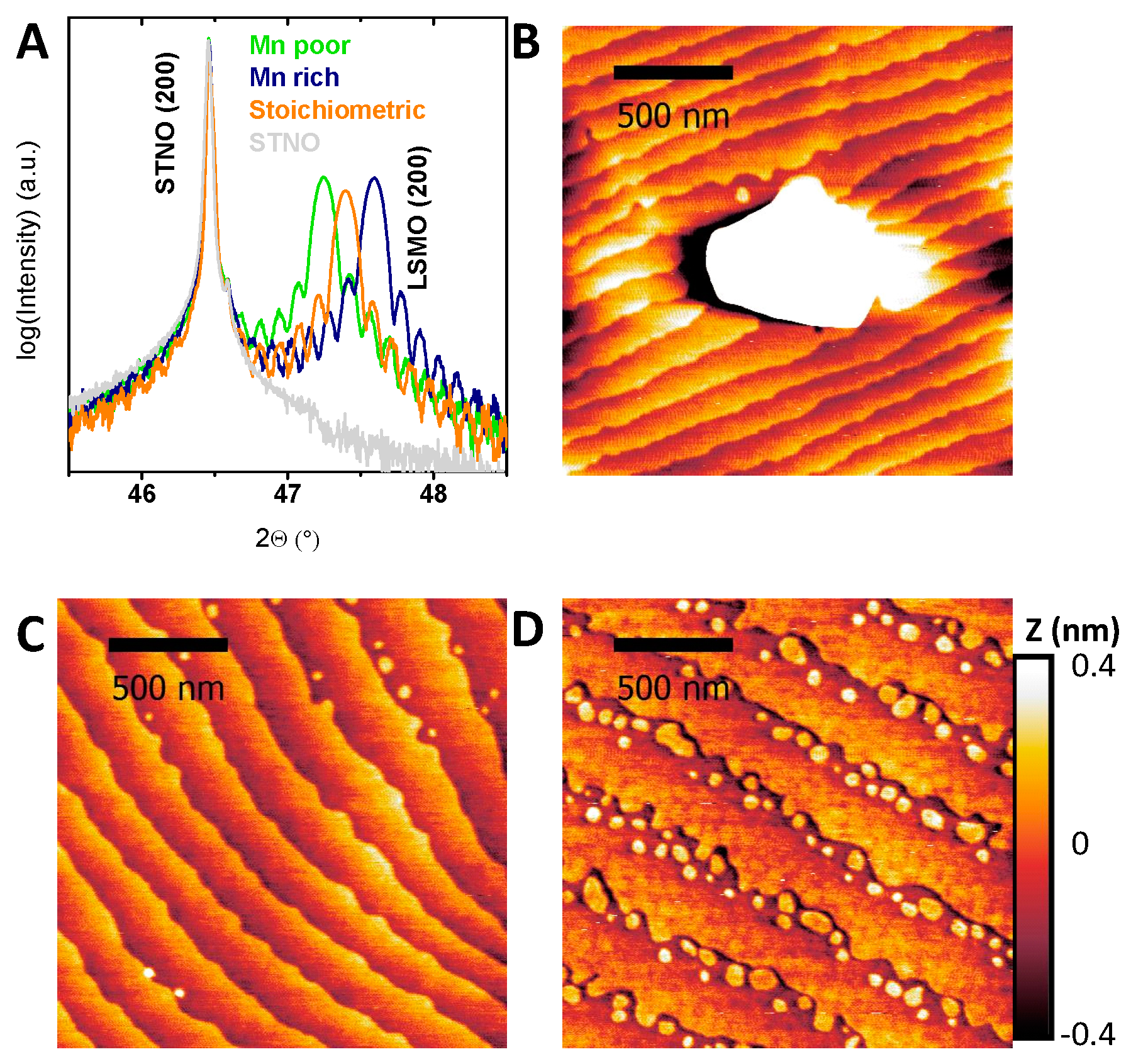
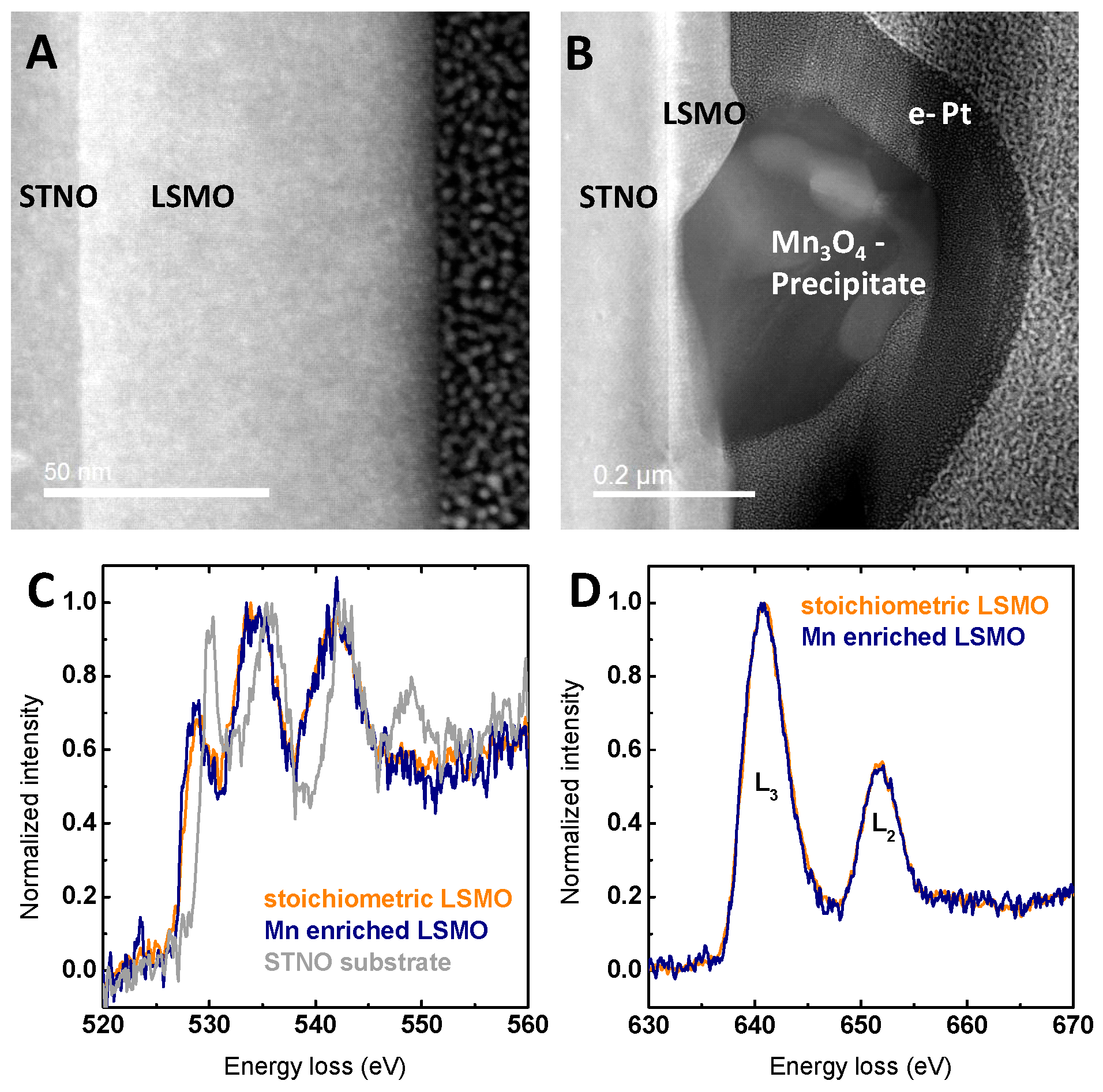
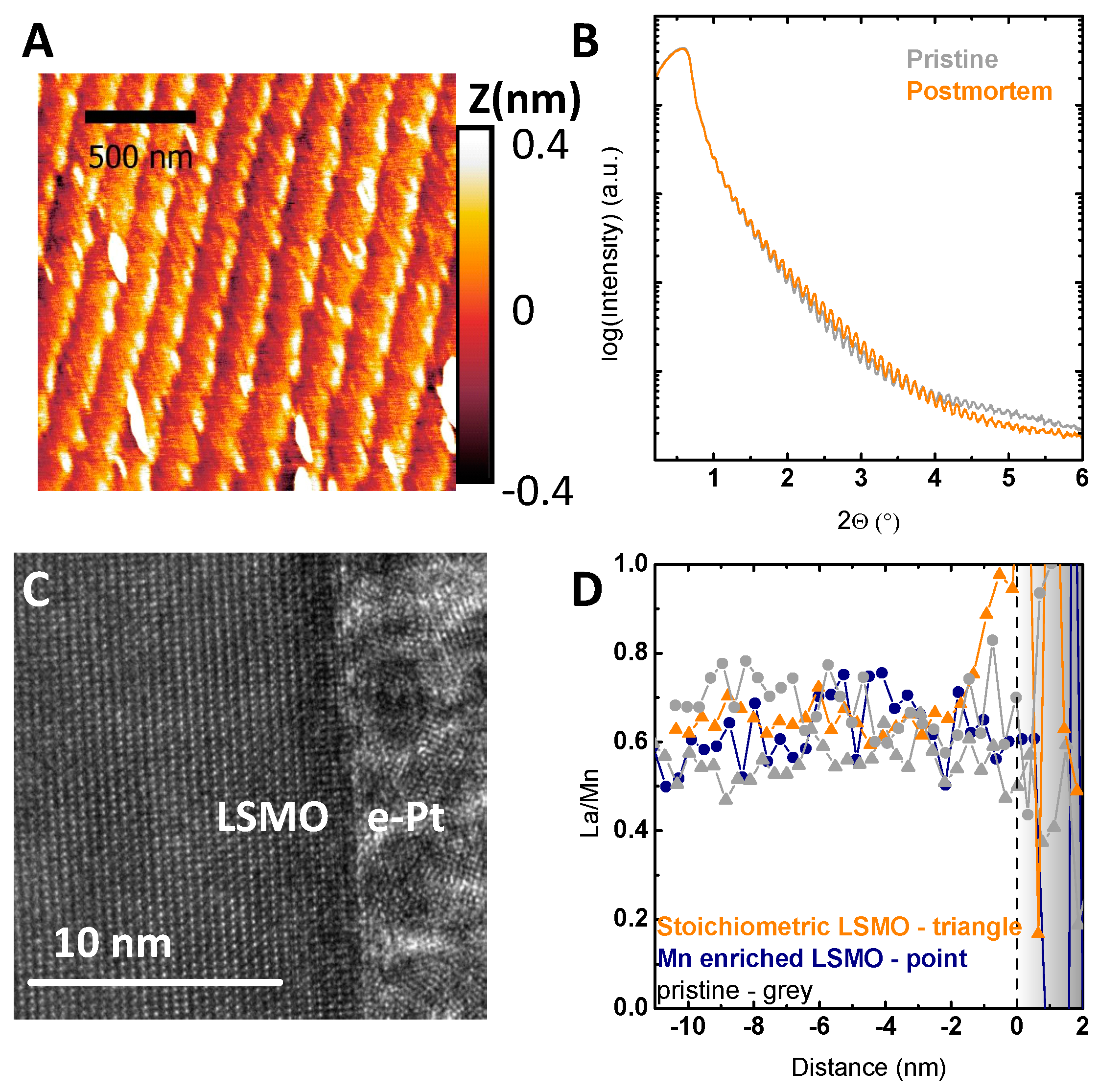
2.1. Structural and Electronic Characterization
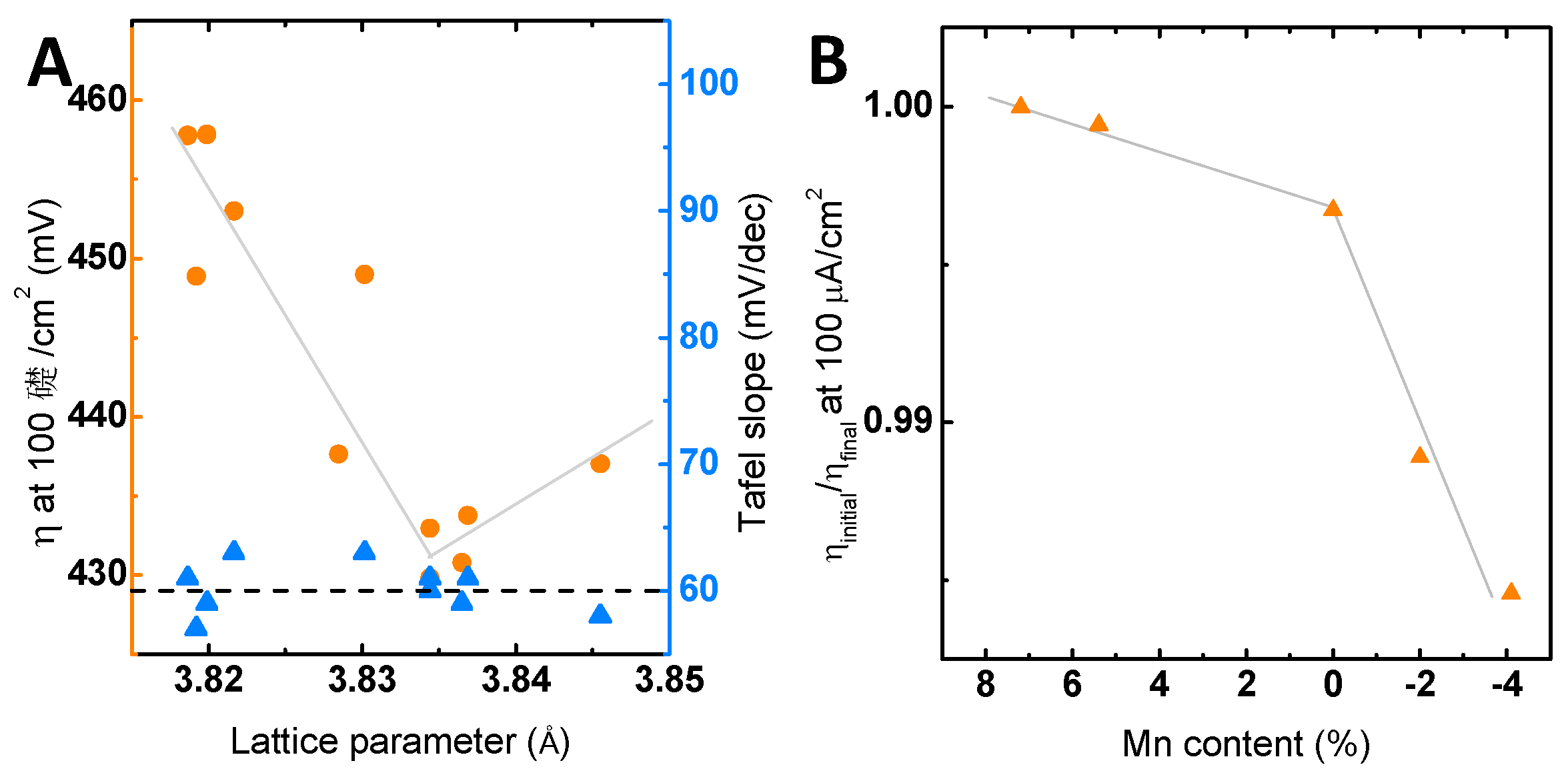
2.2. Electrochemical Characterization
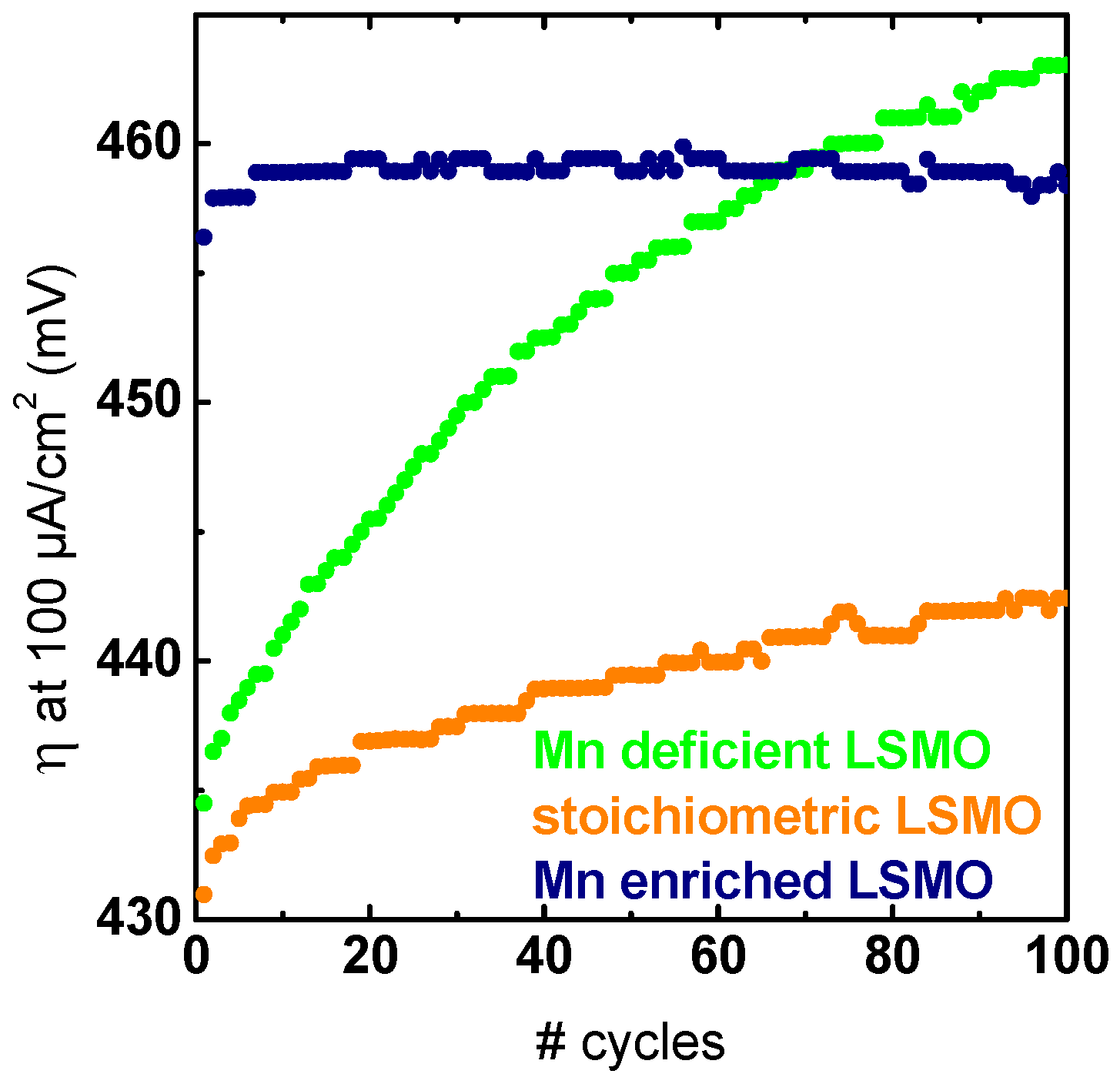
2.3. Stability Characterization
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wiechen, M.; Berends, H.-M.; Kurz, P. Wateroxidation catalysed by manganese compounds: From complexes to “biomimetic rocks”. Dalton Trans. 2012, 41, 21–31. [Google Scholar] [CrossRef] [PubMed]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef] [PubMed]
- Giordano, L.; Han, B.; Risch, M.; Hong, W.T.; Rao, R.R.; Stoerzinger, K.A.; Shao-Horn, Y. pH dependence of OER activity of oxides: Current and future perspectives. Catal. Today 2016, 262, 2–10. [Google Scholar] [CrossRef]
- Hong, W.T.; Risch, M.; Stoerzinger, K.A.; Grimaud, A.; Suntivich, J.; Shao-Horn, Y. Toward the rational design of non-precious transition metal oxides for oxygen electrocatalysis. Energy Environ. Sci. 2015, 8, 1404–1427. [Google Scholar] [CrossRef]
- Rincón, R.A.; Ventosa, E.; Tietz, F.; Masa, J.; Seisel, S.; Kuznetsov, V.; Schuhmann, W. Evaluation of Perovskites as Electrocatalysts for the Oxygen Evolution Reaction. ChemPhysChem 2014, 15, 2810–2816. [Google Scholar] [CrossRef] [PubMed]
- Bockris, J.O.; Otagawa, T. Mechanism of oxygen evolution on perovskites. J. Phys. Chem. 1983, 87, 2960–2971. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Sato, E. Electrocatalytic properties of transition metal oxides for oxygen evolution reaction. Mater. Chem. Phys. 1986, 14, 397–426. [Google Scholar] [CrossRef]
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shao-Horn, Y. A Perovskite Oxide Optimized for Oxygen Evolution Catalysis from Molecular Orbital Principles. Science 2011, 334, 1383–1385. [Google Scholar] [CrossRef] [PubMed]
- Scholz, J.; Risch, M.; Stoerzinger, K.A.; Wartner, G.; Shao-Horn, Y.; Jooss, C. Rotating Ring—Disk Electrode Study of Oxygen Evolution at a Perovskite Surface: Correlating Activity to Manganese Concentration. J. Phys. Chem. C 2016, 120, 27746–27756. [Google Scholar] [CrossRef]
- Burke, M.S.; Zou, S.; Enman, L.J.; Kellon, J.E.; Gabor, C.A.; Pledger, E.; Boettcher, S.W. Revised Oxygen Evolution Reaction Activity Trends for First-Row Transition-Metal (Oxy)hydroxides in Alkaline Media. J. Phys. Chem. Lett. 2015, 6, 3737–3742. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Suntivich, J.; May, K.J.; Perry, E.E.; Shao-Horn, Y. Synthesis and Activities of Rutile IrO2 and RuO2 Nanoparticles for Oxygen Evolution in Acid and Alkaline Solutions. J. Phys. Chem. Lett. 2012, 3, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Stoerzinger, K.A.; Qiao, L.; Biegalski, M.D.; Shao-Horn, Y. Orientation-Dependent Oxygen Evolution Activities of Rutile IrO2 and RuO2. J. Phys. Chem. Lett. 2014, 5, 1636–1641. [Google Scholar] [CrossRef] [PubMed]
- Paoli, E.A.; Masini, F.; Frydendal, R.; Deiana, D.; Schlaup, C.; Malizia, M.; Hansen, T.W.; Horch, S.; Stephens, I.E.L.; Chorkendorff, I. Oxygen evolution on well-characterized mass-selected Ru and RuO2 nanoparticles. Chem. Sci. 2015, 6, 190–196. [Google Scholar] [CrossRef]
- Frydendal, R.; Paoli, E.A.; Knudsen, B.P.; Wickman, B.; Malacrida, P.; Stephens, I.E.L.; Chorkendorff, I. Benchmarking the Stability of Oxygen Evolution Reaction Catalysts: The Importance of Monitoring Mass Losses. ChemElectroChem 2014, 1, 2075–2081. [Google Scholar] [CrossRef]
- Ebrahimizadeh Abrishami, M.; Risch, M.; Scholz, J.; Roddatis, V.; Osterthun, N.; Jooss, C. Oxygen Evolution at Manganite Perovskite Ruddlesden-Popper Type Particles: Trends of Activity on Structure, Valence and Covalence. Materials 2016, 9, 921. [Google Scholar] [CrossRef]
- Hong, W.T.; Welsch, R.E.; Shao-Horn, Y. Descriptors of Oxygen-Evolution Activity for Oxides: A Statistical Evaluation. J. Phys. Chem. C 2016, 120, 78–86. [Google Scholar] [CrossRef]
- Man, I.C.; Su, H.-Y.; Calle-Vallejo, F.; Hansen, H.A.; Martínez, J.I.; Inoglu, N.G.; Kitchin, J.; Jaramillo, T.F.; Nørskov, J.K.; Rossmeisl, J. Universality in Oxygen Evolution Electrocatalysis on Oxide Surfaces. ChemCatChem 2011, 3, 1159–1165. [Google Scholar] [CrossRef]
- Calle-Vallejo, F.; Inoglu, N.G.; Su, H.-Y.; Martínez, J.I.; Man, I.C.; Koper, M.T.M.; Kitchin, J.R.; Rossmeisl, J. Number of outer electrons as descriptor for adsorption processes on transition metals and their oxides. Chem. Sci. 2013, 4, 1245–1249. [Google Scholar] [CrossRef]
- Lee, Y.-L.; Gadre, M.J.; Shao-Horn, Y.; Morgan, D. Ab initio GGA + U study of oxygen evolution and oxygen reduction electrocatalysis on the (001) surfaces of lanthanum transition metal perovskites LaBO3 (B = Cr, Mn, Fe, Co and Ni). Phys. Chem. Chem. Phys. 2015, 17, 21643–21663. [Google Scholar] [CrossRef] [PubMed]
- Mierwaldt, D.; Mildner, S.; Arrigo, R.; Knop-Gericke, A.; Franke, E.; Blumenstein, A.; Hoffmann, J.; Jooss, C. In Situ XANES/XPS Investigation of Doped Manganese Perovskite Catalysts. Catalysts 2014, 4, 129–145. [Google Scholar] [CrossRef]
- Raabe, S.; Mierwaldt, D.; Ciston, J.; Uijttewaal, M.; Stein, H.; Hoffmann, J.; Zhu, Y.; Blöchl, P.; Jooss, C. In Situ Electrochemical Electron Microscopy Study of Oxygen Evolution Activity of Doped Manganite Perovskites. Adv. Funct. Mater. 2012, 22, 3378–3388. [Google Scholar] [CrossRef]
- Stoerzinger, K.A.; Choi, W.S.; Jeen, H.; Lee, H.N.; Shao-Horn, Y. Role of Strain and Conductivity in Oxygen Electrocatalysis on LaCoO3 Thin Films. J. Phys. Chem. Lett. 2015, 6, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Jalili, H.; Han, J.W.; Kuru, Y.; Cai, Z.; Yildiz, B. New insights into the strain coupling to surface chemistry, electronic structure, and reactivity of La0.7Sr0.3MnO3. J. Phys. Chem. Lett. 2011, 2, 801–807. [Google Scholar] [CrossRef]
- Petrie, J.R.; Jeen, H.; Barron, S.C.; Meyer, T.L.; Lee, H.N. Enhancing Perovskite Electrocatalysis through Strain Tuning of the Oxygen Deficiency. J. Am. Chem. Soc. 2016, 138, 7252–7255. [Google Scholar] [CrossRef] [PubMed]
- Petrie, J.R.; Cooper, V.R.; Freeland, J.W.; Meyer, T.L.; Zhang, Z.; Lutterman, D.A.; Lee, H.N. Enhanced Bifunctional Oxygen Catalysis in Strained LaNiO3 Perovskites. J. Am. Chem. Soc. 2016, 138, 2488–2491. [Google Scholar] [CrossRef] [PubMed]
- Augustynski, J.; Koudelka, M.; Sanchez, J.; Conway, B.E. ESCA study of the state of iridium and oxygen in electrochemically and thermally formed iridium oxide films. J. Electroanal. Chem. Interfacial Electrochem. 1984, 160, 233–248. [Google Scholar] [CrossRef]
- Goniakowski, J.; Finocchi, F.; Noguera, C. Polarity of oxide surfaces and nanostructures. Rep. Prog. Phys. 2008, 71, 016501. [Google Scholar] [CrossRef]
- Strmcnik, D.S.; Tripkovic, D.V.; van der Vliet, D.; Chang, K.-C.; Komanicky, V.; You, H.; Karapetrov, G.; Greeley, J.P.; Stamenkovic, V.R.; Marković, N.M. Unique Activity of Platinum Adislands in the CO Electrooxidation Reaction. J. Am. Chem. Soc. 2008, 130, 15332–15339. [Google Scholar] [CrossRef] [PubMed]
- Strmcnik, D.; Li, D.; Lopes, P.P.; Tripkovic, D.; Kodama, K.; Stamenkovic, V.R.; Markovic, N.M. When Small is Big: The Role of Impurities in Electrocatalysis. Top. Catal. 2015, 58, 1174–1180. [Google Scholar] [CrossRef]
- Jeen, H.; Bi, Z.; Choi, W.S.; Chisholm, M.F.; Bridges, C.A.; Paranthaman, M.P.; Lee, H.N. Orienting Oxygen Vacancies for Fast Catalytic Reaction. Adv. Mater. 2013, 25, 6459–6463. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Danilovic, N.; Chang, K.; Subbaraman, R.; Paulikas, A.P.; Fong, D.D.; Highland, M.J.; Baldo, P.M.; Stamenkovic, V.R.; Freeland, J.W.; et al. Functional links between stability and reactivity of strontium ruthenate single crystals during oxygen evolution. Nat. Commun. 2014, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lopes, P.P.; Strmcnik, D.; Tripkovic, D.; Connell, J.G.; Stamenkovic, V.; Markovic, N.M. Relationships between Atomic Level Surface Structure and Stability/Activity of Platinum Surface Atoms in Aqueous Environments. ACS Catal. 2016, 6, 2536–2544. [Google Scholar] [CrossRef]
- Danilovic, N.; Subbaraman, R.; Chang, K.-C.; Chang, S.H.; Kang, Y.J.; Snyder, J.; Paulikas, A.P.; Strmcnik, D.; Kim, Y.-T.; Myers, D.; et al. Activity-Stability Trends for the Oxygen Evolution Reaction on Monometallic Oxides in Acidic Environments. J. Phys. Chem. Lett. 2014, 5, 2474–2478. [Google Scholar] [CrossRef] [PubMed]
- Barbier, A.; Renaud, G.; Stierle, A. The NiO(111)-(1×1) surface. Surf. Sci. 1998, 402–404, 757–760. [Google Scholar] [CrossRef]
- Kitakatsu, N.; Maurice, V.; Marcus, P. Local decomposition of NiO ultra-thin films formed on Ni(111). Surf. Sci. 1998, 411, 215–230. [Google Scholar] [CrossRef]
- Marozau, I.; Das, P.T.; Döbeli, M.; Storey, J.G.; Uribe-Laverde, M.A.; Das, S.; Wang, C.; Rössle, M.; Bernhard, C. Influence of La and Mn vacancies on the electronic and magnetic properties of LaMnO3 thin films grown by pulsed laser deposition. Phys. Rev. B 2014, 89, 174422. [Google Scholar] [CrossRef]
- Töpfer, J.; Goodenough, J.B. LaMnO3+δ Revisited. J. Solid State Chem. 1997, 130, 117–128. [Google Scholar] [CrossRef]
- Kobayashi, S.; Tokuda, Y.; Ohnishi, T.; Mizoguchi, T.; Shibata, N.; Sato, Y.; Ikuhara, Y.; Yamamoto, T. Cation off-stoichiometric SrMnO3−δ thin film grown by pulsed laser deposition. J. Mater. Sci. 2011, 46, 4354–4360. [Google Scholar] [CrossRef]
- Gao, Q.; Ranjan, C.; Pavlovic, Z.; Blume, R.; Schlögl, R. Enhancement of Stability and Activity of MnOx/Au Electrocatalysts for Oxygen Evolution through Adequate Electrolyte Composition. ACS Catal. 2015, 5, 7265–7275. [Google Scholar] [CrossRef]
- Ifland, B.; Hoffmann, J.; Kramer, T.; Scherff, M.; Mildner, S.; Jooss, C. Strain Driven Phase Decomposition in Ion-Beam Sputtered Pr1−xCax MnO3 Films. J. Nanomater. 2015, 2015, 1–12. [Google Scholar] [CrossRef]
- Grundy, A.N.; Hallstedt, B.; Gauckler, L.J. Assessment of the La–Sr–Mn–O system. Calphad 2004, 28, 191–201. [Google Scholar] [CrossRef]
- Töpfer, J.; Goodenough, J.B. Transport and Magnetic Properties of the Perovskites La1−y MnO3 and LaMn1−z O3. Chem. Mater. 1997, 9, 1467–1474. [Google Scholar] [CrossRef]
- Paterson, J.H.; Krivanek, O.L. ELNES of 3d transition-metal oxides. Ultramicroscopy 1990, 32, 319–325. [Google Scholar] [CrossRef]
- Rask, J.H.; Miner, B.A.; Buseck, P.R. Determination of manganese oxidation states in solids by electron energy-loss spectroscopy. Ultramicroscopy 1987, 21, 321–326. [Google Scholar] [CrossRef]
- Riedl, T.; Gemming, T.; Gruner, W.; Acker, J.; Wetzig, K. Determination of manganese valency in La1−xSrxMnO3 using ELNES in the (S)TEM. Micron 2007, 38, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Varela, M.; Oxley, M.P.; Luo, W.; Tao, J.; Watanabe, M.; Lupini, A.R.; Pantelides, S.T.; Pennycook, S.J. Atomic-resolution imaging of oxidation states in manganites. Phys. Rev. B 2009, 79, 085117. [Google Scholar] [CrossRef]
- Nishida, S.; Kobayashi, S.; Kumamoto, A.; Ikeno, H.; Mizoguchi, T.; Tanaka, I.; Ikuhara, Y.; Yamamoto, T. Effect of local coordination of Mn on Mn-L2,3 edge electron energy loss spectrum. J. Appl. Phys. 2013, 114, 054906. [Google Scholar] [CrossRef]
- Norpoth, J.; Mildner, S.; Scherff, M.; Hoffmann, J.; Jooss, C. In situ TEM analysis of resistive switching in manganite based thin-film heterostructures. Nanoscale 2014, 6, 9852–9862. [Google Scholar] [CrossRef] [PubMed]
- Zemni, S.; Dhahri, J.; Cherif, K.; Dhahri, J.; Oummezzine, M.; Ghedira, M.; Vincent, H. The effect of a cation radii on structural, magnetic and electrical properties of doped manganites La0.6−xPrxSr0.4MnO3. J. Solid State Chem. 2004, 177, 2387–2393. [Google Scholar] [CrossRef]
- Izumi, M.; Konishi, Y.; Nishihara, T.; Hayashi, S.; Shinohara, M.; Kawasaki, M.; Tokura, Y. Atomically defined epitaxy and physical properties of strained La0.6Sr0.4MnO3 films. Appl. Phys. Lett. 1998, 73, 2497–2499. [Google Scholar] [CrossRef]
- Mori, M.; Hiei, Y.; Sammes, N.M.; Tompsett, G.A. Thermal-Expansion Behaviors and Mechanisms for Ca- or Sr-Doped Lanthanum Manganite Perovskites under Oxidizing Atmospheres. J. Electrochem. Soc. 2000, 147, 1295–1302. [Google Scholar] [CrossRef]
- Mitchell, R.H.; Chakhmouradian, A.R.; Woodward, P.M. Crystal chemistry of perovskite-type compounds in the tausonite-loparite series, (Sr1−2xNaxLax)TiO3. Phys. Chem. Miner. 2000, 27, 583–589. [Google Scholar] [CrossRef]
- Aruta, C.; Ghiringhelli, G.; Tebano, A.; Boggio, N.G.; Brookes, N.B.; Medaglia, P.G.; Balestrino, G. Strain induced X-ray absorption linear dichroism in La0.7Sr0.3MnO3 thin films. Phys. Rev. B 2006, 73, 235121. [Google Scholar] [CrossRef]
- Méchin, L.; Wu, S.; Guillet, B.; Perna, P.; Fur, C.; Lebargy, S.; Adamo, C.; Schlom, D.G.; Routoure, J.M. Experimental evidence of correlation between 1/f noise level and metal-to-insulator transition temperature in epitaxial La0.7Sr0.3MnO3 thin films. J. Phys. D Appl. Phys. 2013, 46, 202001. [Google Scholar] [CrossRef]
- Hu, Y.; Tolmachev, Y.V.; Scherson, D.A. Rotating ring-disk studies of oxidized nickel hydrous oxide: Oxygen evolution and pseudocapacitance. J. Electroanal. Chem. 1999, 468, 64–69. [Google Scholar] [CrossRef]
- Risch, M.; Stoerzinger, K.A.; Maruyama, S.; Hong, W.T.; Takeuchi, I.; Shao-Horn, Y. La0.8Sr0.2MnO3−δ Decorated with Ba0.5Sr0.5Co0.8Fe0.2O3−δ: A Bifunctional Surface for Oxygen Electrocatalysis with Enhanced Stability and Activity. J. Am. Chem. Soc. 2014, 136, 5229–5232. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, A.; Hillebrand, P.; Stellmach, D.; May, M.M.; Bogdanoff, P.; Fiechter, S. Evaluation of MnOx, Mn2O3, and Mn3O4 Electrodeposited Films for the Oxygen Evolution Reaction of Water. J. Phys. Chem. C 2014, 118, 14073–14081. [Google Scholar] [CrossRef]
- Doyle, R.L.; Godwin, I.J.; Brandon, M.P.; Lyons, M.E.G. Redox and electrochemical water splitting catalytic properties of hydrated metal oxide modified electrodes. Phys. Chem. Chem. Phys. 2013, 15, 13737–13783. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, S. Tafel slopes from first principles. J. Solid State Electrochem. 2009, 13, 537–549. [Google Scholar] [CrossRef]
- Bockris, J.O. Kinetics of Activation Controlled Consecutive Electrochemical Reactions: Anodic Evolution of Oxygen. J. Chem. Phys. 1956, 24, 817–827. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions, 2nd ed.; National Association of Corrosion Engineers: Houston, TX, USA, 1974. [Google Scholar]
- Su, H.; Gorlin, Y.; Man, I.C.; Calle-Vallejo, F.; Nørskov, J.K.; Jaramillo, T.F.; Rossmeisl, J. Identifying active surface phases for metal oxide electrocatalysts: A study of manganese oxide bi-functional catalysts for oxygen reduction and water oxidation catalysis. Phys. Chem. Chem. Phys. 2012, 14, 14010–14022. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Open Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]

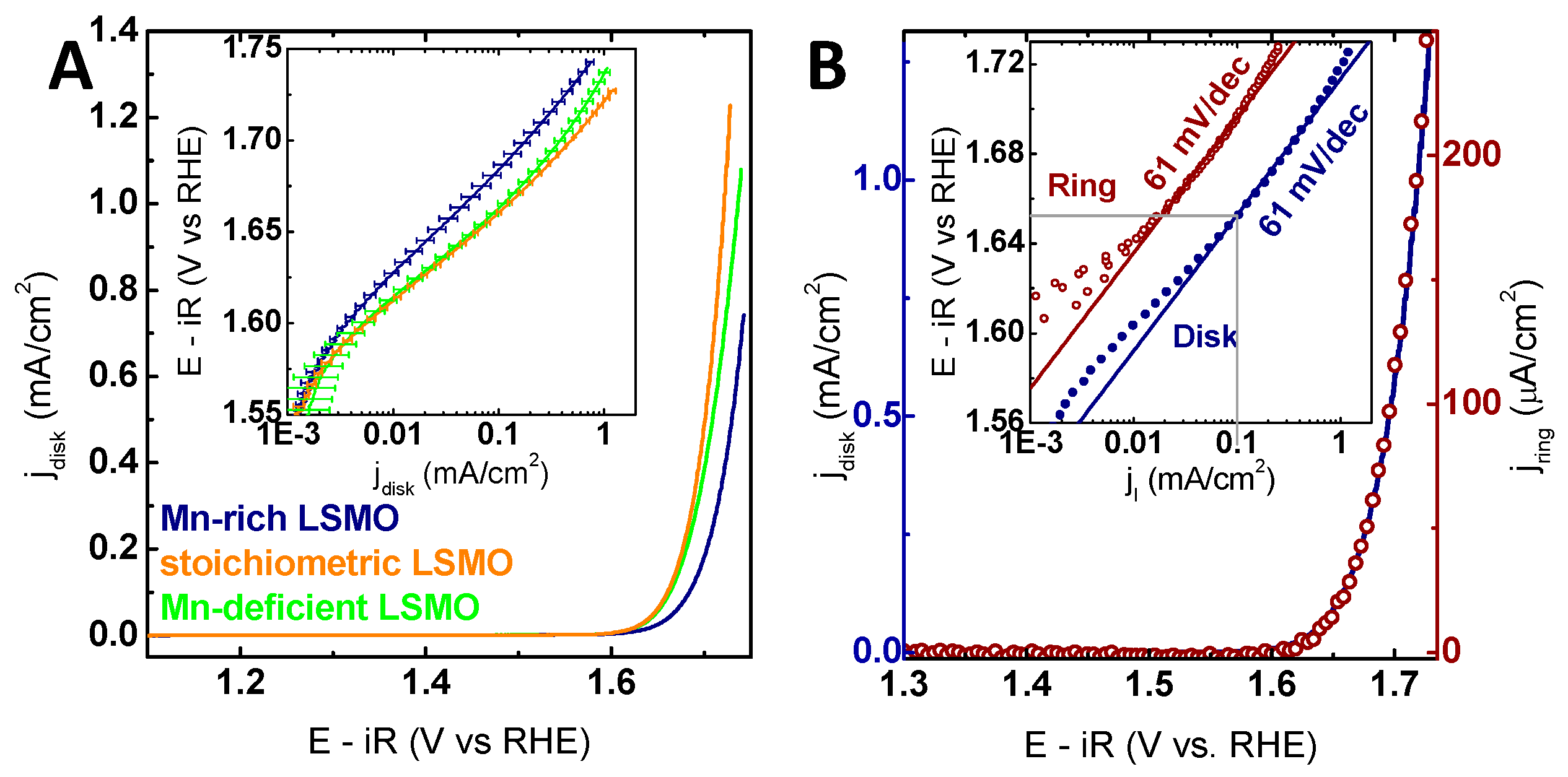
| Sample | Mn-Off-Stoichiometry (%) | Overpotential at 100 µA/cm2 (mV) | JI at 1.7 V vs. RHE (µA/cm2) | Tafel Slope Disk (mV/dec) | Tafel Slope Ring (mV/dec) |
|---|---|---|---|---|---|
| LSM − 2 | −2.0 | 434 | 385 | 61 | 62 |
| LSM − 4 | −4.1 | 437 | 341 | 58 | 59 |
| LSM − 2 | −2.0 | 431 | 460 | 59 | 62 |
| LSM | ~0.0 | 430 | 502 | 60 | 59 |
| LSM | ~0.0 | 433 | 588 | 61 | 61 |
| LSM + 2 a | +1.6 | 453 | 217 | 63 | 64 |
| LSM + 4 a | +3.8 | 449 | 218 | 63 | 63 |
| LSM + 7 | +7.2 | 458 | 162 | 61 | 63 |
| LSM + 5 | +5.4 | 449 | 226 | 57 | 61 |
| LSM + 7 | +7.2 | 458 | 167 | 59 | 62 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scholz, J.; Risch, M.; Wartner, G.; Luderer, C.; Roddatis, V.; Jooss, C. Tailoring the Oxygen Evolution Activity and Stability Using Defect Chemistry. Catalysts 2017, 7, 139. https://doi.org/10.3390/catal7050139
Scholz J, Risch M, Wartner G, Luderer C, Roddatis V, Jooss C. Tailoring the Oxygen Evolution Activity and Stability Using Defect Chemistry. Catalysts. 2017; 7(5):139. https://doi.org/10.3390/catal7050139
Chicago/Turabian StyleScholz, Julius, Marcel Risch, Garlef Wartner, Christoph Luderer, Vladimir Roddatis, and Christian Jooss. 2017. "Tailoring the Oxygen Evolution Activity and Stability Using Defect Chemistry" Catalysts 7, no. 5: 139. https://doi.org/10.3390/catal7050139