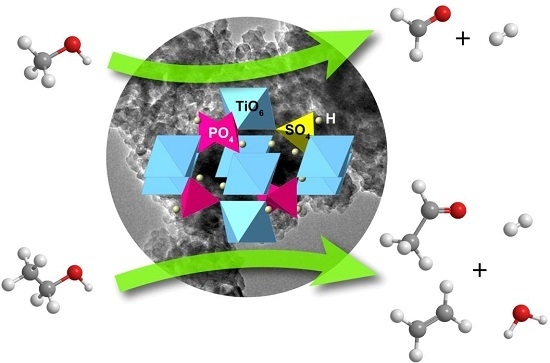
Highly Selective Solid Acid Catalyst H1−xTi2(PO4)3−x(SO4)x for Non-Oxidative Dehydrogenation of Methanol and Ethanol
Abstract
:1. Introduction
2. Results and Discussion
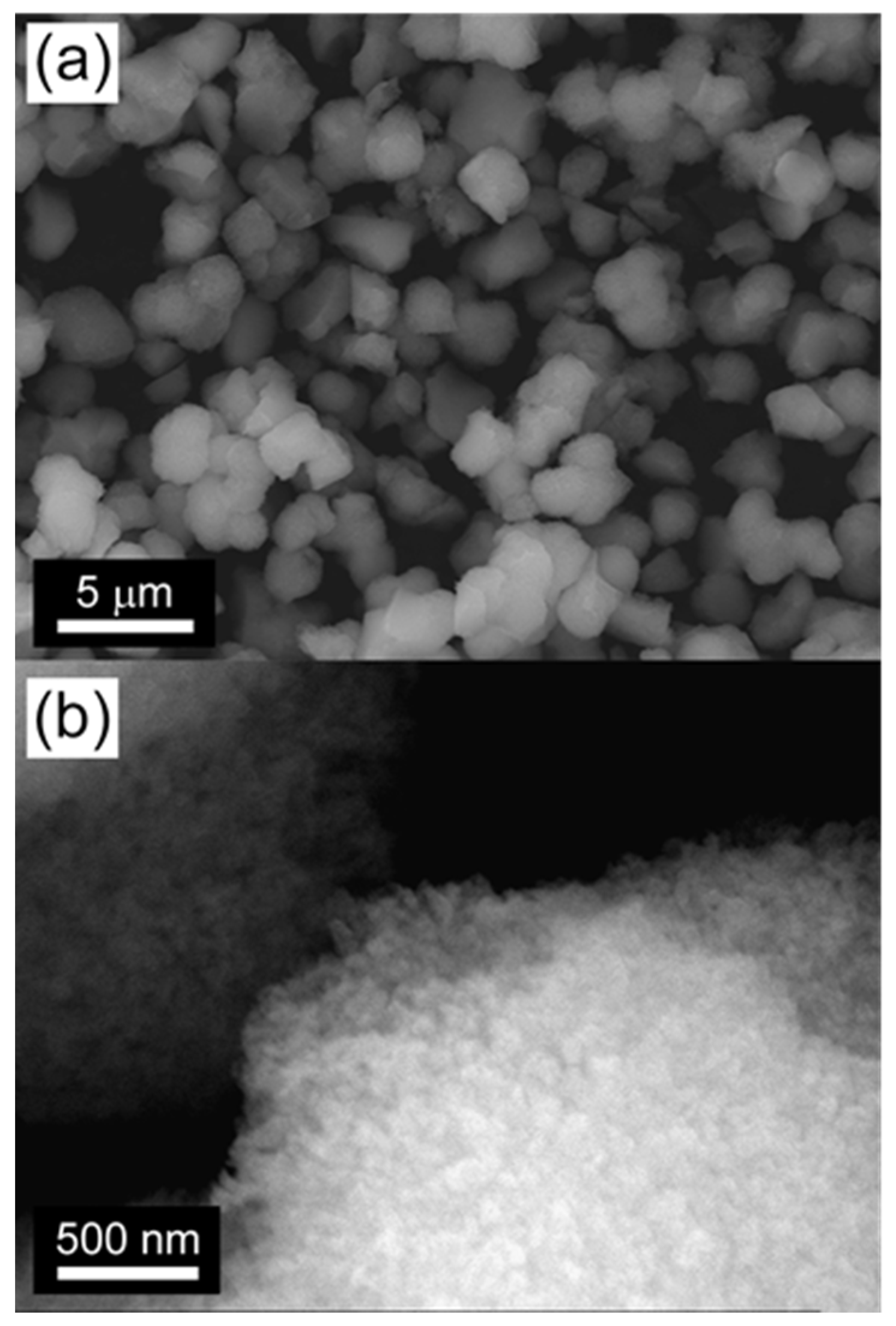
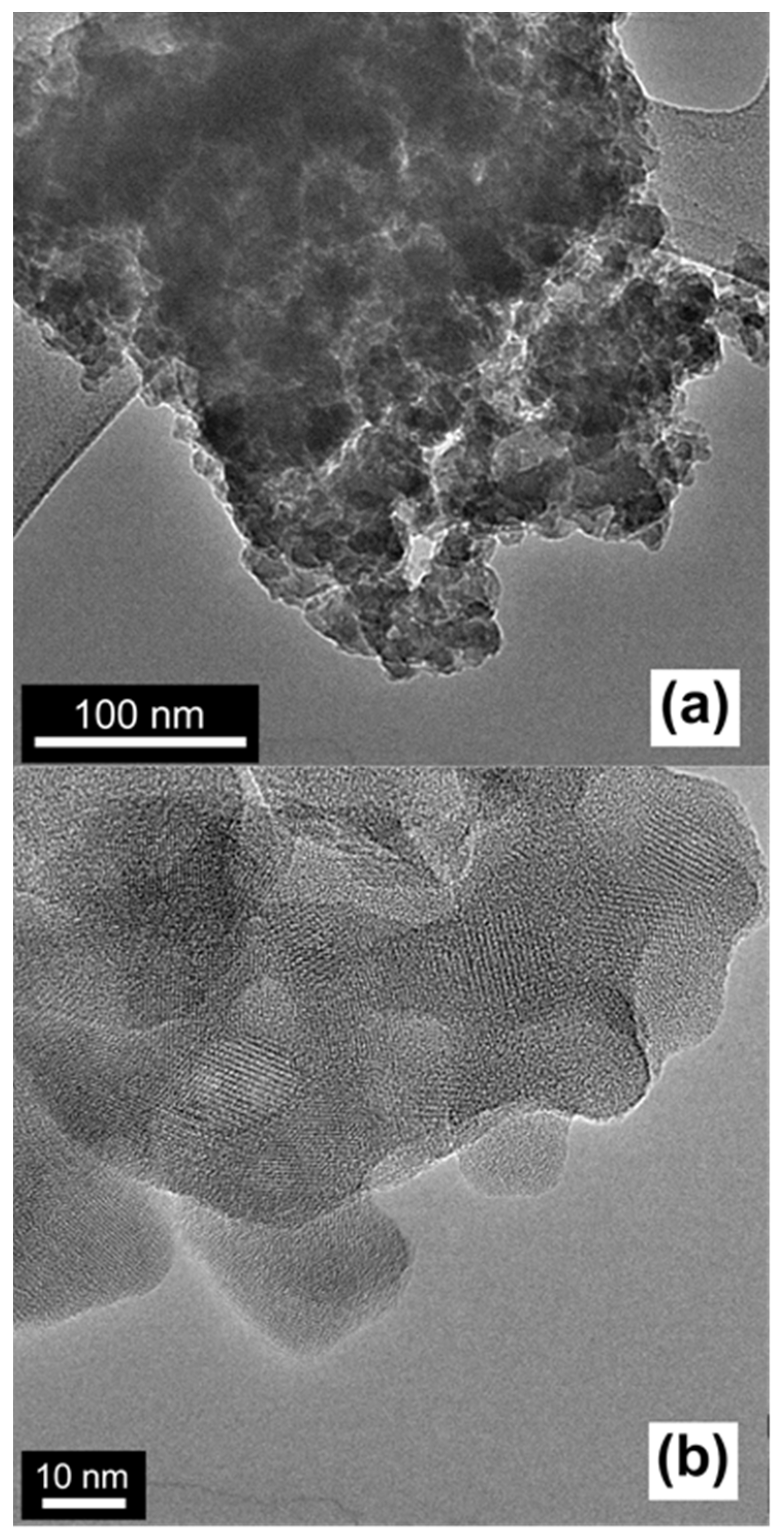
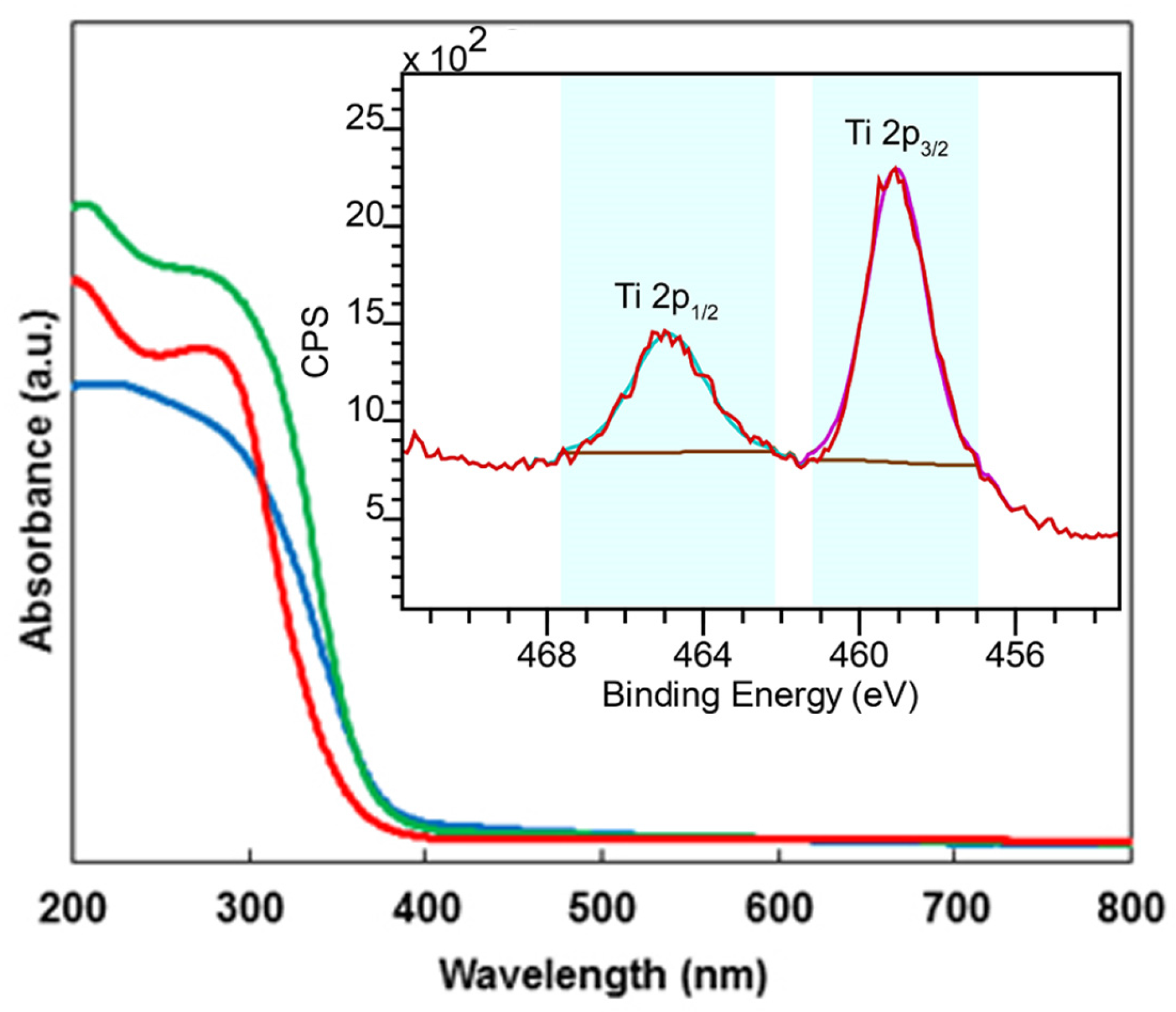
2.1. Characterization of the Catalysts
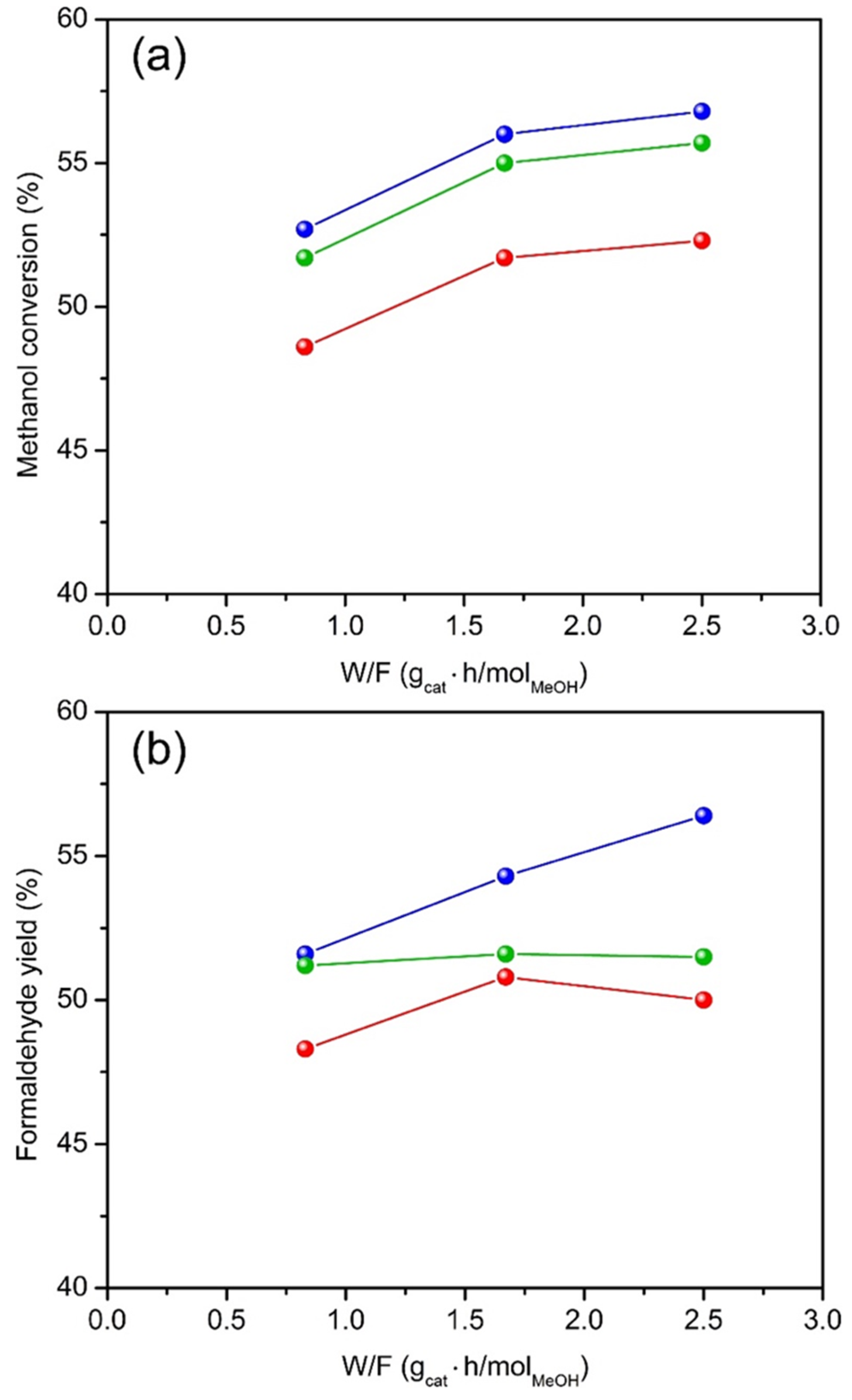
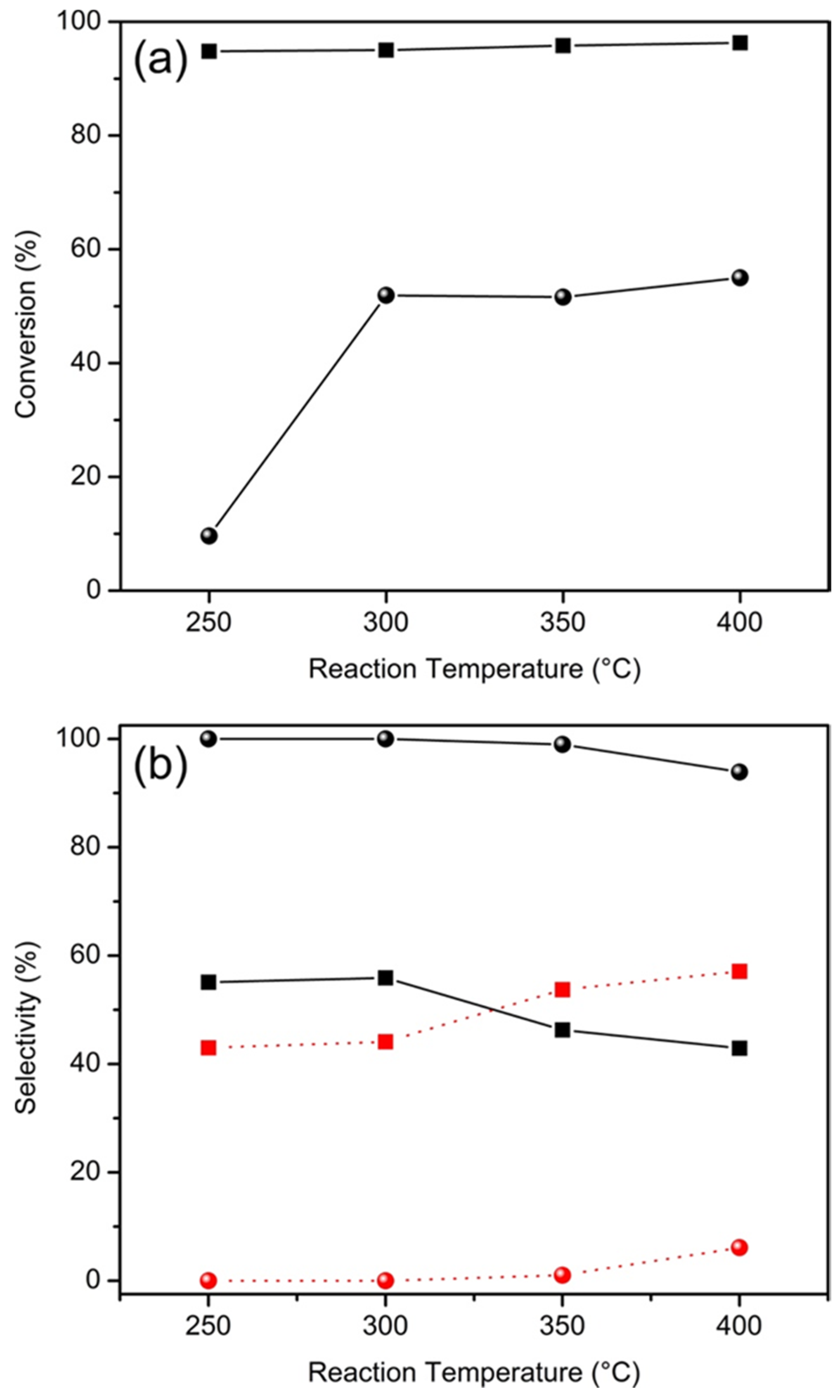
2.2. Catalytic Properties
3. Experimental Methods
3.1. Materials Synthesis
3.2. Materials Characterization
3.3. Measurements of Catalytic Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brookes, C.; Bowker, M.; Wells, P.P. Catalysts for the selective oxidation of methanol. Catalysts 2016, 6, 92. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, B.; Fang, T. A theoretical study on the complete dehydrogenation of methanol on Pd (100) surface. Appl. Surf. Sci. 2016, 364, 613–619. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Y. Recent advances in catalytic conversion of ethanol to chemicals. ACS Catal. 2014, 4, 1078–1090. [Google Scholar] [CrossRef]
- Ruf, S.; May, A.; Emig, G. Anhydrous formaldehyde by sodium catalysis. Appl. Catal. A 2001, 213, 203–215. [Google Scholar] [CrossRef]
- Matsumura, Y.; Hashimoto, K.; Yoshida, S. Active sites for methanol dehydrogenation to formaldehyde on sodium-modified silicalite-1. J. Catal. 1991, 131, 226–233. [Google Scholar] [CrossRef]
- Usachev, N.Y.; Krukovskii, I.M.; Kanaev, S.A. The nonoxidative methanol dehydrogenation to formaldehyde (A review). Pet. Chem. 2004, 44, 379–394. [Google Scholar]
- El-Molla, S.A.; Mahmoud, H.R. Synthesis, textural and catalytic properties of nanosized Fe2O3/MgO system. Mater. Res. Bull. 2013, 48, 4105–4111. [Google Scholar] [CrossRef]
- Takagi, K.; Morikawa, Y.; Ikawa, T. Catalytic activities of coppers in the various oxidation states for the dehydrogenation of methanol. Chem. Lett. 1985, 14, 527–530. [Google Scholar] [CrossRef]
- Muŝiĉ, A.; Batista, J.; Levec, J. Gas-phase catalytic dehydrogenation of methanol to formaldehyde over ZnO/SiO2 based catalysts, zeolites, and phosphates. Appl. Catal. A 1997, 165, 115–131. [Google Scholar]
- Ilolov, A.M.; Tret’yakov, V.F.; Talyshinskii, R.M.; Lermontov, A.S. Thermodynamic analysis of the feasibility of coupled nonoxidative dehydrogenation of methanol into formaldehyde. Pet. Chem. 2009, 49, 127–132. [Google Scholar] [CrossRef]
- Yamamoto, T.; Shimoda, A.; Okuhara, T.; Misono, M.A. A promoting effect of phosphorus-addition to Cu/SiO2 on selective synthesis of formaldehyde by dehydrogenation of methanol. Chem. Lett. 1988, 17, 273–276. [Google Scholar] [CrossRef]
- Wang, Q.N.; Shi, L.; Lu, A.H. Highly selective copper catalyst supported on mesoporous carbon for the dehydrogenation of ethanol to acetaldehyde. ChemCatChem 2015, 7, 2846–2852. [Google Scholar] [CrossRef]
- Takei, T.; Iguchi, N.; Haruta, M. Support effect in the gas phase oxidation of ethanol over nanoparticulate gold catalysts. New J. Chem. 2011, 35, 2227–2233. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, X.; Yang, S.; Li, T.; Hensen, E.J.M. On the metal–support synergy for selective gas-phase ethanol oxidation over MgCuCr2O4 supported metal nanoparticle catalysts. J. Catal. 2015, 331, 138–146. [Google Scholar] [CrossRef]
- Cao, Y.; Dai, W.-L.; Deng, J.-F. The synthesis, characterization and application of Ag–SiO2–Al2O3 sol–gel composites. Mat. Lett. 2001, 50, 12–17. [Google Scholar] [CrossRef]
- Ren, L.-P.; Dai, W.-L.; Cao, Y.; Li, H.; Fan, K. First observation of highly efficient dehydrogenation of methanol to anhydrous formaldehyde over novel Ag–SiO2–MgO–Al2O3 catalyst. Chem. Commun. 2003, 24, 3030–3031. [Google Scholar] [CrossRef]
- Dong, Y.; Dai, W.-L.; Li, J.-L.; Deng, J.-F. Direct dehydrogenation of methanol to formaldehyde over novel Ag-containing ceramics. Chem. Lett. 2001, 30, 534–535. [Google Scholar] [CrossRef]
- Li, H.-J.; Lausche, A.C.; Peterson, A.A.; Hansen, H.A.; Studt, F.; Bligaard, T. Using microkinetic analysis to search for novel anhydrous formaldehyde production catalysts. Surf. Sci. 2015, 641, 105–111. [Google Scholar] [CrossRef]
- Wiesgickl, G.; Beck, H.P.; Emig, G. Novel type of catalyst for the pure dehydrogenation of methanol to formaldehyde. Appl. Catal. 1990, 59, L1–L7. [Google Scholar] [CrossRef]
- Su, S.; Prairie, M.R.; Renken, A. Reaction mechanism of methanol dehydrogenation on a sodium carbonate catalyst. Appl. Catal. A 1992, 91, 131–142. [Google Scholar] [CrossRef]
- Ren, L.-P.; Dai, W.-L.; Yang, X.-L.; Cao, Y.; Li, H.; Fan, K.-N. Novel highly active Ag–SiO2–Al2O3–ZnO catalyst for the production of anhydrous HCHO from direct dehydrogenation of CH3OH. Appl. Catal. A 2004, 273, 83–88. [Google Scholar] [CrossRef]
- Asabina, E.A.; Orekhova, N.V.; Ermilova, M.M.; Pet’kov, V.I.; Glukhova, I.O.; Zhilyaeva, N.A.; Yaroslavtsev, A.B. Synthesis and catalytic properties of MFeTi(PO) (M = Co, Ni, Cu; 0 ≤ x ≤ 2) for methanol conversion reactions. Inorg. Mater. 2015, 51, 793–798. [Google Scholar] [CrossRef]
- Mieritz, D.; Davidowski, S.K.; Seo, D.-K. Accessing alkali-free NASICON-type compounds through mixed oxoanion sol–gel chemistry: Hydrogen titanium phosphate sulfate, H1−xTi2(PO4)3−x(SO4)x (x = 0.5–1). J. Solid State Chem. 2016, 242, 116–125. [Google Scholar]
- Alothman, Z. A review: Fundamental aspects of silicate mesoporous materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Zhao, H.; Jiang, P.; Dong, Y.; Huang, M.; Liu, B. A high-surface-area mesoporous sulfated nano-titania solid superacid catalyst with exposed (101) facets for esterification: facile preparation and catalytic performance. New J. Chem. 2014, 38, 4541–4548. [Google Scholar] [CrossRef]
- Cruz, P.; Pérez, Y.; del Hierro, I. Titanium alkoxides immobilized on magnetic mesoporous silica nanoparticles and their characterization by solid state voltammetry techniques: Application in ring opening polymerization. Micro. Meso. Mater. 2017, 240, 227–235. [Google Scholar] [CrossRef]
- Kimbombo, H.; Peng, R.; Rasalingam, S.; Koodali, R. Versatility of heterogeneous photocatalysis: synthetic methodologies epitomizing the role of silica support in TiO2 based mixed oxides. Catal. Sci. Technol. 2012, 2, 1737–1766. [Google Scholar] [CrossRef]
- Zhao, R.; Mieritz, D.; Seo, D.-K.; Chan, C.K. New hydrogen titanium phosphate sulfate electrodes for Li-ion and Na-ion batteries. J. Power Sources 2017, 343, 197–206. [Google Scholar] [CrossRef]
- Ilin, A.B.; Orekhova, N.V.; Ermilova, M.M.; Yaroslavtsev, A.B. Catalytic activity of LiZr2(PO4)3 nasicon-type phosphates in ethanol conversion process in conventional and membrane reactors. Catal. Today 2016, 268, 29–36. [Google Scholar] [CrossRef]
- Florek-Milewska, J.; Decyk, P.; Ziolek, M. Catalytic properties of Cu/SBA-3 in oxidative dehydrogenation of methanol—The effect of the support composition. Appl. Catal. A 2011, 393, 215–224. [Google Scholar] [CrossRef]
- Hattori, H.; Ono, Y. Solid Acid Catalysis: From Fundamentals to Applications; Pan Stanford Publishing: Singapore, Singapore, 2015. [Google Scholar]
- Il’in, A.B.; Ermilova, M.M.; Orekhova, N.V.; Yaroslavtsev, A.B. Synthesis of framework lithium zirconium molybdate phosphates and their catalytic properties in ethanol conversion reactions. Inorg. Mater. 2015, 51, 711–717. [Google Scholar] [CrossRef]
- Halsey, G. Physical adsorption on non-uniform surfaces. J. Chem. Phys. 1948, 16, 931–938. [Google Scholar] [CrossRef]
- Liu, D.; Yuan, P.; Liu, H.; Cai, J.; Tan, D.; He, H.; Zhu, J.; Chen, T. Quantitative characterization of the solid acidity of montmorillonite using combined FTIR and TPD based on the NH3 adsorption system. Appl. Clay Sci. 2013, 80–81, 407–412. [Google Scholar] [CrossRef]





| Sample | a (Å) | c (Å) | Crystallite Size (nm) | SBET (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) | Surface Acid Site (µmol/g) | |
|---|---|---|---|---|---|---|---|---|
| Medium | Total | |||||||
| HTPS-600 | 8.472 | 21.66 | 16 | 56 | 0.22 | 16 | 21 | 37.6 |
| HTPS-650 | 8.482 | 21.64 | 15 | 50 | 0.25 | 20 | 25 | 32.5 |
| HTPS-700 | 8.484 | 21.76 | 17 | 32 | 0.24 | 29 | - | 4.8 |
| Catalyst | Temperature (°C) | Methanol Conversion (%) | H2CO Selectivity (%) | TON 1 (10−4) | Reference |
|---|---|---|---|---|---|
| HTPS-600 | 400 | 53 | 98 | 1.5 | This work |
| HTPS-650 | 400 | 52 | 99 | 1.2 | This work |
| HTPS-700 | 400 | 49 | 99 | 0.6 | This work |
| Cu(0)-TSM 2 | 400 | 6 | 100 | - | [8] |
| Cu/SiO2 | 500 | 30 | 82 | - | [11] |
| Ag/SiO2-Al2O3 | 650 | 70 | 97.5 | - | [16] |
| Ce-ZnO/SiO2 | 600 | 40 | 32 | - | [9] |
| Na-ZnO/SiO2 | 600 | 38 | 30 | - | [9] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitran, G.; Mieritz, D.G.; Seo, D.-K. Highly Selective Solid Acid Catalyst H1−xTi2(PO4)3−x(SO4)x for Non-Oxidative Dehydrogenation of Methanol and Ethanol. Catalysts 2017, 7, 95. https://doi.org/10.3390/catal7030095
Mitran G, Mieritz DG, Seo D-K. Highly Selective Solid Acid Catalyst H1−xTi2(PO4)3−x(SO4)x for Non-Oxidative Dehydrogenation of Methanol and Ethanol. Catalysts. 2017; 7(3):95. https://doi.org/10.3390/catal7030095
Chicago/Turabian StyleMitran, Gheorghiţa, Daniel G. Mieritz, and Dong-Kyun Seo. 2017. "Highly Selective Solid Acid Catalyst H1−xTi2(PO4)3−x(SO4)x for Non-Oxidative Dehydrogenation of Methanol and Ethanol" Catalysts 7, no. 3: 95. https://doi.org/10.3390/catal7030095