Efficient Degradation of Refractory Organics Using Sulfate Radicals Generated Directly from WO3 Photoelectrode and the Catalytic Reaction of Sulfate
Abstract
:1. Introduction
2. Results and Discussion
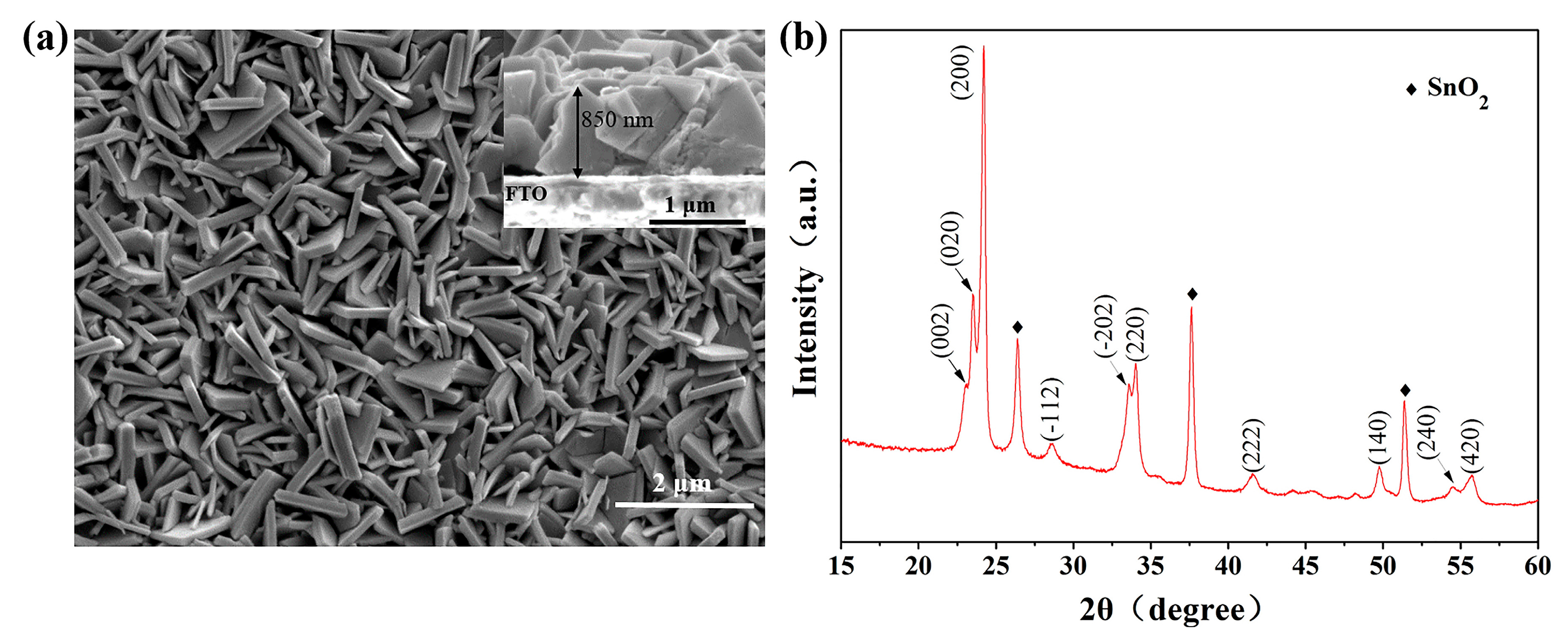
2.1. Characterization of WO3 Photoanodes
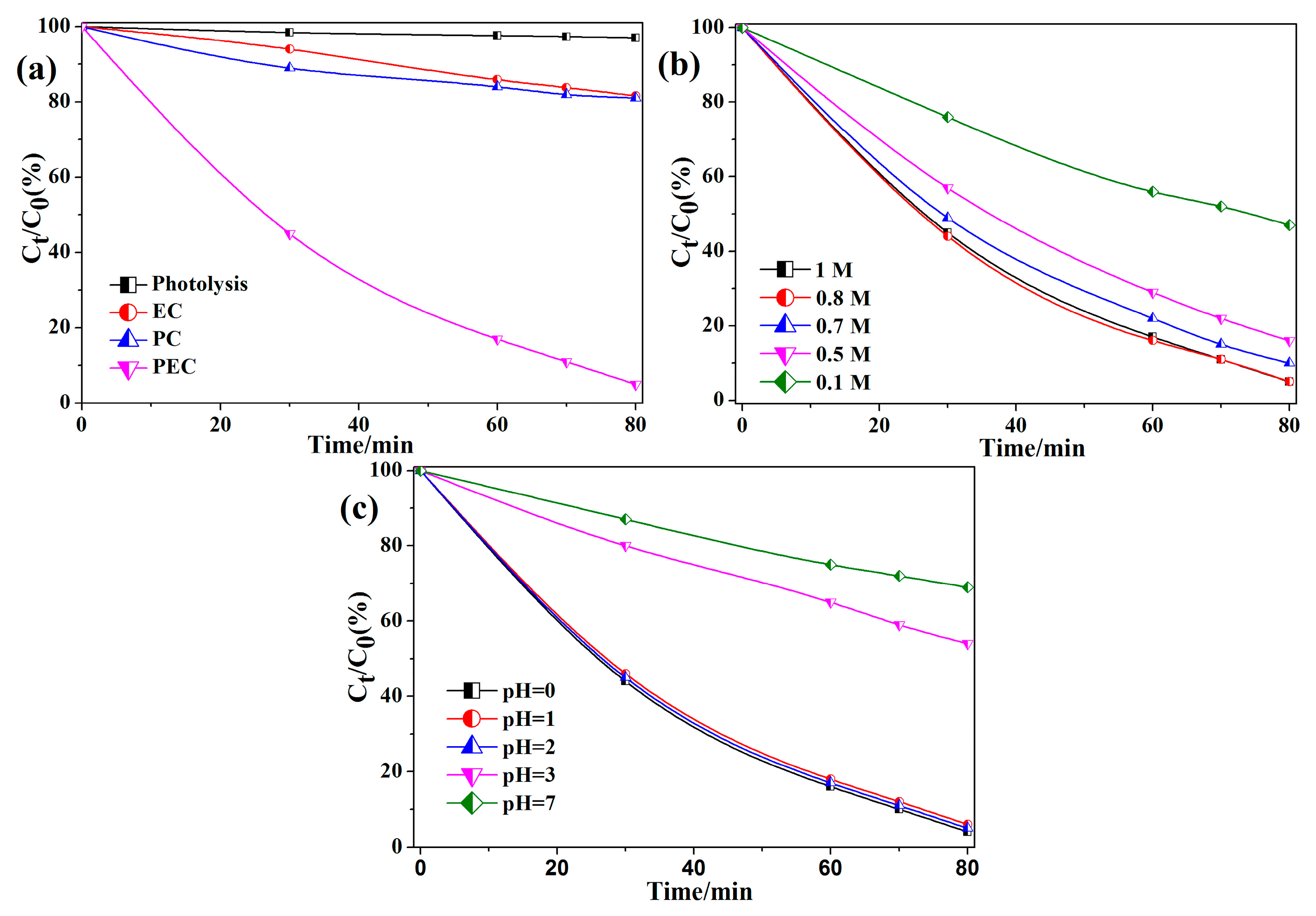
2.2. PEC Degradation of Methyl Orange in Sulfate Medium
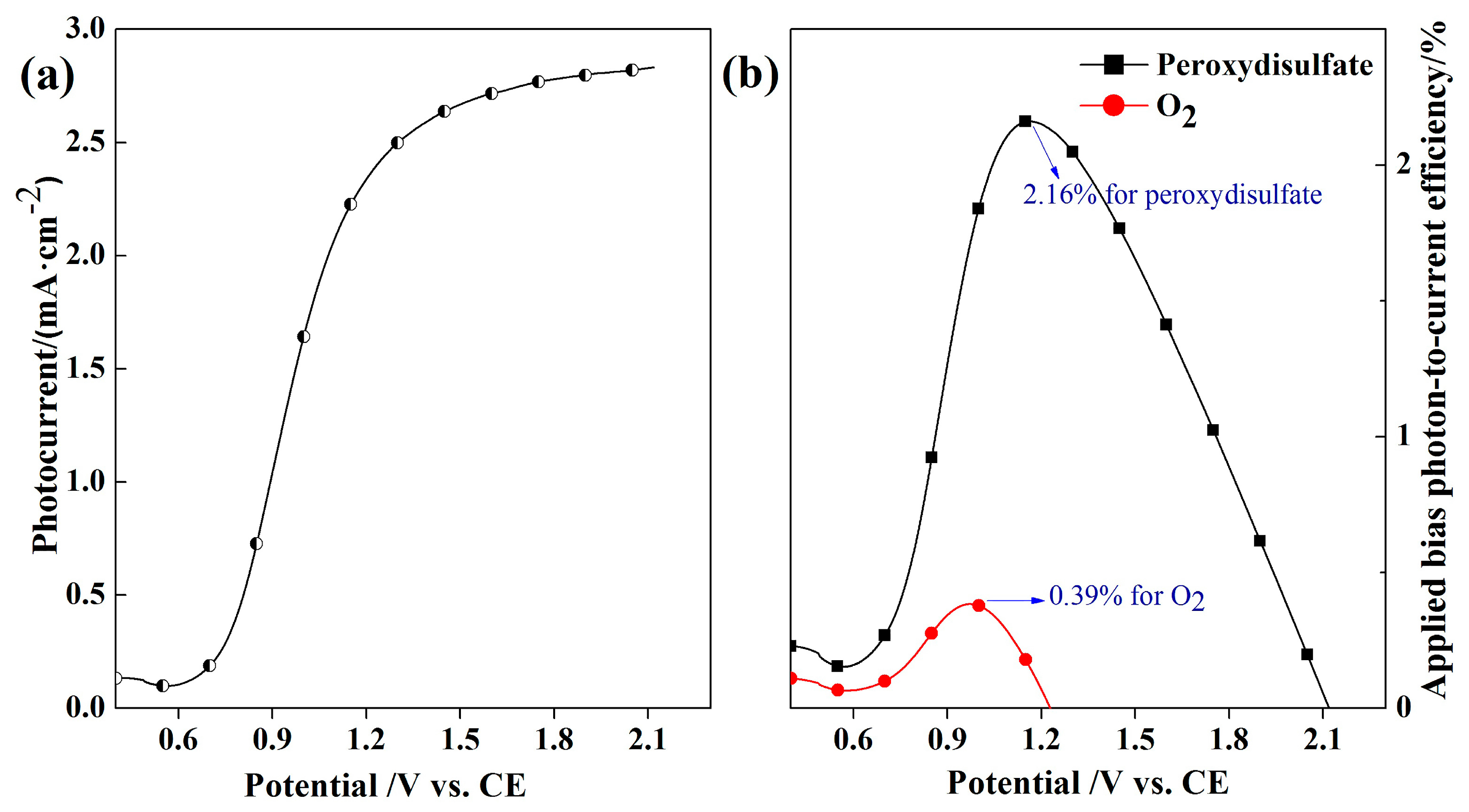
2.3. Identification of Main Active Radical in the PEC System
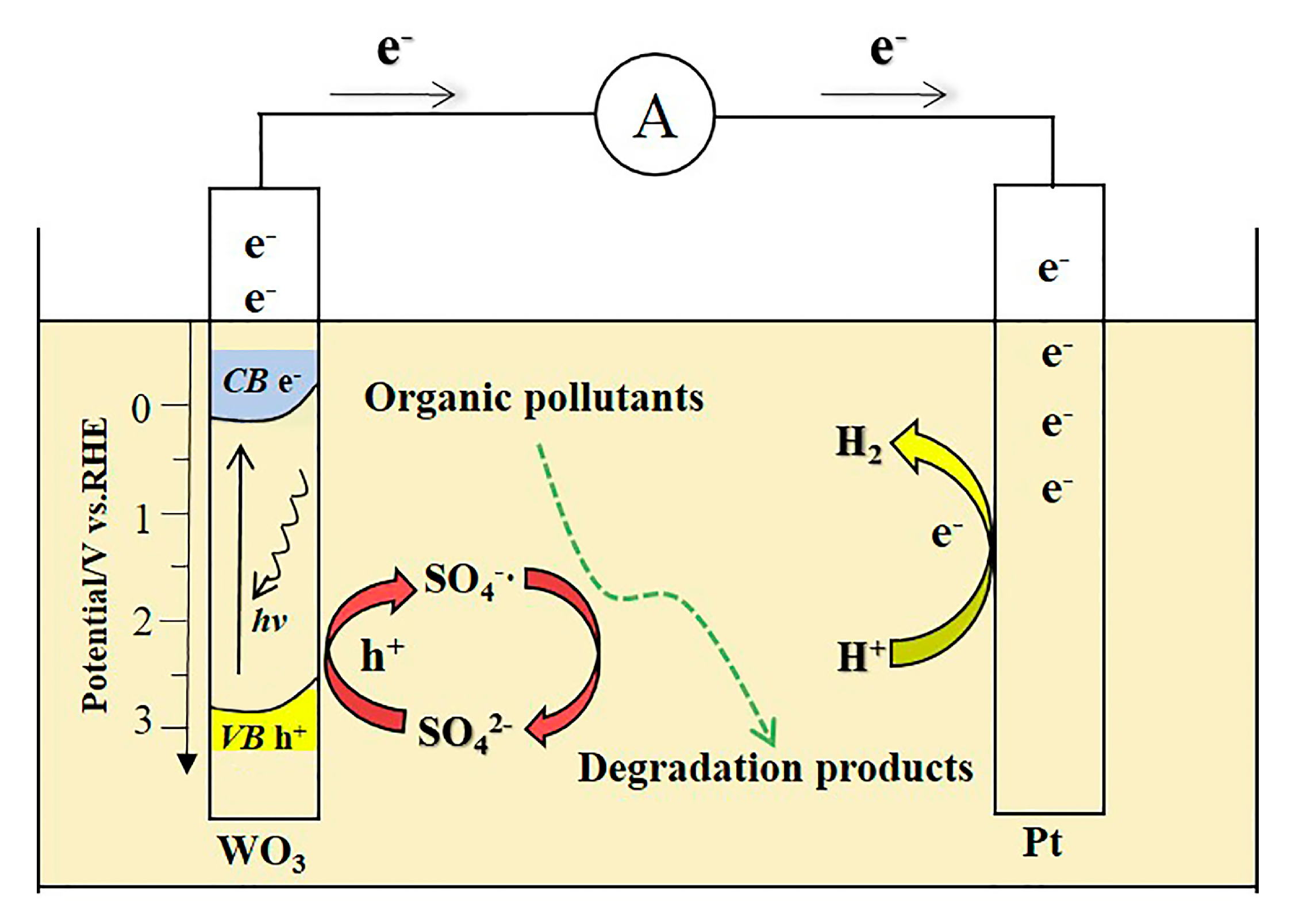
2.4. The PEC Degradation Mechanism of MO
2.5. Recycling Performance
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of WO3 Photoanodes
3.3. Apparatus and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fuku, K.; Wang, N.; Miseki, Y.; Funaki, T.; Sayama, K. Photoelectrochemical reaction for the efficient production of hydrogen and high-value-added oxidation reagents. ChemSusChem 2015, 8, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, Z.; Fang, C.; Liu, W.; Lou, X.; Liu, J. Importance of reagent addition order in contaminant degradation in an Fe(II)/PMS system. RSC Adv. 2016, 6, 70271–72026. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical generation by the interaction of transition metals with common oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef] [PubMed]
- Mi, Z.; Chen, X.; He, Z.; Murugananthan, M.; Zhang, Y. Degradation of p-nitrophenol by heat and metal ions co-activated persulfate. Chem. Eng. J. 2015, 264, 39–47. [Google Scholar]
- Wang, C.W.; Liang, C. Oxidative degradation of TMAH solution with UV persulfate activation. Chem. Eng. J. 2014, 254, 472–478. [Google Scholar] [CrossRef]
- Huang, K.C.; Zhao, Z.; Hoag, G.E.; Dahmani, A.; Block, P.A. Degradation of volatile organic compounds with thermally activated persulfate oxidation. Chemosphere 2005, 61, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Waldemer, R.H.; Tratnyek, P.G.; Johnson, R.L.; Nurmi, J.T. Oxidation of chlorinated ethenes by heat-activated persulfate: Kinetics and products. Environ. Sci. Technol. 2007, 41, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Du, X.Z.; Huang, W.L. Temperature effect on the kinetics of persulfate oxidation of p-chloroaniline. Chin. Chem. Lett. 2011, 22, 358–361. [Google Scholar] [CrossRef]
- Criquet, J.; Leitner, N.K.V. Degradation of acetic acid with sulfate radical generated by persulfate ions photolysis. Chemosphere 2009, 77, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Saien, J.; Ojaghloo, Z. Homogeneous and heterogeneous AOPs for rapid degradation of Triton X-100 in aqueous media via UV light, nano titania hydrogen peroxide and potassium persulfate. Chem. Eng. J. 2011, 167, 172–182. [Google Scholar] [CrossRef]
- Do, S.H.; Kwon, Y.J.; Kong, S.H. Effect of metal oxides on the reactivity of persulfate/Fe(II) in the remediation of diesel-contaminated soil and sand. J. Hazard. Mater. 2010, 182, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ye, Q.; Tang, T.; Wang, D. Hg0 oxidative absorption by K2S2O8 solution catalyzed by Ag+ and Cu2+. J. Hazard. Mater. 2008, 158, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.R.; Li, X.Z. Degradation of azo dye Orange G in aqueous solutions by persulfate with ferrous ion. Sep. Purif. Technol. 2010, 72, 105–111. [Google Scholar] [CrossRef]
- Yuchi, L.; Shanglien, L.; Peite, C.; Chang, D.G. Efficient decomposition of perfluorocarboxylic acids in aqueous solution using microwave-induced persulfate. Water Res. 2009, 43, 2811–2816. [Google Scholar]
- Bai, J.; Zhou, B. Titanium Dioxide Nanomaterials for Sensor Applications. Chem. Rev. 2014, 114, 10131–10176. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wang, F.; Chen, Z.F.; Zhang, Q.; Su, Y.; Shen, L.; Yao, K.; Liu, Y.; Cai, Z.; Lv, W.; et al. Study on the photocatalytic mechanism and detoxicity of gemfibrozil by a sunlight-driven TiO2/carbon dots photocatalyst: The significant roles of reactive oxygen species. Appl. Catal. B 2017, 204, 250–259. [Google Scholar] [CrossRef]
- Fang, W.; Xing, M.; Zhang, J. Modifications on reduced titanium dioxide photocatalysts: A review. J. Photochem. Photobiol. C Photochem. Rev. 2017, 32, 21–39. [Google Scholar] [CrossRef]
- Antoniadou, M.; Lianos, P. Production of electricity by photoelectrochemical oxidation of ethanol in a PhotoFuelCell. Appl. Catal. B 2010, 99, 307–313. [Google Scholar] [CrossRef]
- Pop, L.C.; Sfaelou, S.; Lianos, P. Cation adsorption by mesoporous titania photoanodes and its effect on the current-voltage characteristics of photoelectrochemical cells. Electrochim. Acta 2015, 156, 223–227. [Google Scholar] [CrossRef]
- Song, X.N.; Wang, C.Y.; Wang, W.K.; Zhang, X.; Hou, N.N.; Yu, H.Q. A Dissolution-Regeneration Route to Synthesize Blue Tungsten Oxide Flowers and their Applications in Photocatalysis and Gas Sensing. Adv. Mater. Interfaces 2016, 3. [Google Scholar] [CrossRef]
- Altomare, M.; Pfoch, O.; Tighineanu, A.; Kirchgeorg, R.; Lee, K.; Selli, E.; Schmuki, P. Molten o-H3PO4: A New Electrolyte for the Anodic Synthesis of Self-Organized Oxide Structures: WO3 Nanochannel Layers and Others. J. Am. Chem. Soc. 2015, 137, 5646–5649. [Google Scholar] [CrossRef] [PubMed]
- Kalanur, S.S.; Yun, J.H.; Sang, Y.C.; Joo, O.S. Facile growth of aligned WO3 nanorods on FTO substrate for enhanced photoanodic water oxidation activity. J. Mater. Chem. A 2013, 1, 3479–3488. [Google Scholar] [CrossRef]
- Kim, Y.O.; Yu, S.H.; Ahn, K.S.; Lee, S.K.; Kang, S.H. Photoelectrochemical Behavior of Compact and Inverse Opal Tungsten Trioxide Films: Surface Area and Charge Transfer Properties. J. Electrochem. Soc. 2015, 162, H449–H452. [Google Scholar] [CrossRef]
- Li, W.; Da, P.; Zhang, Y.; Wang, Y.; Lin, X.; Gong, X.; Zheng, G. WO3 Nanoflakes for Enhanced Photoelectrochemical Conversion. ACS Nano 2014, 8, 11770–11777. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.M.; Cho, I.S.; Zheng, X. Flame synthesis of WO3 nanotubes and nanowires for efficient photoelectrochemical water-splitting. Proc. Combust. Inst. 2013, 34, 2187–2195. [Google Scholar] [CrossRef]
- Smith, Y.R.; Sarma, B.; Mohanty, S.K.; Misra, M. Formation of TiO2-WO3 nanotubular composite via single-step anodization and its application in photoelectrochemical hydrogen generation. Electrochem. Commun. 2012, 19, 131–134. [Google Scholar] [CrossRef]
- Thind, S.S.; Rozic, K.; Amano, F.; Chen, A. Fabrication and photoelectrochemical study of WO3-based bifunctional electrodes for environmental applications. Appl. Catal. B 2015, 176, 464–471. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Liu, H.; Wang, C.; Liu, S.; Sun, P.; Wang, L.; Liu, Y. Heterostructured TiO2/WO3 porous microspheres: Preparation, characterization and photocatalytic properties. Catal. Today 2013, 201, 195–202. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Song, G.; Hong, J.; Van, T.K.; Pawar, A.U.; Kim, D.Y.; Chang, W.K.; Haider, Z.; Kang, Y.S. Facile Fabrication of WO3 Nanoplates Thin Films with Dominant Crystal Facet of (002) for Water Splitting. Cryst. Growth Des. 2014, 14, 6057–6066. [Google Scholar] [CrossRef]
- Wang, G.; Ling, Y.; Wheeler, D.A.; George, K.E.; Horsley, K.; Heske, C.; Zhang, J.Z.; Li, Y. Facile synthesis of highly photoactive α-Fe2O3-based films for water oxidation. Nano Lett. 2011, 11, 3503–3509. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Bai, J.; Li, J.; Xia, L.; Huang, K.; Li, X.; Zhou, B. A novel in situ preparation method for nanostructured a-Fe2O3 films from electrodeposited Fe films for efficient photoelectrocatalytic water splitting and the degradation of organic pollutants. J. Mater. Chem. A 2015, 3, 4345–4353. [Google Scholar] [CrossRef]
- Yousefi, M.; Amiri, M.; Azimirad, R.; Moshfegh, A.Z. Enhanced photoelectrochemical activity of Ce doped ZnO nanocomposite thin films under visible light. J. Electroanal. Chem. 2011, 661, 106–112. [Google Scholar] [CrossRef]
- Zhao, Q.D.; Wang, D.J.; Peng, L.L.; Lin, Y.H.; Yang, M.; Xie, T.F. Surface photovoltage study of photogenerated charges in ZnO nanorods array grown on ITO. Chem. Phys. Lett. 2007, 434, 96–100. [Google Scholar] [CrossRef]
- Sayama, K.; Nomura, A.; Arai, T.; Sugita, T.; Abe, R.; Yanagida, M.; Oi, T.; Iwasaki, Y.; Abe, Y.; Sugihara, H. Photoelectrochemical decomposition of water into H2 and O2 on porous BiVO4 thin-film electrodes under visible light and significant effect of Ag ion treatment. J. Phys. Chem. B 2006, 110, 11352–11360. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Kudo, A. Effects of Structural Variation on the Photocatalytic Performance of Hydrothermally Synthesized BiVO4. Adv. Funct. Mater. 2006, 16, 2163–2169. [Google Scholar] [CrossRef]
- Zhang, X.; Du, L.; Wang, H.; Dong, X.; Zhang, X.; Ma, C.; Ma, H. Highly ordered mesoporous BiVO4: Controllable ordering degree and super photocatalytic ability under visible light. Microporous Mesoporous Mater. 2013, 173, 175–180. [Google Scholar] [CrossRef]
- Zheng, H.; Ou, J.Z.; Strano, M.S.; Kaner, R.B.; Mitchell, A.; Kalantar-Zadeh, K. Nanostructured Tungsten Oxide—Properties, Synthesis, and Applications. Adv. Funct. Mater. 2011, 21, 2175–2196. [Google Scholar] [CrossRef]
- Hill, J.C.; Choi, K.S. Effect of Electrolytes on the Selectivity and Stability of n-type WO3 Photoelectrodes for Use in Solar Water Oxidation. J. Phys. Chem. C 2012, 116, 7612–7620. [Google Scholar] [CrossRef]
- Ghigliazza, R.; Lodi, A.; Rovatti, M. Kinetic and process considerations on biological reduction of soluble and scarcely soluble sulfates. Resour. Conserv. Recycl. 2000, 29, 181–194. [Google Scholar] [CrossRef]
- Benatti, C.T.; Tavares, C.R.; Lenzi, E. Sulfate removal from waste chemicals by precipitation. J. Environ. Manag. 2009, 90, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Li, J.; Bai, J.; Li, X.; Xia, L.; Zhou, B. Preparation of vertically aligned WO3 nanoplate array films based on peroxotungstate reduction reaction and their excellent photoelectrocatalytic performance. Appl. Catal. B 2017, 202, 388–396. [Google Scholar] [CrossRef]
- Mi, Q.; Coridan, R.H.; Brunschwig, B.S.; Gray, H.B.; Lewis, N.S. Photoelectrochemical oxidation of anions by WO3 in aqueous and nonaqueous electrolytes. Energy Environ. Sci. 2013, 6, 2646–2653. [Google Scholar] [CrossRef]
- Mi, Q.; Zhanaidarova, A.; Brunschwig, B.S.; Gray, H.B.; Lewis, N.S. A quantitative assessment of the competition between water and anion oxidation at WO3 photoanodes in acidic aqueous electrolytes. Energy Environ. Sci. 2012, 5, 5694–5700. [Google Scholar] [CrossRef]
- Kelsall, G.H.; Thompson, I. Redox Chemistry of H2S Oxidation in the British-Gas Stretford Process 1: Thermodynamics of Sulfur-Water Systems at 298 K. J. Appl. Electrochem. 1993, 23, 279–286. [Google Scholar] [CrossRef]
- Murray, R.C.; Cubicciotti, D. Thermodynamics of Aqueous Sulfur Species to 300 °C and Potential-pH Diagrams. J. Electrochem. Soc. 1983, 130, 866–869. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Degradation of organic contaminants in water with sulfate radicals generated by the conjunction of peroxymonosulfate with cobalt. Environ. Sci. Technol. 2003, 37, 4790–4797. [Google Scholar] [CrossRef] [PubMed]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (⋅OH/⋅O-) in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Solarska, R.; Santato, C.; Jorand-Sartoretti, C.; Ulmann, M.; Augustynski, J. Photoelectrolytic oxidation of organic species at mesoporous tungsten trioxide film electrodes under visible light illumination. J. Appl. Electrochem. 2005, 35, 715–721. [Google Scholar] [CrossRef]
- Nakajima, T.; Hagino, A.; Nakamura, T.; Tsuchiya, T.; Sayama, K. WO3 nanosponge photoanodes with high applied bias photon-to-current efficiency for solar hydrogen and peroxydisulfate production. J. Mater. Chem. A 2016, 4, 17809–17818. [Google Scholar] [CrossRef]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.; Li, J.; Bai, J.; Tan, X.; Zeng, Q.; Li, L.; Zhou, B. Efficient Degradation of Refractory Organics Using Sulfate Radicals Generated Directly from WO3 Photoelectrode and the Catalytic Reaction of Sulfate. Catalysts 2017, 7, 346. https://doi.org/10.3390/catal7110346
Zheng J, Li J, Bai J, Tan X, Zeng Q, Li L, Zhou B. Efficient Degradation of Refractory Organics Using Sulfate Radicals Generated Directly from WO3 Photoelectrode and the Catalytic Reaction of Sulfate. Catalysts. 2017; 7(11):346. https://doi.org/10.3390/catal7110346
Chicago/Turabian StyleZheng, Jingyuan, Jinhua Li, Jing Bai, Xiaohan Tan, Qingyi Zeng, Linsen Li, and Baoxue Zhou. 2017. "Efficient Degradation of Refractory Organics Using Sulfate Radicals Generated Directly from WO3 Photoelectrode and the Catalytic Reaction of Sulfate" Catalysts 7, no. 11: 346. https://doi.org/10.3390/catal7110346




