First-Row-Transition Ion Metals(II)-EDTA Functionalized Magnetic Nanoparticles as Catalysts for Solvent-Free Microwave-Induced Oxidation of Alcohols
Abstract
:1. Introduction
2. Results
2.1. Catalysts Characterization
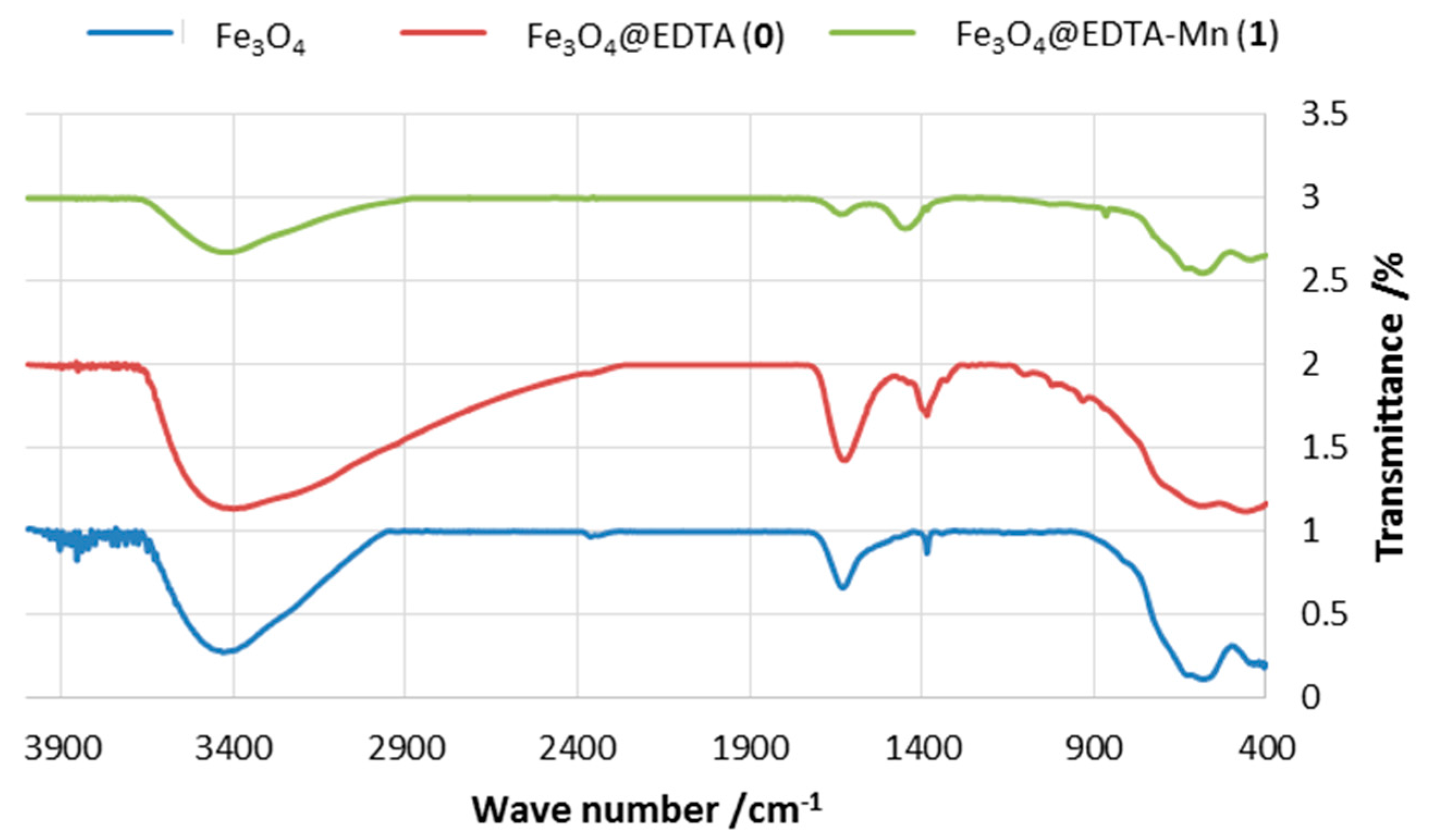
2.1.1. FT-IR Spectra
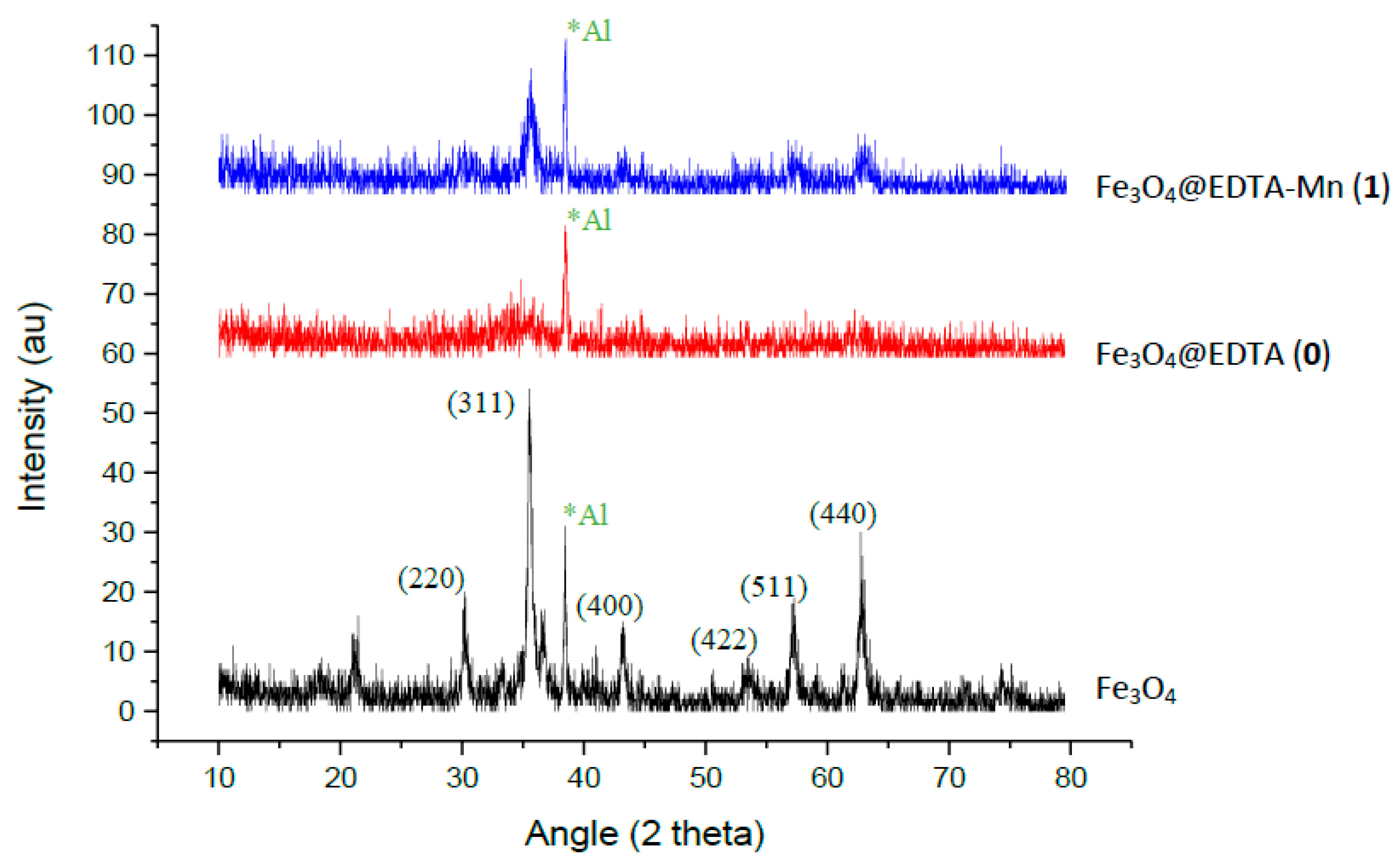
2.1.2. XRD
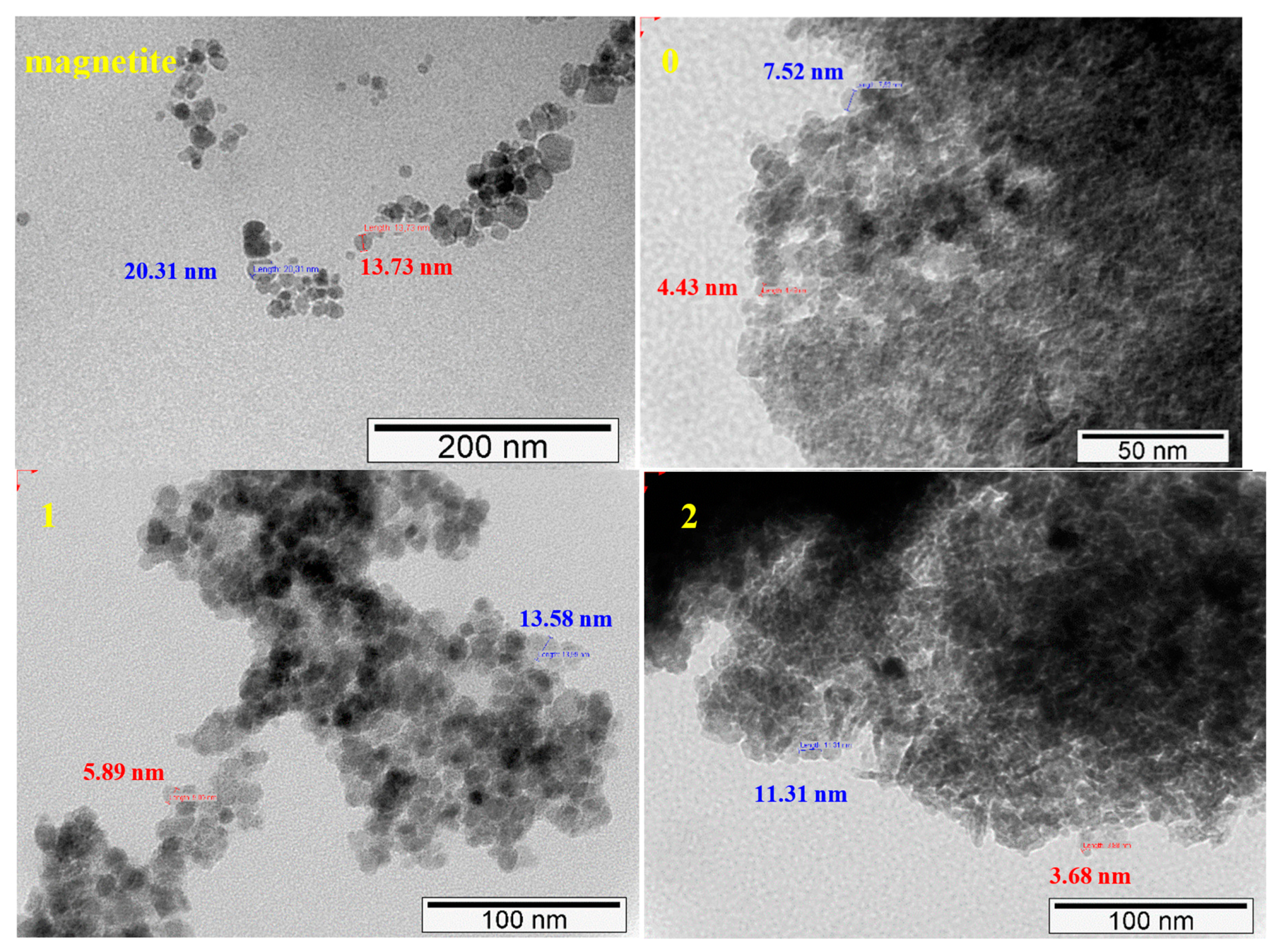
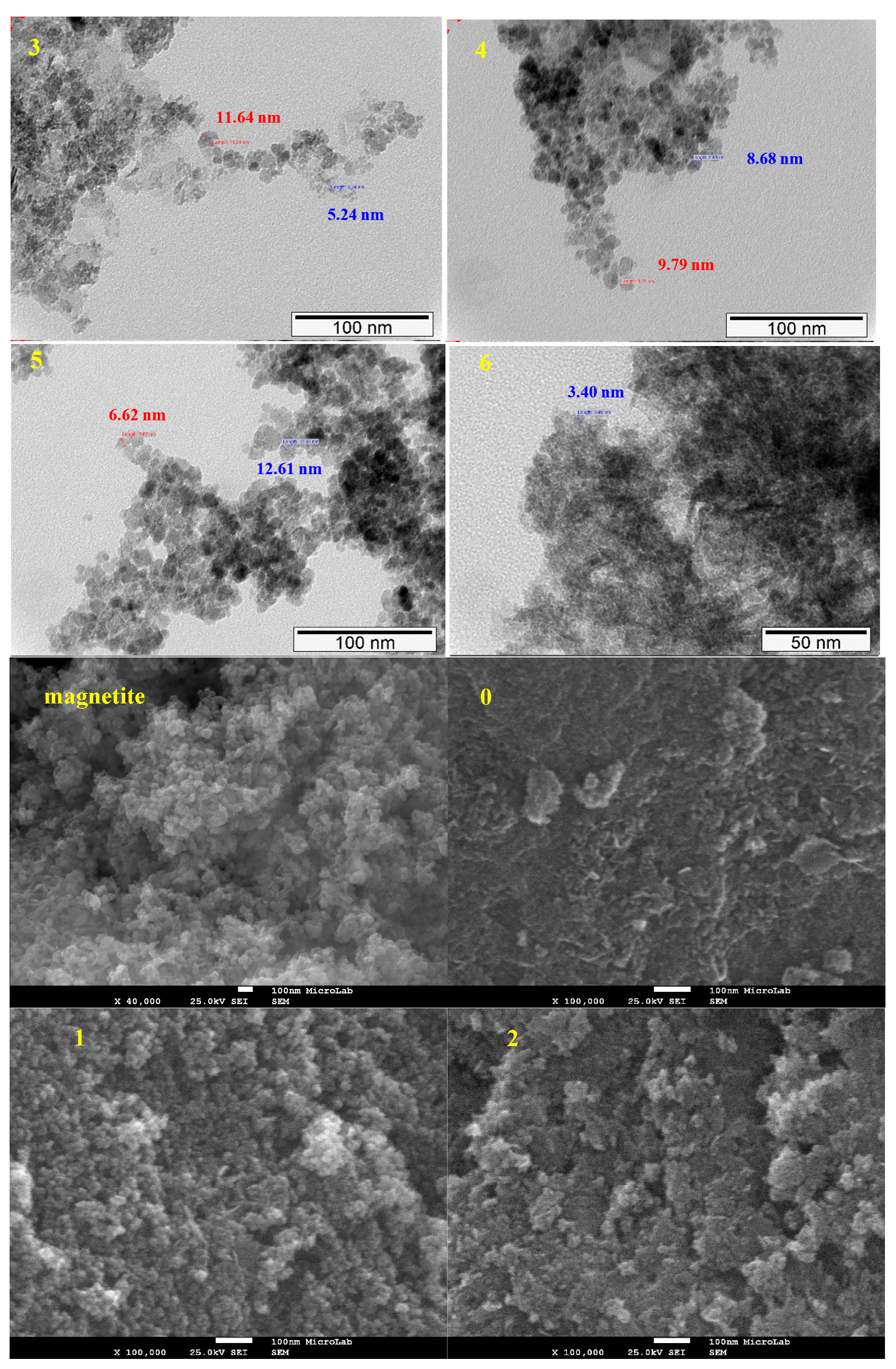
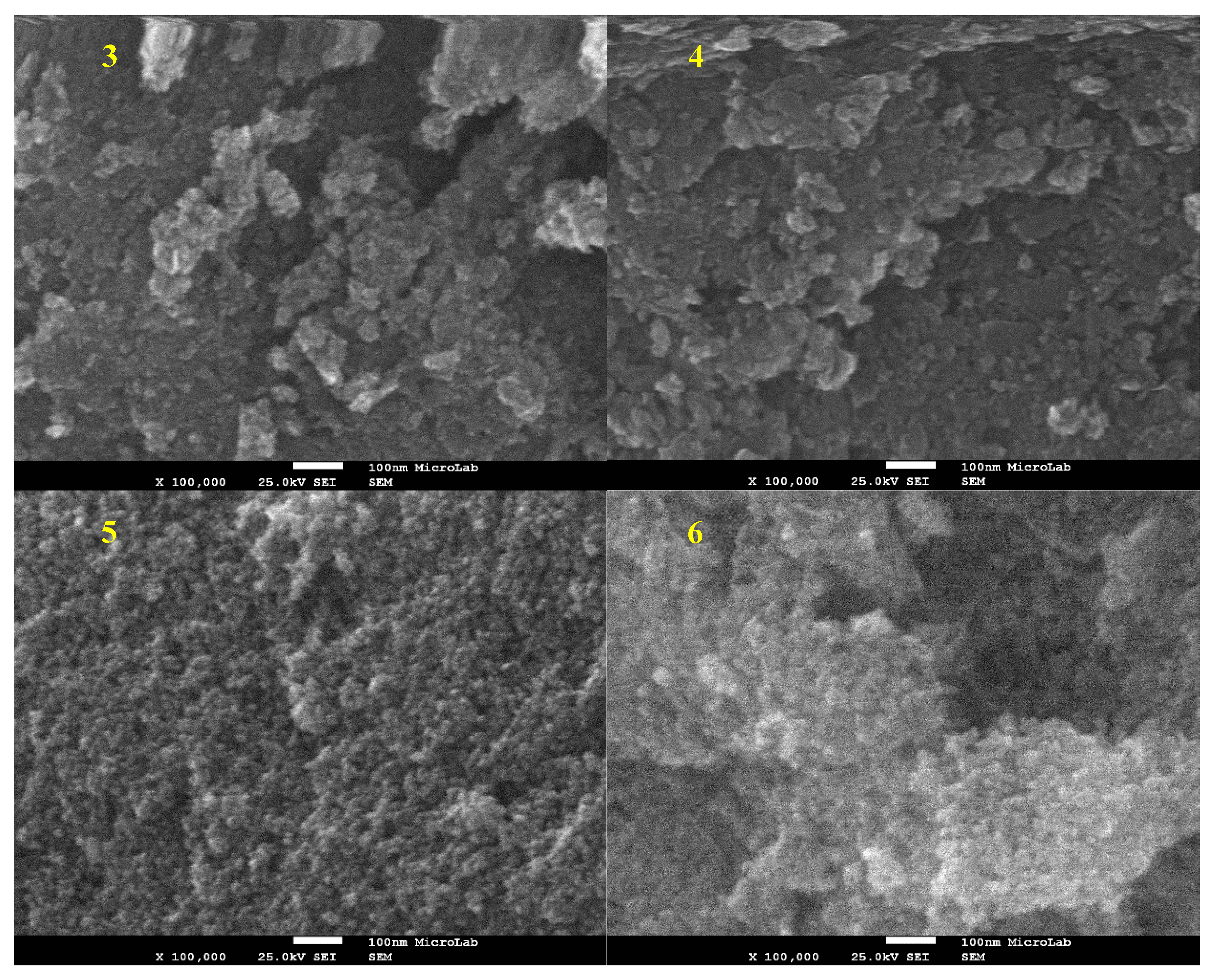
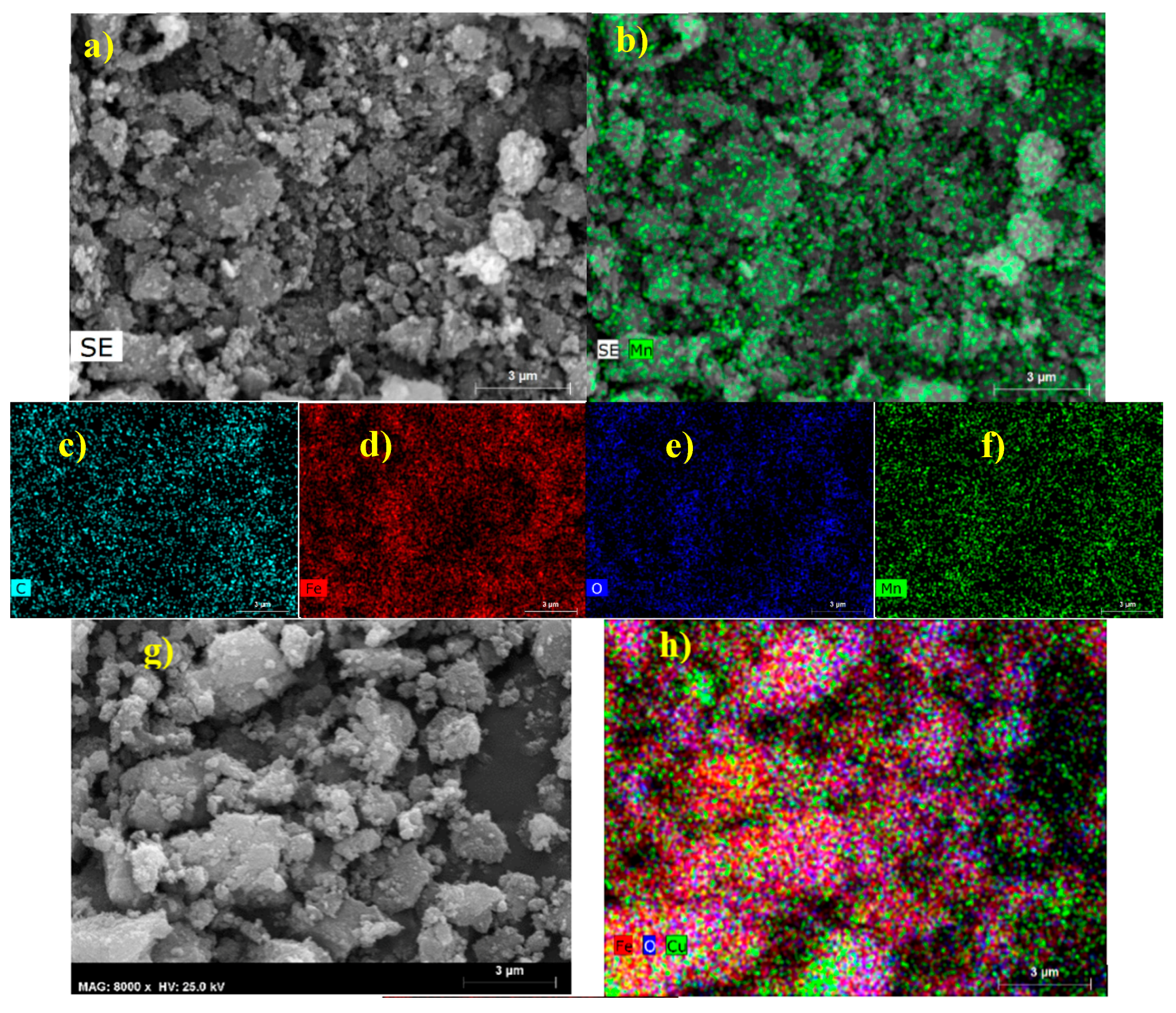
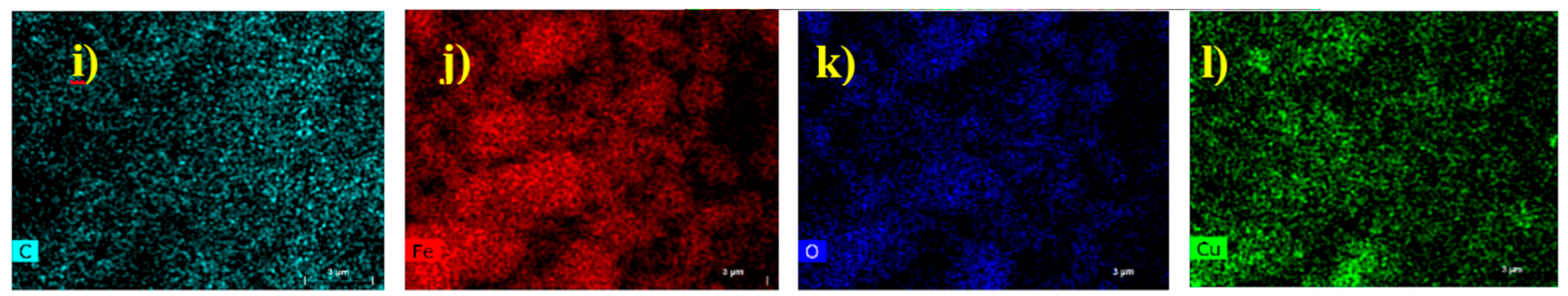
2.1.3. TEM and SEM-EDS (Scanning Electron Microscopy-Energy Dispersive X-ray Spectroscopy
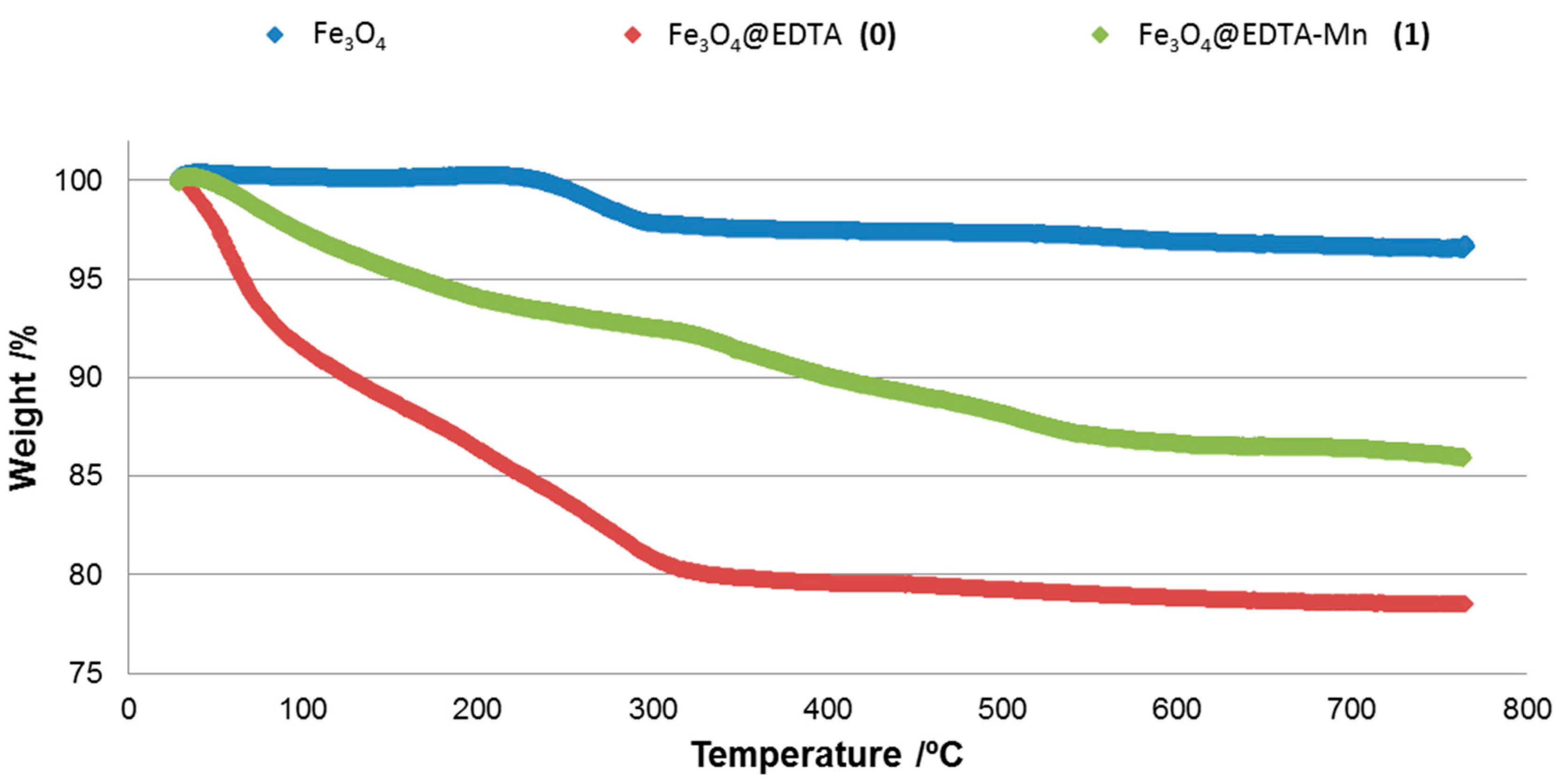
2.1.4. TGA (Thermal Gravimetric Analysis)
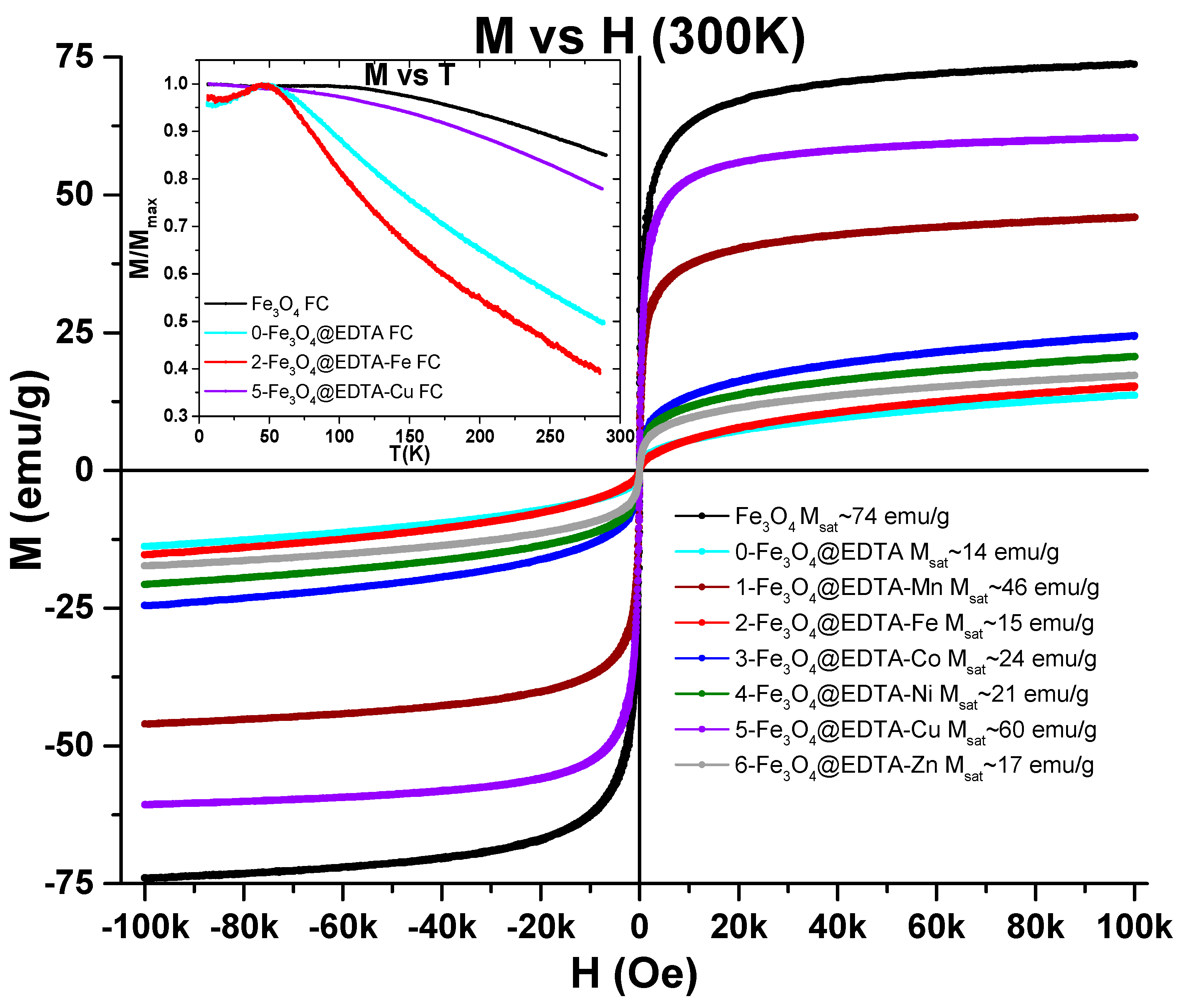
2.1.5. VSM (Vibrating Sample Magnetometry)
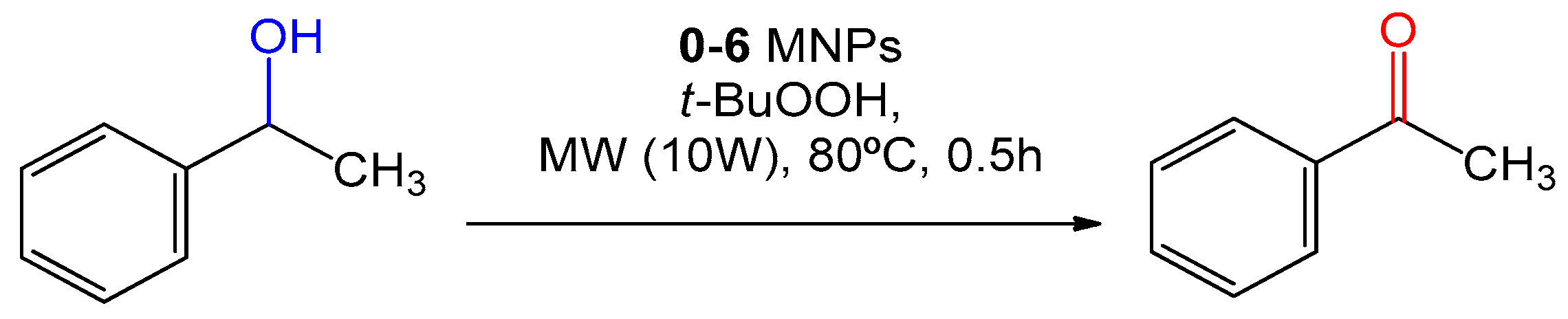
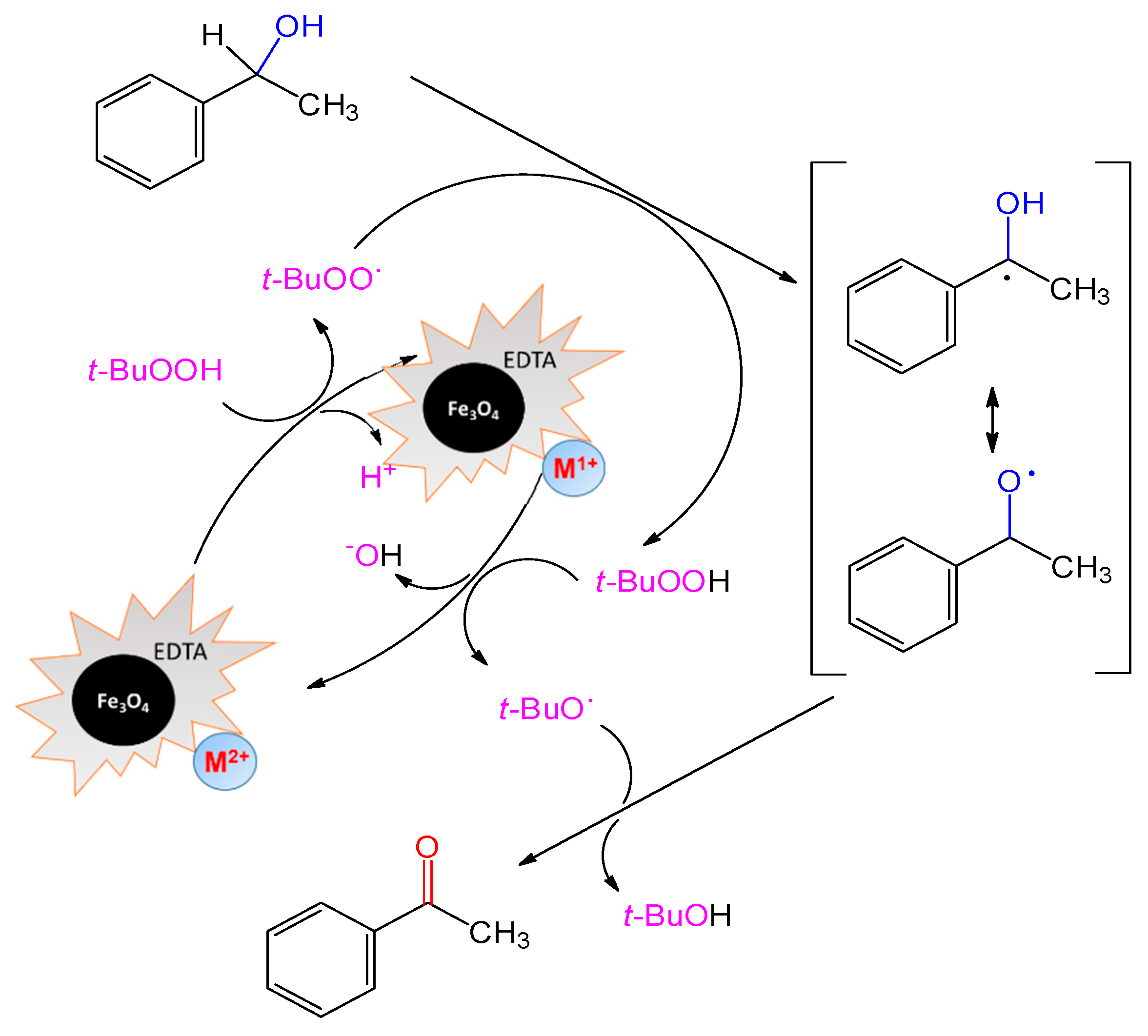
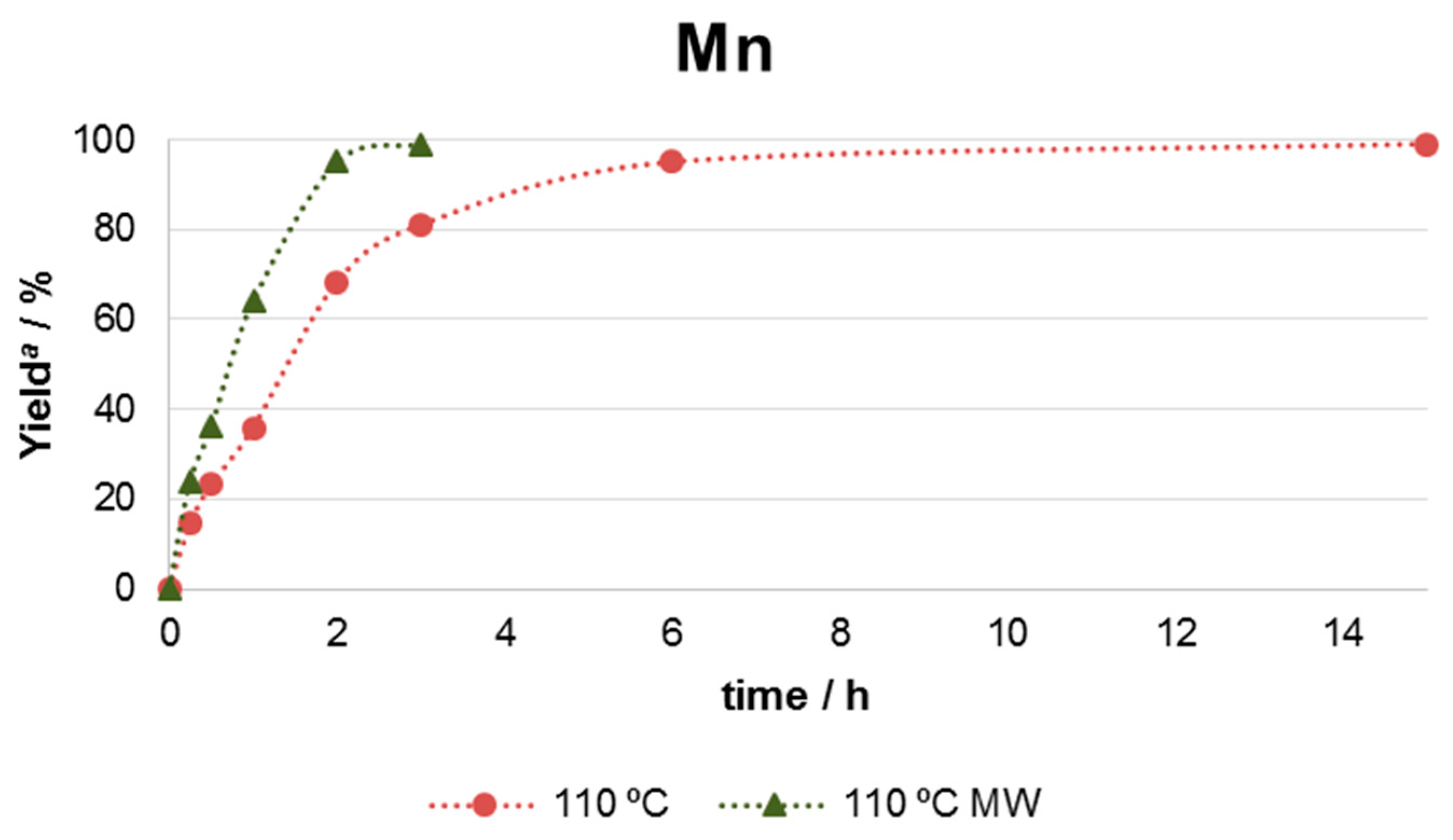
2.2. Catalytic Performance
3. Experimental Section
3.1. Materials and Instrumentation
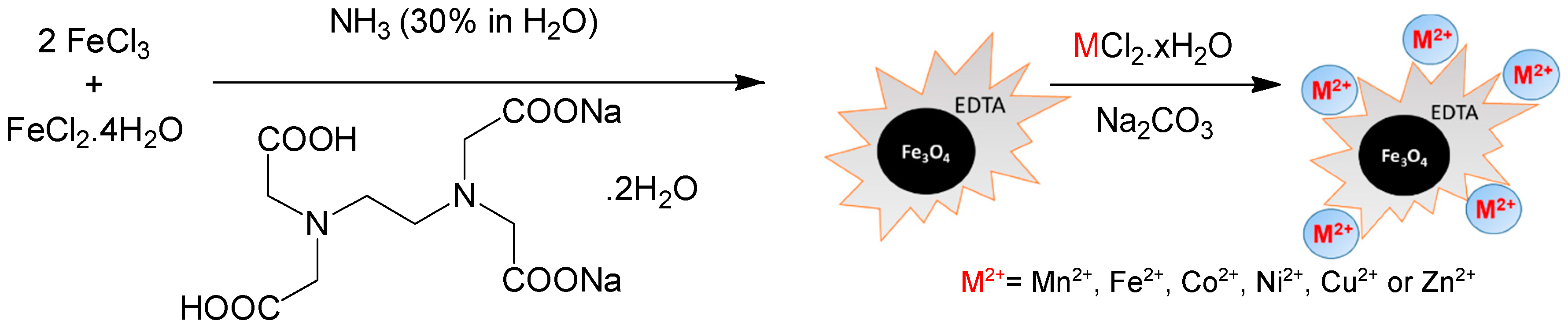
3.2. Catalyst Preparation
3.2.1. Synthesis of Fe3O4@EDTA
3.2.2. Functionalization of Fe3O4@EDTA
3.3. General Procedure for the Peroxidative Oxidation of Alcohols
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bäckvall, J.E. Modern Oxidation Methods; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Tojo, G.; Fernandez, M.I. Oxidation of Alcohols to Aldehydes and Ketones: A Guide to Current Common Practice; Springer: Berlin, Germnay, 2006. [Google Scholar]
- Parmeggiani, C.; Cardona, F. Transition metal based catalysts in the aerobic oxidation of alcohols. Green Chem. 2012, 14, 547–564. [Google Scholar] [CrossRef]
- González-Arellano, C.; Campelo, J.M.; Macquarrie, D.J.; Marinas, J.M.; Romero, A.A.; Luque, R. Efficient microwave oxidation of alcohols using low-loaded supported metallic iron nanoparticles. ChemSusChem 2008, 1, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Mahyari, M.; Shaabani, A. Graphene oxide-iron phthalocyanine catalyzed aerobic oxidation of alcohols. Appl. Catal. A 2014, 469, 524–531. [Google Scholar] [CrossRef]
- Karabach, Y.Y.; Kopylovich, M.N.; Mahmudov, K.T.; Pombeiro, A.J.L. Microwave-assisted catalytic oxidation of alcohols to carbonyl compounds. In Advances in Organometallic Chemistry and Catalysis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 233–245. [Google Scholar]
- Kopylovich, M.N.; Ribeiro, A.P.C.; Alegria, E.C.B.A.; Martins, N.M.R.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Chapter three—Catalytic oxidation of alcohols: Recent advances. In Advances in Organometallic Chemistry; Pedro, J.P., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 63, pp. 91–174. [Google Scholar]
- Polshettiwar, V.; Varma, R.S. Green chemistry by nano-catalysis. Green Chem. 2010, 12, 743–754. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Luque, R.; Fihri, A.; Zhu, H.; Bouhrara, M.; Basset, J.-M. Magnetically recoverable nanocatalysts. Chem. Rev. 2011, 111, 3036–3075. [Google Scholar] [CrossRef] [PubMed]
- Albonetti, S.; Mazzoni, R.; Cavani, F. Chapter 1 homogeneous, heterogeneous and nanocatalysis. In Transition Metal Catalysis in Aerobic Alcohol Oxidation; The Royal Society of Chemistry: London, UK, 2015; pp. 1–39. [Google Scholar]
- Ghandoor, H.E.; Zidan, H.M.; Khalil, M.M.H.; Ismail, M.I.M. Synthesis and some physical properties of magnetite (Fe3O4) nanoparticles. Int. J. Electrochem. Sci. 2012, 7, 5734–5745. [Google Scholar]
- Maaz, K.; Mumtaz, A.; Hasanain, S.K.; Ceylan, A. Synthesis and magnetic properties of cobalt ferrite (CoFe2O4) nanoparticles prepared by wet chemical route. J. Magn. Magn. Mater. 2007, 308, 289–295. [Google Scholar] [CrossRef]
- Deng, H.; Li, X.; Peng, Q.; Wang, X.; Chen, J.; Li, Y. Monodisperse magnetic single-crystal ferrite microspheres. Angew. Chem. Int. Ed. 2005, 44, 2782–2785. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Martins, L.; Amorim, C.; Amaral, V.; Pombeiro, A. Solvent-free microwave-induced oxidation of alcohols catalyzed by ferrite magnetic nanoparticles. Catalysts 2017, 7, 222. [Google Scholar] [CrossRef]
- Banerjee, S.S.; Chen, D.-H. Fast removal of copper ions by gum arabic modified magnetic nano-adsorbent. J. Hazard. Mater. 2007, 147, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Chen, D.-H. Preparation and adsorption properties of monodisperse chitosan-bound Fe3O4 magnetic nanoparticles for removal of Cu(II) ions. J. Colloid Interface Sci. 2005, 283, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-F.; Zhao, Z.-S.; Jiang, G.-B. Coating Fe3O4 magnetic nanoparticles with humic acid for high efficient removal of heavy metals in water. Environ. Sci. Technol. 2008, 42, 6949–6954. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-H.; Chen, D.-H. Rapid removal of heavy metal cations and anions from aqueous solutions by an amino-functionalized magnetic nano-adsorbent. J. Hazard. Mater. 2009, 163, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Naeimi, H.; Mohamadabadi, S. Sulfonic acid-functionalized silica-coated magnetic nanoparticles as an efficient reusable catalyst for the synthesis of 1-substituted 1h-tetrazoles under solvent-free conditions. Dalton Trans. 2014, 43, 12967–12973. [Google Scholar] [CrossRef] [PubMed]
- Habibi, D.; Faraji, A.R. Synthesis, characterization and application of a nano-manganese-catalyst as an efficient solid catalyst for solvent free selective oxidation of ethylbenzene, cyclohexene, and benzylalcohol. Appl. Surf. Sci. 2013, 276, 487–496. [Google Scholar] [CrossRef]
- Azgomi, N.; Mokhtary, M. Nano-Fe3O4@SiO2 supported ionic liquid as an efficient catalyst for the synthesis of 1,3-thiazolidin-4-ones under solvent-free conditions. J. Mol. Catal. A 2015, 398, 58–64. [Google Scholar] [CrossRef]
- Jiang, S.; Yan, J.; Habimana, F.; Ji, S. Preparation of magnetically recyclable MIL-53(Al)@SiO2@Fe3O4 catalysts and their catalytic performance for friedel–crafts acylation reaction. Catal. Today 2016, 264, 83–90. [Google Scholar] [CrossRef]
- Esmaeilpour, M.; Javidi, J.; Nowroozi Dodeji, F.; Mokhtari Abarghoui, M. Facile synthesis of 1- and 5-substituted 1h-tetrazoles catalyzed by recyclable ligand complex of Copper(ii) supported on superparamagnetic Fe3O4@SiO2 nanoparticles. J. Mol. Catal. A 2014, 393, 18–29. [Google Scholar] [CrossRef]
- Kakavandi, B.; Takdastan, A.; Jaafarzadeh, N.; Azizi, M.; Mirzaei, A.; Azari, A. Application of Fe3O4@C catalyzing heterogeneous uv-fenton system for tetracycline removal with a focus on optimization by a response surface method. J. Photochem. Photobiol. A 2016, 314, 178–188. [Google Scholar] [CrossRef]
- Fan, W.; Gao, W.; Zhang, C.; Tjiu, W.W.; Pan, J.; Liu, T. Hybridization of graphene sheets and carbon-coated Fe3O4 nanoparticles as a synergistic adsorbent of organic dyes. J. Mater. Chem. 2012, 22, 25108–25115. [Google Scholar] [CrossRef]
- Zhang, D.-H.; Li, G.-D.; Li, J.-X.; Chen, J.-S. One-pot synthesis of Ag-Fe3O4 nanocomposite: A magnetically recyclable and efficient catalyst for epoxidation of styrene. Chem. Commun. 2008, 29, 3414–3416. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, M.; Hao, Y. Study on the adsorption of Cu(II) by edta functionalized Fe3O4 magnetic nano-particles. Chem. Eng. J. 2013, 218, 46–54. [Google Scholar] [CrossRef]
- Azizi, K.; Karimi, M.; Nikbakht, F.; Heydari, A. Direct oxidative amidation of benzyl alcohols using EDTA@Cu(II) functionalized superparamagnetic nanoparticles. Appl. Catal. A 2014, 482, 336–343. [Google Scholar] [CrossRef]
- Azizi, K.; Karimi, M.; Heydari, A. Oxidative coupling of formamides with β-dicarbonyl compounds and the synthesis of 2-aminobenzothiazole using Cu(II)-functionalized Fe3O4 nanoparticles. Tetrahedron Lett. 2015, 56, 812–816. [Google Scholar] [CrossRef]
- Spargo, P.L. Microwave assisted organic synthesis edited by J. P. Tierney and P. Lidstrom. Blackwell publishing: Oxford. 2005. 280 pp. £89.50. ISBN 1-4051-1560-2. (Also published by CRC Press in USA and Canada, ISBN 0-8493-2371-1.). Organ. Process Res. Dev. 2005, 9, 697. [Google Scholar] [CrossRef]
- Dallinger, D.; Kappe, C.O. Microwave-assisted synthesis in water as solvent. Chem. Rev. 2007, 107, 2563–2591. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; An, S.; Shi, W.; Yang, Q.; Zhang, L. Microwave-induced catalytic oxidation of malachite green under magnetic Cu-ferrites: New insight into the degradation mechanism and pathway. J. Mol. Catal. A 2014, 395, 243–250. [Google Scholar] [CrossRef]
- Roy, S.; Ghose, J. Effect of polymer matrix on the phase transitions of CuFe2O4. J. Solid State Chem. 1999, 144, 159–162. [Google Scholar] [CrossRef]
- De la Hoz, A.; Diaz-Ortiz, A.; Moreno, A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 2005, 34, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Kappe, C.O.; Dallinger, D.; Murphree, S.S. Experimental protocols. In Practical Microwave Synthesis for Organic Chemists; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009; pp. 203–290. [Google Scholar]
- Nasani, R.; Saha, M.; Mobin, S.M.; Martins, L.M.D.R.S.; Pombeiro, A.J.L.; Kirillov, A.M.; Mukhopadhyay, S. Copper-organic frameworks assembled from in situ generated 5-(4-pyridyl)tetrazole building blocks: Synthesis, structural features, topological analysis and catalytic oxidation of alcohols. Dalton Trans. 2014, 43, 9944–9954. [Google Scholar] [CrossRef] [PubMed]
- Mahmudov, K.T.; Sutradhar, M.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Ribera, A.; Nunes, A.V.M.; Gahramanova, S.I.; Marchetti, F.; Pombeiro, A.J.L. Mnii and cuii complexes with arylhydrazones of active methylene compounds as effective heterogeneous catalysts for solvent- and additive-free microwave-assisted peroxidative oxidation of alcohols. RSC Adv. 2015, 5, 25979–25987. [Google Scholar] [CrossRef]
- Sutradhar, M.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Oxidovanadium complexes with tridentate aroylhydrazone as catalyst precursors for solvent-free microwave-assisted oxidation of alcohols. Appl. Catal. A 2015, 493, 50–57. [Google Scholar] [CrossRef]
- Tăbăcaru, A.; Xhaferaj, N.; Martins, L.M.D.R.S.; Alegria, E.C.B.A.; Chay, R.S.; Giacobbe, C.; Domasevitch, K.V.; Pombeiro, A.J.L.; Galli, S.; Pettinari, C. Metal azolate/carboxylate frameworks as catalysts in oxidative and C–C coupling reactions. Inorgan. Chem. 2016, 55, 5804–5817. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, A.; Martins, L.M.D.R.S.; Hazra, S.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Metal–organic frameworks with pyridyl-based isophthalic acid and their catalytic applications in microwave assisted peroxidative oxidation of alcohols and henry reaction. Cryst. Growth Des. 2016, 16, 1837–1849. [Google Scholar] [CrossRef]
- Martins, N.M.R.; Mahmudov, K.T.; Guedes da Silva, M.F.C.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Copper(II) and Iron(III) complexes with arylhydrazone of ethyl 2-cyanoacetate or formazan ligands as catalysts for oxidation of alcohols. New J. Chem. 2016, 40, 10071–10083. [Google Scholar] [CrossRef]
- Moiseeva, N.I.; Gekhman, A.E.; Minin, V.V.; Larin, G.M.; Bashtanov, M.E.; Krasnovskii, A.A.; Moiseev, I.I. Free radical/singlet dioxygen system under the conditions of catalytic hydrogen peroxide decomposition. Kinet. Catal. 2000, 41, 170–182. [Google Scholar] [CrossRef]
- Mattalia, J.M.; Vacher, B.; Samat, A.; Chanon, M. Mechanistic investigation of the reaction between Alpha-sulfonyl carbanions and polyhalogenmethanes. Electron transfer versus polar pathways. J. Am. Chem. Soc. 1992, 114, 4111–4119. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Alegria, E.C.B.A.; Smoleński, P.; Kuznetsov, M.L.; Pombeiro, A.J.L. Oxorhenium complexes bearing the water-soluble tris(pyrazol-1-yl)methanesulfonate, 1,3,5-triaza-7-phosphaadamantane or related ligands, as catalysts for the Baeyer-Villiger oxidation of ketones. Inorgan. Chem. 2013, 52, 4534–4546. [Google Scholar] [CrossRef] [PubMed]
- Barton, D.H.R.; Le Gloahec, N.V.; Patin, H.; Launay, F. Radical chemistry of tert-butyl hydroperoxide (tbhp). Parts 1 and 2. Studies of the feiii-tbhp mechanism. New J. Chem. 1998, 22, 559–568. [Google Scholar] [CrossRef]
- Kopylovich, M.N.; Mahmudov, K.T.; Silva, M.F.C.G.; Martins, L.M.D.R.S.; Kuznetsov, M.L.; Silva, T.F.S.; Fraústo da Silva, J.J.R.; Pombeiro, A.J.L. Trends in properties of para-substituted 3-(phenylhydrazo)pentane-2,4-diones. J. Phys. Organ. Chem. 2011, 24, 764–773. [Google Scholar] [CrossRef]
- Minisci, F.; Fontana, F.; Araneo, S.; Recupero, F.; Banfi, S.; Quici, S. Kharasch and metalloporphyrin catalysis in the functionalization of alkanes, alkenes, and alkylbenzenes by t-buooh. Free radical mechanisms, solvent effect, and relationship with the gif reaction. J. Am. Chem. Soc. 1995, 117, 226–232. [Google Scholar] [CrossRef]
- Boess, E.; Wolf, L.M.; Malakar, S.; Salamone, M.; Bietti, M.; Thiel, W.; Klussmann, M. Competitive hydrogen atom transfer to oxyl- and peroxyl radicals in the Cu-catalyzed oxidative coupling of N-aryl tetrahydroisoquinolines using tert-butyl hydroperoxide. ACS Catal. 2016, 6, 3253–3261. [Google Scholar] [CrossRef]
- Ten Brink, G.J.; Arends, I.W.C.E.; Sheldon, R.A. The baeyer−villiger reaction: New developments toward greener procedures. Chem. Rev. 2004, 104, 4105–4124. [Google Scholar] [CrossRef] [PubMed]
- Vafaeezadeh, M.; Mahmoodi Hashemi, M. Simple and green oxidation of cyclohexene to adipic acid with an efficient and durable silica-functionalized ammonium tungstate catalyst. Catal. Commun. 2014, 43, 169–172. [Google Scholar] [CrossRef]
- Shaikh, M.; Satanami, M.; Ranganath, K.V.S. Efficient aerobic oxidation of alcohols using magnetically recoverable catalysts. Catal. Commun. 2014, 54, 91–93. [Google Scholar] [CrossRef]
- Hou, J.; Luan, Y.; Yu, J.; Qi, Y.; Wang, G.; Lu, Y. Fabrication of hierarchical composite microspheres of copper-doped Fe3O4@P4VP@ZIF-8 and their application in aerobic oxidation. New J. Chem. 2016, 40, 10127–10135. [Google Scholar] [CrossRef]
- Li, J.; Gao, H.; Tan, L.; Luan, Y.; Yang, M. Superparamagnetic core–shell metal–organic framework Fe3O4/Cu3(BTC)2 microspheres and their catalytic activity in the aerobic oxidation of alcohols and olefins. Eur. J. Inorgan. Chem. 2016, 2016, 4906–4912. [Google Scholar] [CrossRef]
- Shaabani, A.; BoroujenI, M.B.; Sangachin, M.H. Cobalt-chitosan: Magnetic and biodegradable heterogeneous catalyst for selective aerobic oxidation of alkyl arenes and alcohols. J. Chem. Sci. 2015, 127, 1927–1935. [Google Scholar] [CrossRef]
- Zhou, Q.; Wan, Z.; Yuan, X.; Luo, J. A new magnetic nanoparticle-supported schiff base complex of manganese: An efficient and recyclable catalyst for selective oxidation of alcohols. Appl. Organomet. Chem. 2016, 30, 215–220. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, X.; Wu, P.; Zhang, Y.; Liu, B.; Hu, H.; Xue, G. Divanadium-substituted phosphotungstate supported on magnetic mesoporous silica nanoparticles as effective and recyclable catalysts for the selective oxidation of alcohols. ChemCatChem 2016, 8, 3680–3687. [Google Scholar] [CrossRef]


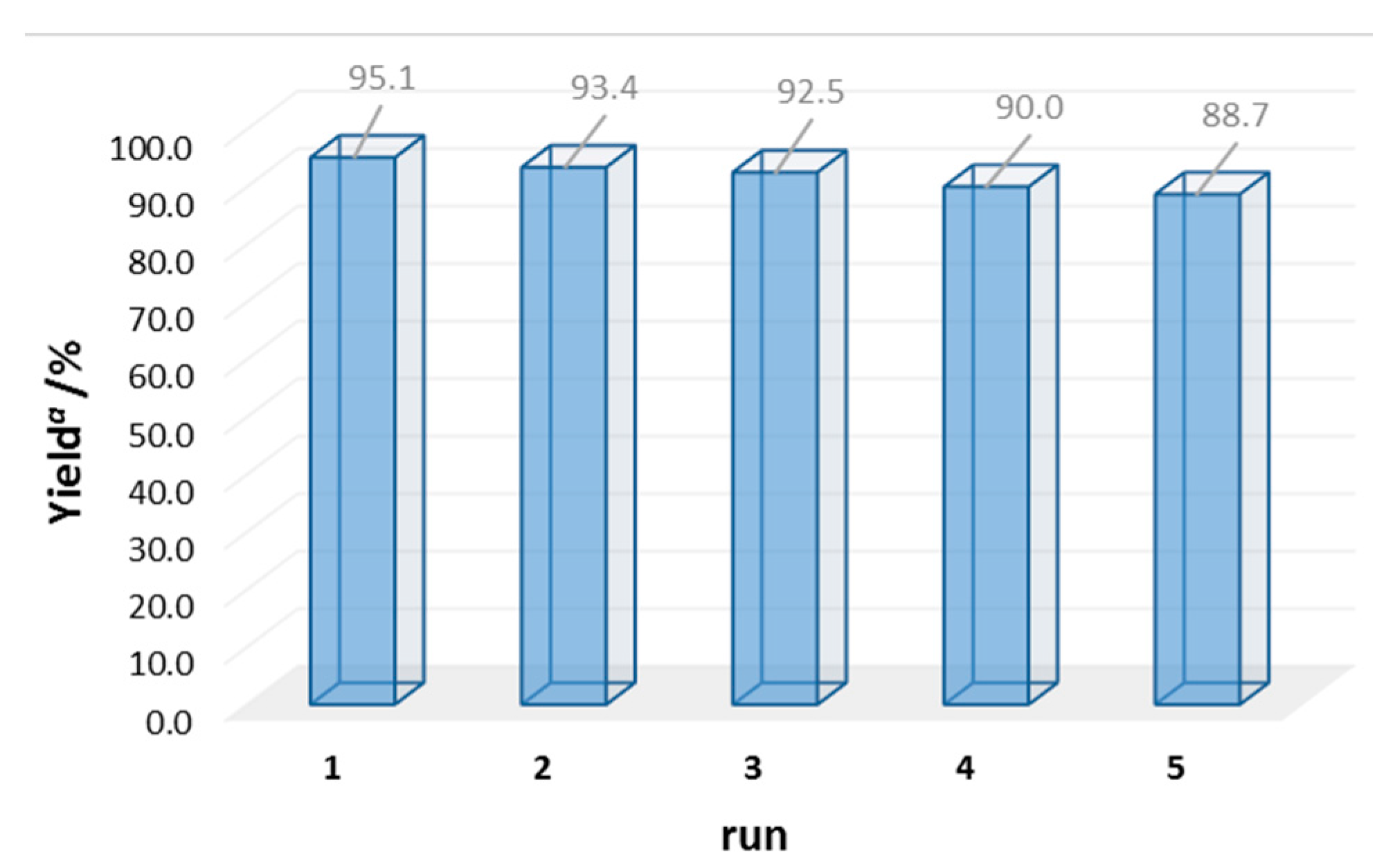
| Entry | Catalyst | m/mg | Yield b/% |
|---|---|---|---|
| 1 | none | - | 1.7 |
| 2 | Fe3O4@EDTA (0) | 30.1 | 6.5 |
| 3 | Fe3O4@EDTA-Mn2+ (1) | 29.1 | 27.4 |
| 4 | Fe3O4@EDTA-Fe2+ (2) | 29.9 | 10.5 |
| 5 | Fe3O4@EDTA-Co2+ (3) | 29.8 | 31.1 |
| 6 | Fe3O4@EDTA-Ni2+ (4) | 30.0 | 7.2 |
| 7 | Fe3O4@EDTA-Cu2+ (5) | 30.0 | 14.3 |
| 8 | Fe3O4@EDTA-Zn2+ (6) | 29.9 | 7.0 |
| Entry | Substrate | Product | Yield b/% |
|---|---|---|---|
| 1 | 1-Phenylethanol | Acetophenone | 95.1 |
| 2 | Benzyl alcohol | Benzaldehyde | 69.8 |
| 3 | Benzhydrol | Benzophenone | 87.5 |
| 4 | Cinnamyl alcohol | Cinnamylaldehyde | 68.3 |
| 5 | Cyclopentanol | Cyclopentanone | 96.2 |
| 6 | Cyclohexanol | Cyclohexanone | 96.7 |
| 7 | Cycloheptanol | Cycloheptanone | 96.8 |
| 8 | Cyclooctanol | Cyclooctanone | 98.8 |
| 9 | Isoborneol | Camphor | 90.2 |
| 10 | Fenchyl alcohol | Fenchylaldehyde | 66.6 |
| 11 | 1,2-Cyclohexanediol | 1,2-Cyclohexanedione | 91.3 c |
| 12 | 1,4-Cyclohexanediol | 1,4-Cyclohexanedione | 73.2 c |
| Entry | Catalyst | Reaction Conditions | Yield a/% | Selectivity b/% | Reference |
|---|---|---|---|---|---|
| 1 | Fe3O4 | O2, 80 °C, 18 h, toluene | 76 | 100 | [51] |
| 2 | Fe3O4 | t-BuOOH, MW 120 °C, 2 h | 51.2 | >99 | [14] |
| 3 | Cu-FPZ | NaHCO3, TEMPO, O2, 60 °C, 12 h, acetonitrile | 54 | 99 | [52] |
| 4 | Fe3O4@Cu3(BTC)2 | Na2CO3, TEMPO, O2, 75 °C, 12 h, acetonitrile | 7 | >99 | [53] |
| 5 | Cobalt-chitosan | K2CO3, O2, 80 °C, 4 h, p-xylene | 95 | 100 | [54] |
| 6 | Mn@MNP | t-BuOOH, 110 °C, 4 h, dimethyl sulfoxide | 4 | 86 | [55] |
| 7 | Fe3O4@mSiO2/NH-PV2W | H2O2, 80 °C, 8 h, toluene | 40 | 99 | [56] |
| 8 | Fe3O4@EDTA-Mn2+ (1) | t-BuOOH, MW 110 °C, 2 h | 95.1 | >99 | This work |
| 9 | Fe3O4@EDTA-Cu2+ (5) | t-BuOOH, MW 110 °C, 2 h | 93.3 | >99 | This work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, N.M.R.; Martins, L.M.D.R.S.; Amorim, C.O.; Amaral, V.S.; Pombeiro, A.J.L. First-Row-Transition Ion Metals(II)-EDTA Functionalized Magnetic Nanoparticles as Catalysts for Solvent-Free Microwave-Induced Oxidation of Alcohols. Catalysts 2017, 7, 335. https://doi.org/10.3390/catal7110335
Martins NMR, Martins LMDRS, Amorim CO, Amaral VS, Pombeiro AJL. First-Row-Transition Ion Metals(II)-EDTA Functionalized Magnetic Nanoparticles as Catalysts for Solvent-Free Microwave-Induced Oxidation of Alcohols. Catalysts. 2017; 7(11):335. https://doi.org/10.3390/catal7110335
Chicago/Turabian StyleMartins, Nuno M. R., Luísa M. D. R. S. Martins, Carlos O. Amorim, Vitor S. Amaral, and Armando J. L. Pombeiro. 2017. "First-Row-Transition Ion Metals(II)-EDTA Functionalized Magnetic Nanoparticles as Catalysts for Solvent-Free Microwave-Induced Oxidation of Alcohols" Catalysts 7, no. 11: 335. https://doi.org/10.3390/catal7110335









