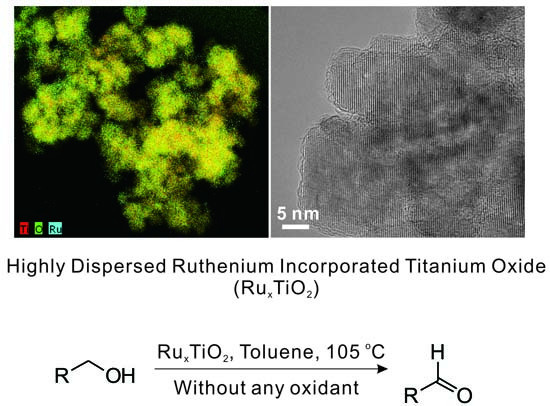
Dehydrogenative Oxidation of Alcohols Catalyzed by Highly Dispersed Ruthenium Incorporated Titanium Oxide
Abstract
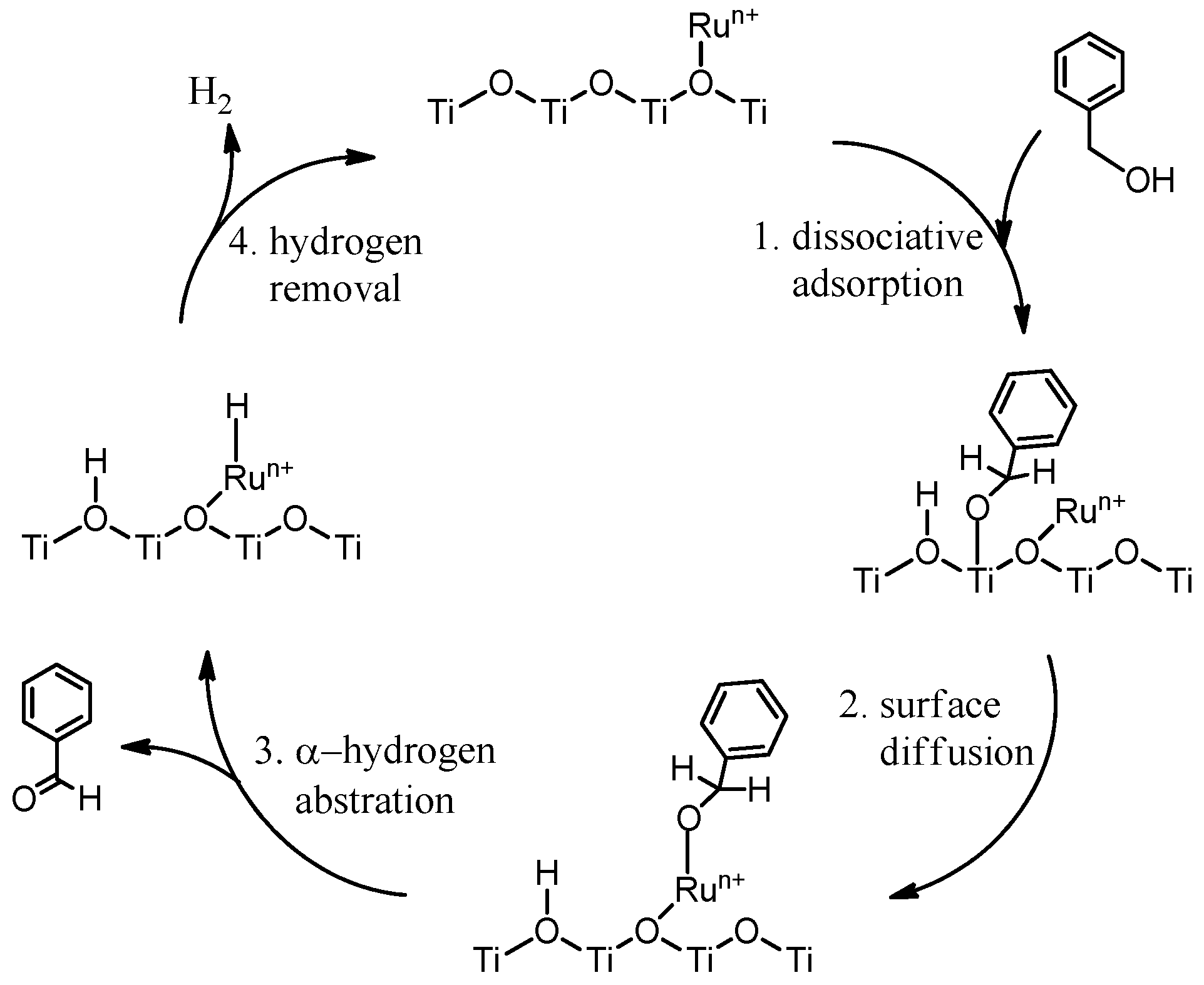
:1. Introduction
2. Results and Discussion
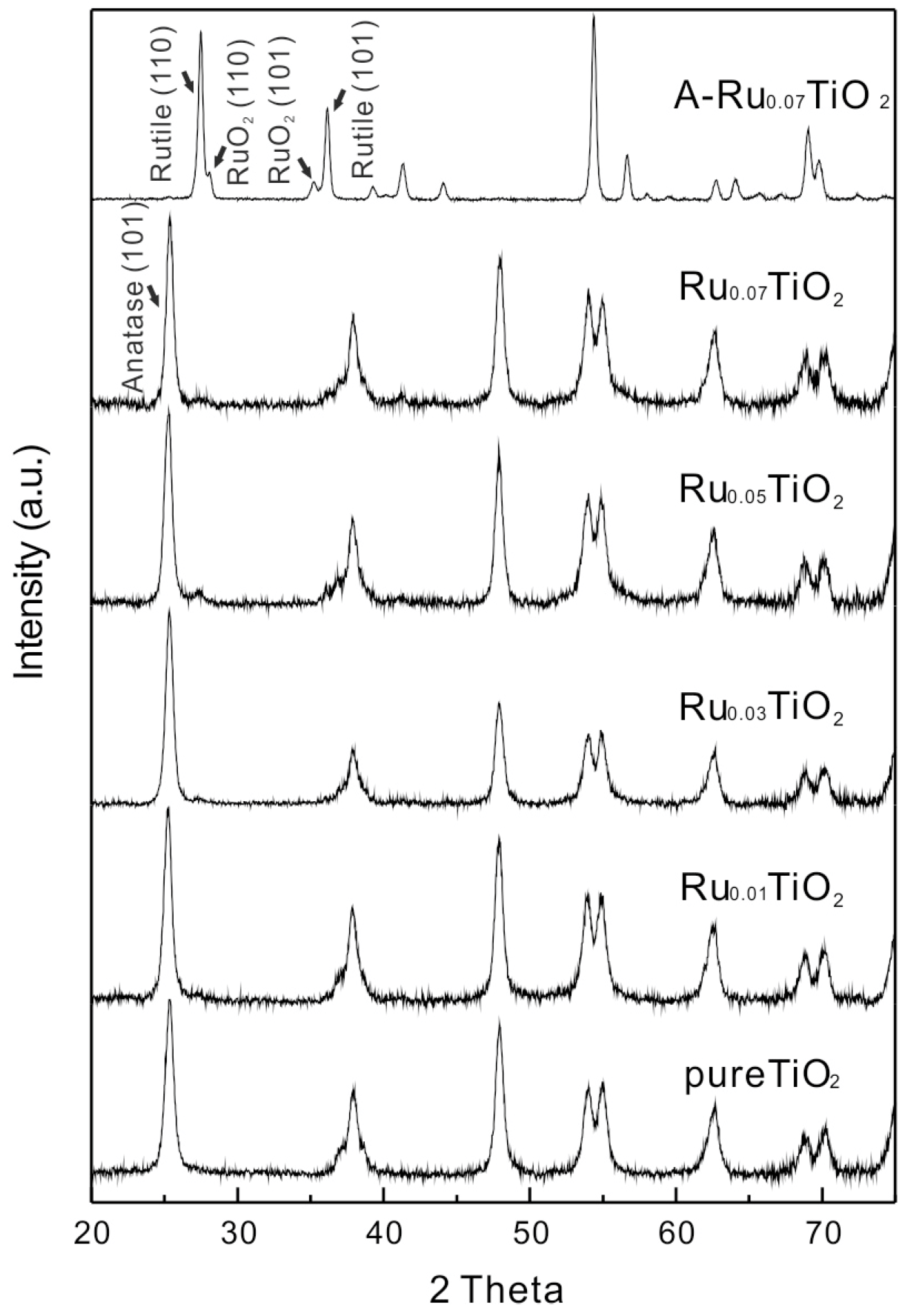
2.1. Catalyst Characterization
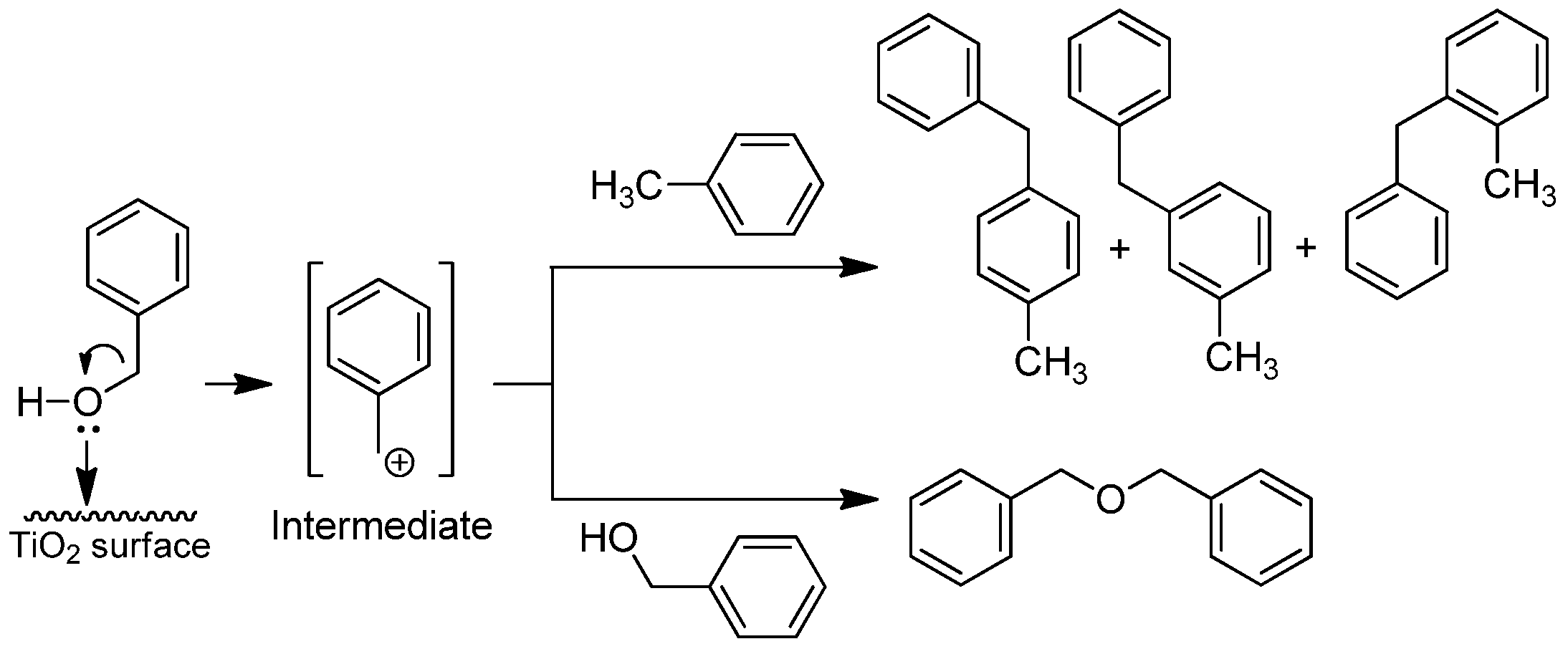
2.2. Catalytic Tests
3. Materials and Methods
3.1. Synthesis of TiO2 and Ruthenium-Incorporated TiO2 (RuxTiO2)
3.2. Characterization of Catalysts
3.3. Catalytic Tests
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dijksman, A.; Marino-González, A.; Payeras, A.M.; Arends, I.W.C.E.; Sheldon, R.A. Efficient and selective aerobic oxidation of alcohols into aldehydes and ketones using ruthenium/TEMPO as the catalytic system. J. Am. Chem. Soc. 2001, 123, 6826–6833. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Mizuno, N. Efficient heterogeneous aerobic oxidation of amines by a supported ruthenium catalyst. Angew. Chem. Int. Ed. 2003, 42, 1480–1483. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Hara, T.; Mizugaki, T.; Ebitani, K.; Kaneda, K. Hydroxyapatite-supported palladium nanoclusters: A highly active heterogeneous catalyst for selective oxidation of alcohols by use of molecular oxygen. J. Am. Chem. Soc. 2004, 126, 10657–10666. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.I.; Tanino, N.; Yamaguchi, R. Ligand-promoted dehydrogenation of alcohols catalyzed by Cp*Ir complexes. A new catalytic system for oxidant-free oxidation of alcohols. Org. Lett. 2007, 9, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, M.; Choualeb, A.; Lough, A.J.; Moore, B.; Spasyuk, D.; Gusev, D.G. Osmium and ruthenium catalysts for dehydrogenation of alcohols. Organometallics 2011, 30, 3479–3482. [Google Scholar] [CrossRef]
- Adair, G.R.A.; Williams, J.M.J. Oxidant-free oxidation: Ruthenium catalysed dehydrogenation of alcohols. Tetrahedron Lett. 2005, 46, 8233–8235. [Google Scholar] [CrossRef]
- Baratta, W.; Bossi, G.; Putignano, E.; Rigo, P. Pincer and diamine Ru and Os diphosphane complexes as efficient catalysts for the dehydrogenation of alcohols to ketones. Chem. Eur. J. 2011, 17, 3474–3481. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Piszel, P.E.; Brennessel, W.W.; Jones, W.D. A single nickel catalyst for the acceptorless dehydrogenation of alcohols and hydrogenation of carbonyl compounds. Organometallics 2015, 34, 5203–5206. [Google Scholar] [CrossRef]
- Shimizu, K.I.; Sugino, K.; Sawabe, K.; Satsuma, A. Oxidant-free dehydrogenation of alcohols heterogeneously catalyzed by cooperation of silver clusters and acid-base sites on alumina. Chem. Eur. J. 2009, 15, 2341–2351. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, F.; Ma, J.; Li, M.; Zhang, Z.; Wang, Y.; Zhang, X.; Xu, J. Investigations on the crystal plane effect of ceria on gold catalysis in the oxidative dehydrogenation of alcohols and amines in the liquid phase. Chem. Commun. 2014, 50, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Mitsudome, T.; Mikami, Y.; Funai, H.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. Oxidant-free alcohol dehydrogenation using a reusable hydrotalcite-supported silver nanoparticle catalyst. Angew. Chem. Int. Ed. 2008, 47, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Kon, K.; Hakim Siddiki, S.M.A.; Shimizu, K.I. Size- and support-dependent Pt nanocluster catalysis for oxidant-free dehydrogenation of alcohols. J. Catal. 2013, 304, 63–71. [Google Scholar] [CrossRef]
- Kon, K.; Onodera, W.; Toyao, T.; Shimizu, K.I. Supported rhenium nanoparticle catalysts for acceptorless dehydrogenation of alcohols: structure-activity relationship and mechanistic studies. Catal. Sci. Technol. 2016, 6, 5864–5870. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Al-Ekabi, H.; Serpone, N. Kinetic studies in heterogeneous photocatalysis. 1. Photocatalytic degradation of chlorinated phenols in aerated aqueous solutions over TiO2 supported on a glass matrix. J. Phys. Chem. 1988, 92, 5726–5731. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Tang, J.; Durrant, J.R.; Klug, D.R. Mechanism of photocatalytic water splitting in TiO2. Reaction of water with photoholes, importance of charge carrier dynamics, and evidence for four-hole chemistry. J. Am. Chem. Soc. 2008, 130, 13885–13891. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Kim, J.W.; He, J.; Mizuno, N. Aerobic alcohol oxidation catalyzed by supported ruthenium hydroxides. J. Catal. 2009, 268, 343–349. [Google Scholar] [CrossRef]
- Köckritz, A.; Sebek, M.; Dittmar, A.; Radnik, J.; Brückner, A.; Bentrup, U.; Pohl, M.M.; Hugl, H.; Mägerlein, W. Ru-catalyzed oxidation of primary alcohols. J. Mol. Catal. A 2006, 246, 85–99. [Google Scholar] [CrossRef]
- Grosjean, N.; Descorme, C.; Besson, M. Catalytic wet air oxidation of N,N-dimethylformamide aqueous solutions: Deactivation of TiO2 and ZrO2-supported noble metal catalysts. Appl. Catal. B 2010, 97, 276–283. [Google Scholar] [CrossRef]
- Besson, M.; Descorme, C.; Bernardi, M.; Gallezot, P.; Di Gregorio, F.; Grosjean, N.; Pham Minh, D.; Pintar, A. Supported noble metal catalysts in the catalytic wet air oxidation of industrial wastewaters and sewage sludges. Environ. Technol. 2010, 31, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Pintar, A.; Batista, J.; Tišler, T. Catalytic wet-air oxidation of aqueous solutions of formic acid, acetic acid and phenol in a continuous-flow trickle-bed reactor over Ru/TiO2 catalysts. Appl. Catal. B 2008, 84, 30–41. [Google Scholar] [CrossRef]
- Buonaiuto, M.; De Crisci, A.G.; Jaramillo, T.F.; Waymouth, R.M. Electrooxidation of alcohols with electrode-supported transfer hydrogenation catalysts. ACS Catal. 2015, 5, 7343–7349. [Google Scholar] [CrossRef]
- Park, H.K.; Moon, Y.T.; Kim, D.K.; Kim, C.H. Formation of monodisperse spherical TiO2 powders by thermal hydrolysis of Ti(SO4)2. J. Am. Ceram. Soc. 1996, 79, 2727–2732. [Google Scholar] [CrossRef]
- Paunović, P.; Gogovska, D.S.; Popovski, O.; Stoyanova, A.; Slavcheva, E.; Lefterova, E.; Iliev, P.; Dimitrov, A.T.; Jordanov, S.H. Preparation and characterization of Co-Ru/TiO2/MWCNTs electrocatalysts in PEM hydrogen electrolyzer. Int. J. Hydrogen Energy 2011, 36, 9405–9414. [Google Scholar] [CrossRef]
- Lin, X.; Yang, K.; Si, R.; Chen, X.; Dai, W.; Fu, X. Photo-assisted catalytic methanation of CO in H2-rich stream over Ru/TiO2. Appl. Catal. B 2014, 147, 585–591. [Google Scholar] [CrossRef]
- Rizzi, G.A.; Magrin, A.; Granozzi, G. Substitutional Ti(1-x)RuxO2 surface alloys obtained from the decomposition of Ru3(CO)12 on TiO2(110). Phys. Chem. Chem. Phys. 1999, 1, 709–711. [Google Scholar] [CrossRef]
- Di, L.; Wu, G.; Dai, W.; Guan, N.; Li, L. Ru/TiO2 for the preferential oxidation of CO in H2-rich stream: Effects of catalyst pre-treatments and reconstruction of Ru sites. Fuel 2015, 143, 318–326. [Google Scholar] [CrossRef]
- Luo, J.; Yang, X.; Li, D. Preparation of TiO2 nanoparticles doped with Cs+ and Sr2+ and their photocatalytic activity under solar light. Adv. Mat. Res. 2010, 113, 1945–1950. [Google Scholar] [CrossRef]
- Nam, H.J.; Amemiya, T.; Murabayashi, M.; Itoh, K. The influence of Na+ on the crystallite size of TiO2 and the photocatalytic activity. Res. Chem. Intermed. 2005, 31, 365–370. [Google Scholar] [CrossRef]
- Devi, L.G.; Murthy, B.N.; Kumar, S.G. Photocatalytic activity of TiO2 doped with Zn2+ and V5+ transition metal ions: Influence of crystallite size and dopant electronic configuration on photocatalytic activity. Mater. Sci. Eng. B 2009, 166, 1–6. [Google Scholar] [CrossRef]
- Safari, M.; Talebi, R.; Rostami, M.H.; Nikazar, M.; Dadvar, M. Synthesis of iron-doped TiO2 for degradation of reactive Orange16. J. Environ. Sci. Eng. 2014, 12. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Li, Z.; Cheng, B.; Zhang, L.; Zhang, M.; Ming, J. The studies on the Friedel-Crafts acylation of toluene with acetic anhydride over HPW/TiO2. Fuel Process. Technol. 2011, 92, 2011–2015. [Google Scholar] [CrossRef]
- Hosseini-Sarvari, M.; Safary, E. Nano-sulfated titania (TiO2/SO2–4) as a new solid acid catalyst for Friedel-Crafts acylation and Beckman rearrangement in solvent-free conditions. J. Sulfur Chem. 2011, 32, 463–473. [Google Scholar] [CrossRef]
- Farfan-Arribas, E.; Madix, R.J. Role of defects in the adsorption of aliphatic alcohols on the TiO2(110) surface. J. Phys. Chem. B 2002, 106, 10680–10692. [Google Scholar] [CrossRef]
- Chen, L.; Li, Z.; Smith, R.S.; Kay, B.D.; Dohnálek, Z. Conversion of 1,2-propylene glycol on rutile TiO2(110). J. Phys. Chem. C 2014, 118, 15339–15347. [Google Scholar] [CrossRef]
- Hansen, J.Ø.; Huo, P.; Martinez, U.; Lira, E.; Wei, Y.Y.; Streber, R.; Lægsgaard, E.; Hammer, B.; Wendt, S.; Besenbacher, F. Direct evidence for ethanol dissociation on rutile TiO2(110). Phys. Rev. Lett. 2011, 107, 136102. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Kay, B.D.; White, J.M.; Dohnálek, Z. Alcohol chemistry on rutile TiO2(110): The influence of alkyl substituents on reactivity and selectivity. J. Phys. Chem. C 2007, 111, 18236–18242. [Google Scholar] [CrossRef]
- Kim, W.H.; Park, I.S.; Park, J. Acceptor-free alcohol dehydrogenation by recyclable ruthenium catalyst. Org. Lett. 2006, 8, 2543–2545. [Google Scholar] [CrossRef] [PubMed]
- Mitsudome, T.; Mikami, Y.; Ebata, K.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. Copper nanoparticles on hydrotalcite as a heterogeneous catalyst for oxidant-free dehydrogenation of alcohols. Chem. Commun. 2008, 39, 4804–4806. [Google Scholar] [CrossRef] [PubMed]
- Damodara, D.; Arundhathi, R.; Likhar, P.R. Copper nanoparticles from copper aluminum hydrotalcite: An efficient catalyst for acceptor- and oxidant-free dehydrogenation of amines and alcohols. Adv. Synth. Catal. 2014, 356, 189–198. [Google Scholar] [CrossRef]

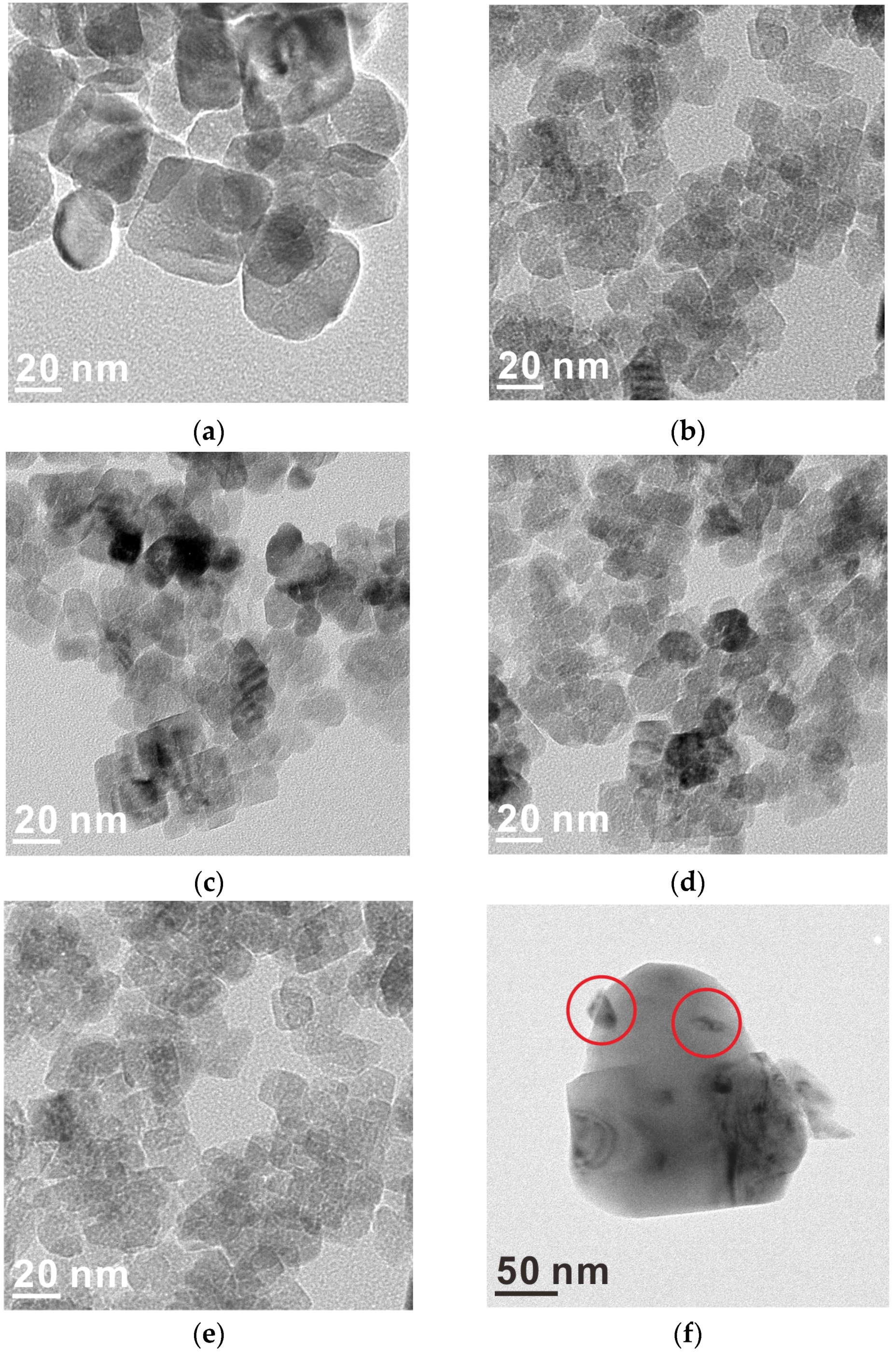

| Samples | Size (nm) | Surface Area | Ru Content | |
|---|---|---|---|---|
| XRD | TEM | (m2/g) | (μmol/g) | |
| Pure TiO2 | 26.4 | 45.7 ± 5.3 | 48 | - |
| Ru0.01TiO2 | 13.2 | 16.7 ± 0.9 | 104 | 9 |
| Ru0.03TiO2 | 13.3 | 16.9 ± 1.0 | 111 | 24 |
| Ru0.05TiO2 | 13.4 | 17.0 ± 1.4 | 125 | 37 |
| Ru0.07TiO2 | 14.7 | 17.3 ± 1.1 | 126 | 51 |
| Entry | Catalysts | Substrates | Products | Conversion (%) | Yield to Aldehyde (%) | Selectivity (%) |
|---|---|---|---|---|---|---|
| 1 | None |  |  | 0 | - | - |
| 2 | Degussa, P25 | 2 | 1 | 60 | ||
| 3 | pure TiO2 | 49 | 1 | 2 | ||
| 4 | Ru0.01TiO2 | 41 | 34 | 86 | ||
| 5 | Ru0.03TiO2 | 79 | 72 | 93 | ||
| 6 | Ru0.05TiO2 | 88 | 82 | 93 | ||
| 7 | Ru0.07TiO2 | >99 | 92 | 93 | ||
| 8 | Reuse 7 | 99 | 92 | 93 | ||
| 9 | Reuse 8 | 98 | 90 | 92 | ||
| 10 | A-Ru0.07TiO2 | 2 | 2 | 99 | ||
| 11 | None |  |  | 0 | - | - |
| 12 | Degussa, P25 | 3 | 3 | 99 | ||
| 13 | pure TiO2 | 44 | 43 | 98 | ||
| 14 | Ru0.01TiO2 | 78 | 78 | >99 | ||
| 15 | Ru0.03TiO2 | 85 | 84 | >99 | ||
| 16 | Ru0.05TiO2 | 96 | 94 | 98 | ||
| 17 | Ru0.07TiO2 | >99 | 98 | 99 | ||
| 18 | None |  |  | 0 | - | - |
| 19 | Degussa, P25 | 3 | 2 | 93 | ||
| 20 | pure TiO2 | 4 | 4 | 98 | ||
| 21 | Ru0.01TiO2 | 12 | 12 | >99 | ||
| 22 | Ru0.03TiO2 | 23 | 22 | 97 | ||
| 23 | Ru0.05TiO2 | 23 | 22 | >99 | ||
| 24 | Ru0.07TiO2 | 24 | 23 | 98 |
| Entry | Catalysts | Substrates | Products | Conversion (%) | Selectivity (%) |
|---|---|---|---|---|---|
| 1 | None |  |  | 0 | - |
| 2 | Degussa, P25 | 3 | >99 | ||
| 3 | pure TiO2 | 3 | >99 | ||
| 4 | Ru0.01TiO2 | 38 | >99 | ||
| 5 | Ru0.03TiO2 | 77 | >99 | ||
| 6 | Ru0.05TiO2 | 94 | >99 | ||
| 7 | Ru0.07TiO2 | >99 | >99 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Ahn, S.; Hwang, J.Y.; Ko, D.-H.; Kwon, K.-Y. Dehydrogenative Oxidation of Alcohols Catalyzed by Highly Dispersed Ruthenium Incorporated Titanium Oxide. Catalysts 2017, 7, 7. https://doi.org/10.3390/catal7010007
Kim Y, Ahn S, Hwang JY, Ko D-H, Kwon K-Y. Dehydrogenative Oxidation of Alcohols Catalyzed by Highly Dispersed Ruthenium Incorporated Titanium Oxide. Catalysts. 2017; 7(1):7. https://doi.org/10.3390/catal7010007
Chicago/Turabian StyleKim, Youngyong, Seokhoon Ahn, Jun Yeon Hwang, Doo-Hyun Ko, and Ki-Young Kwon. 2017. "Dehydrogenative Oxidation of Alcohols Catalyzed by Highly Dispersed Ruthenium Incorporated Titanium Oxide" Catalysts 7, no. 1: 7. https://doi.org/10.3390/catal7010007