Aziridine- and Azetidine-Pd Catalytic Combinations. Synthesis and Evaluation of the Ligand Ring Size Impact on Suzuki-Miyaura Reaction Issues
Abstract
:1. Introduction
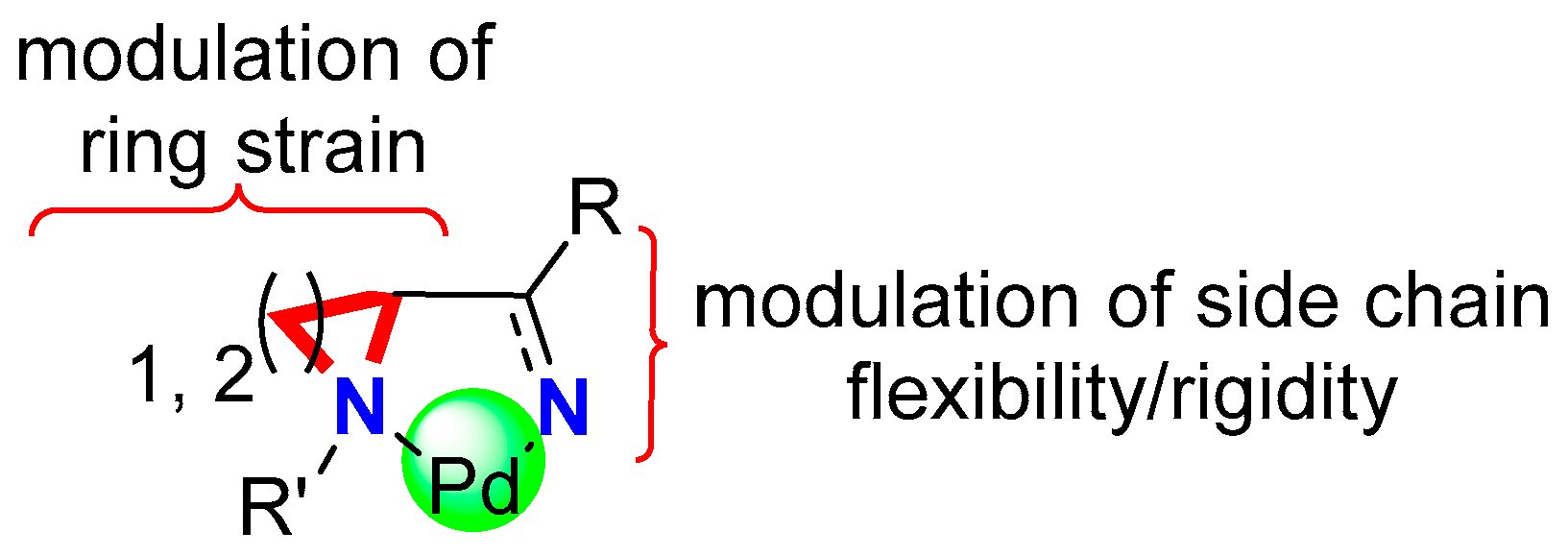
2. Results and Discussion
2.1. Synthesis of Catalytic System
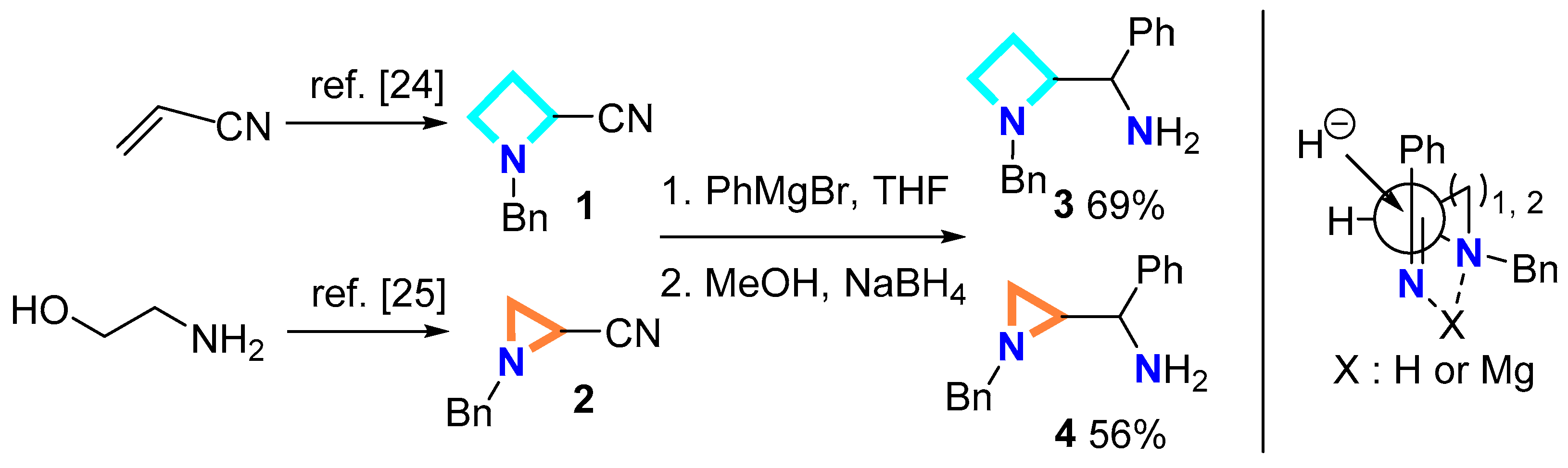
2.1.1. Synthesis of Ligand Precursors
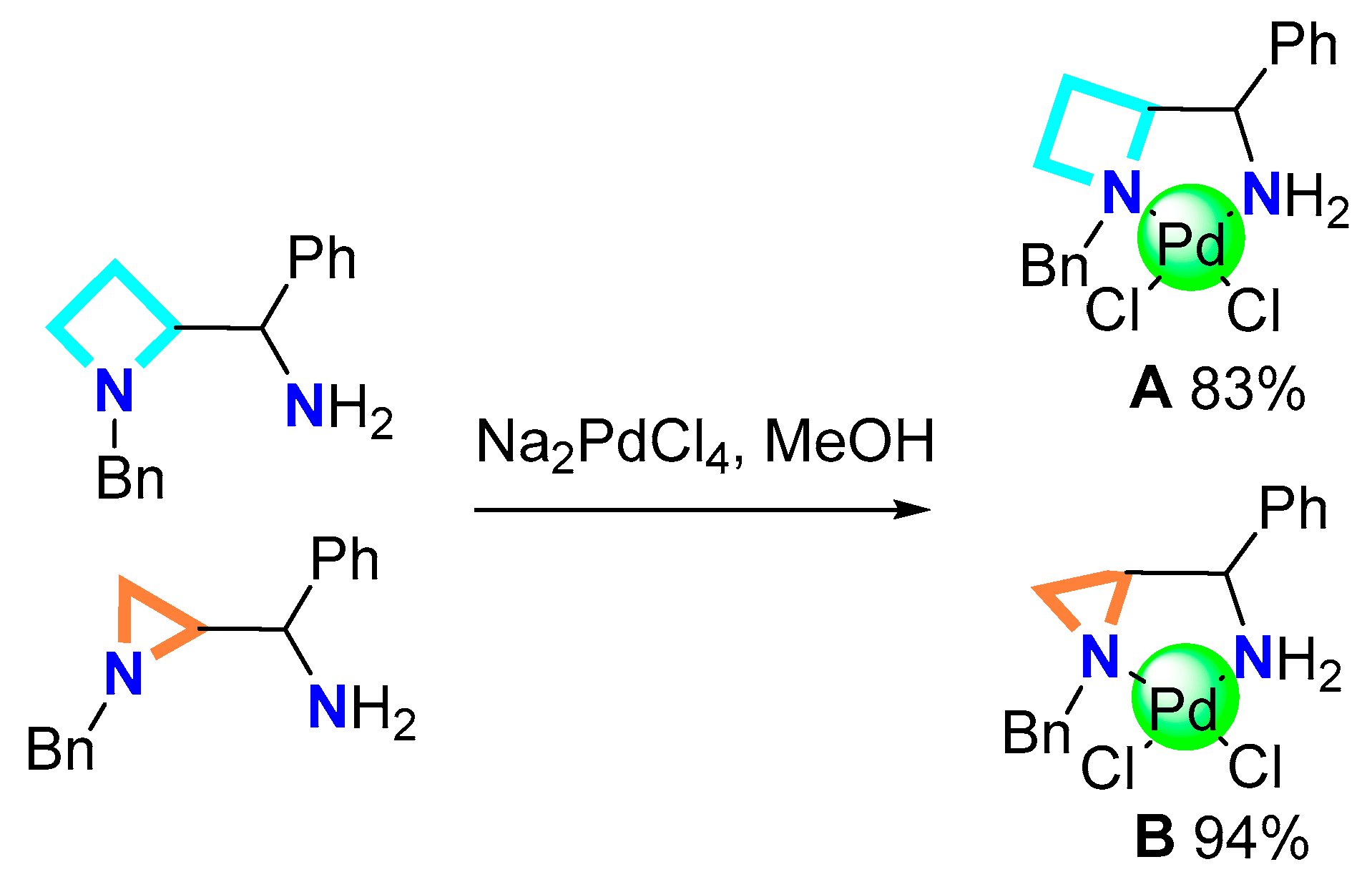
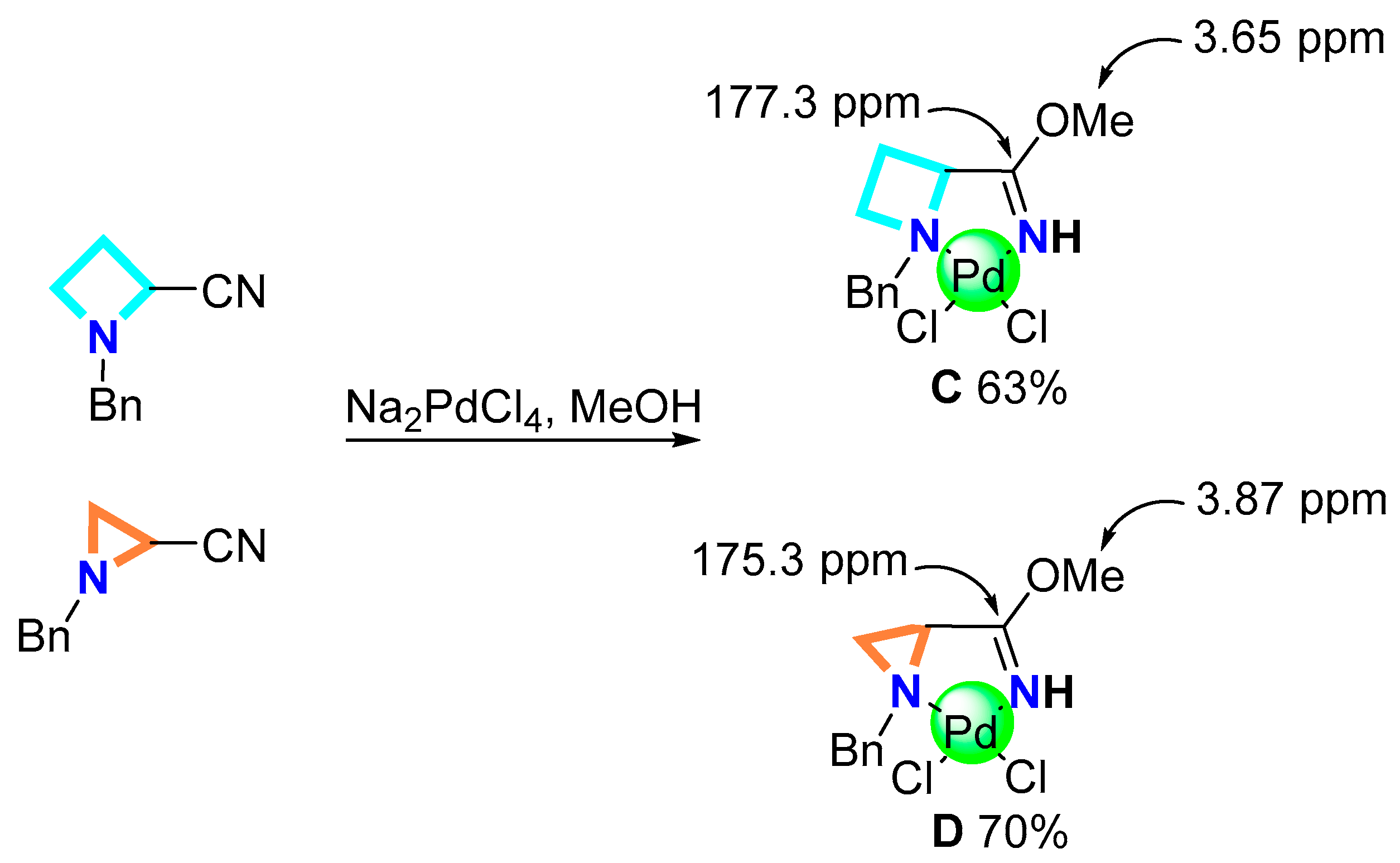
2.1.2. Synthesis of Pd Complexes
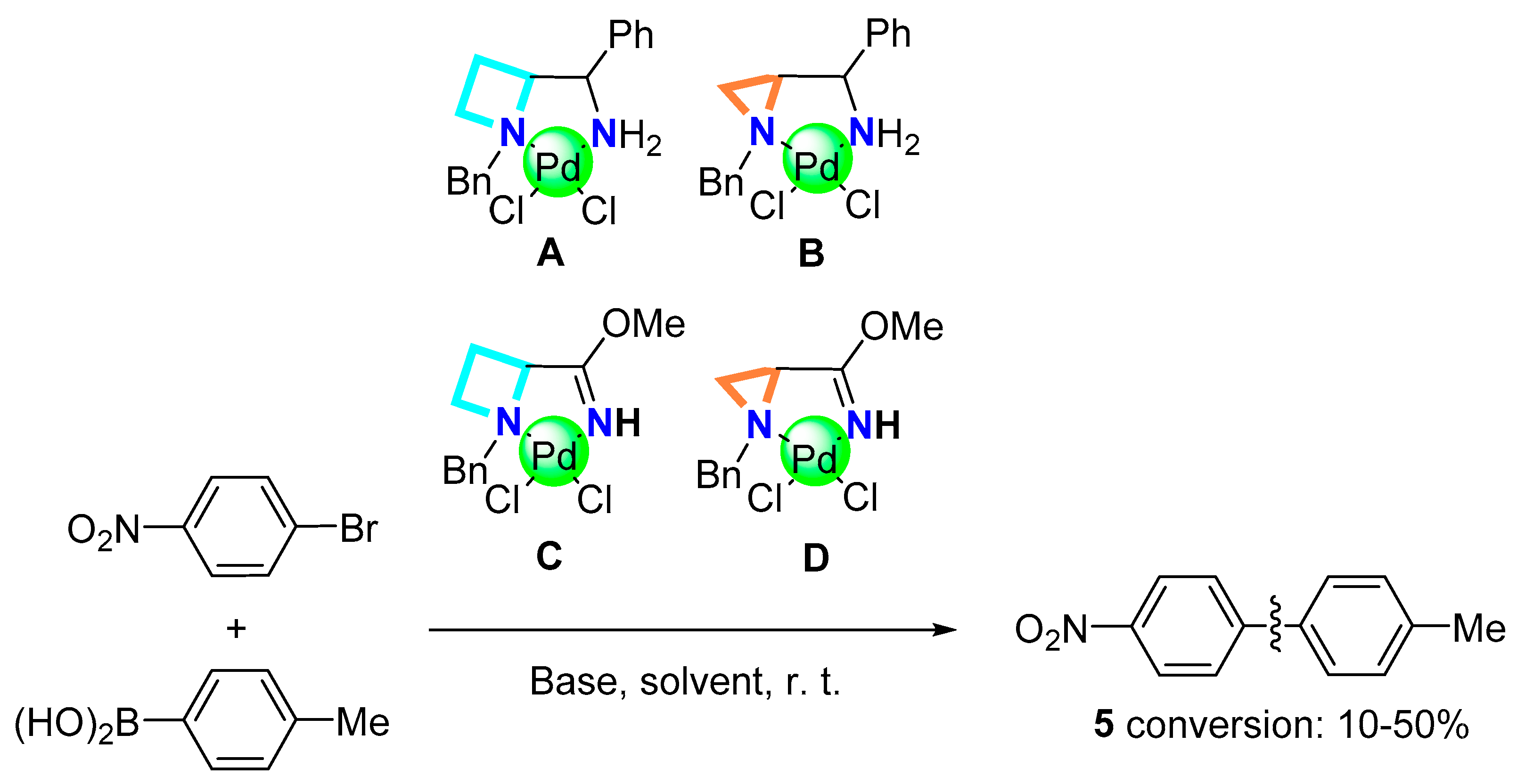
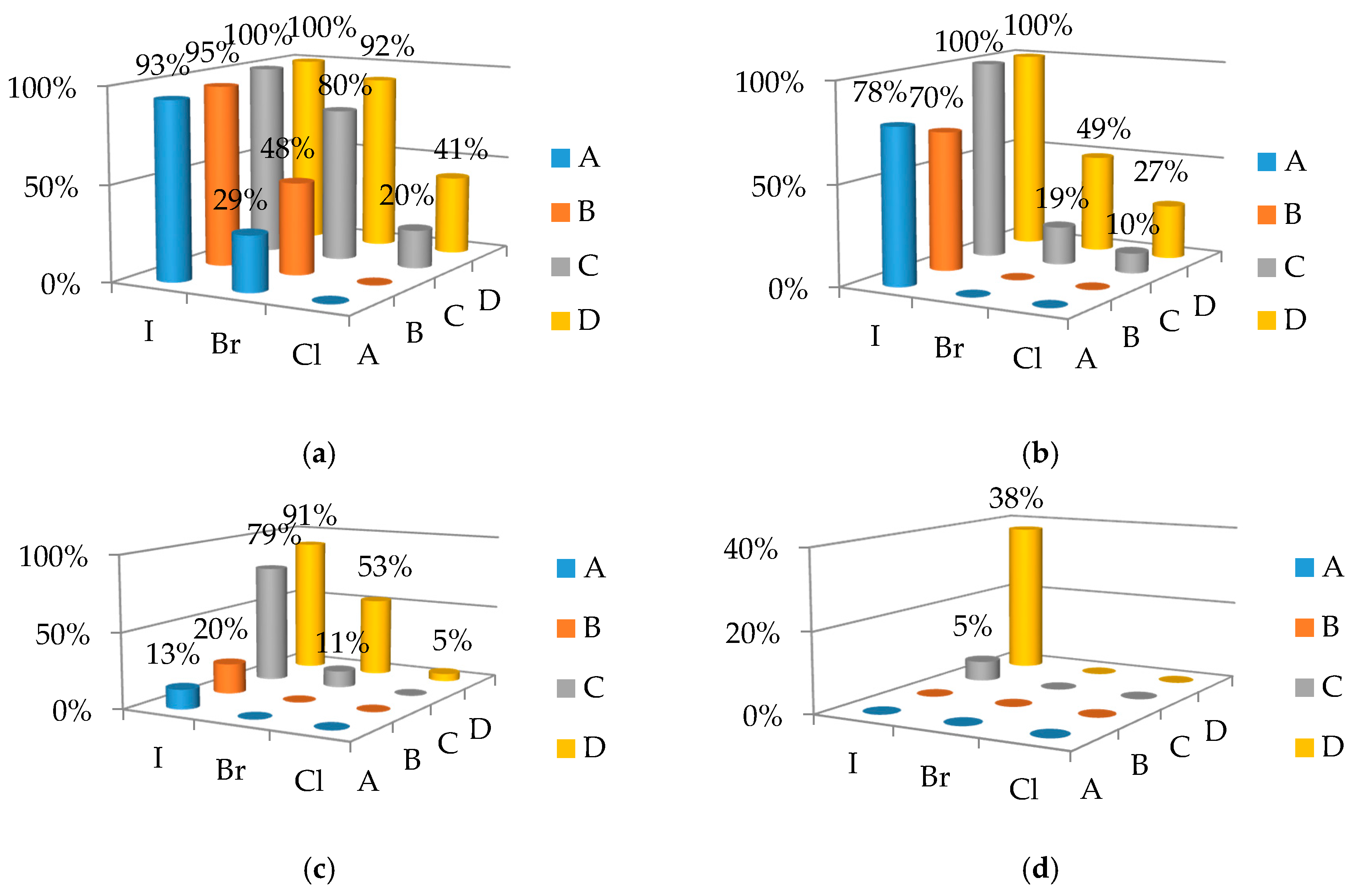
2.2. Evaluation of Catalytic Properties
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. General Procedure for Addition/Reduction Sequence
3.2.2. General Complexation Procedure
3.2.3. General Suzuki Coupling Procedure
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Suzuki, A. Cross-coupling reactions of organoboranes: An easy way to construct C–C bonds (Nobel Lecture). Angew. Chem. Int. Ed. 2011, 50, 6722–6737. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Ward, T.R. Recent advances in the palladium catalyzed Suzuki-Miyaura cross-coupling reaction in water. Catal. Lett. 2016. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Q. Palladium catalyzed asymmetric Suzuki-Miyaura coupling reactions to axially chiral birayl compounds: Chiral ligands and recent advances. Coord. Chem. Rev. 2015, 286, 1–16. [Google Scholar] [CrossRef]
- Maluenda, I.; Navarro, O. Recent developments in the Suzuki-Miyaura reaction: 2010–2014. Molécules 2015, 20, 7528–7557. [Google Scholar] [CrossRef] [PubMed]
- Han, F.-Y. Transition-metal-catalyzed Suzuki-Miyaura cross-coupling reactions: A remarkable advance from palladium to nickel catalysts. Chem. Soc. Rev. 2013, 42, 5270–5298. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar Rao, G.; Kumar, S.; Singh, A.K. Organosulphur and related ligands in Suzuki-Miyaura C–C coupling. Dalton Trans. 2013, 42, 5200–5223. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Bellina, F.; Lessi, M. Selective palladium-catalyzed Suzuki–Miyaura reactions of polyhalogenated heteroarenes. Adv. Synth. Catal. 2012, 354, 1181–1255. [Google Scholar] [CrossRef]
- Li, H.; Seechurn, C.C.C.J.; Colacot, T.J. Development of preformed Pd catalysts for cross-coupling reactions, beyond the 2010 Nobel prize. ACS Catal. 2012, 2, 1147–1164. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Li, H.-Y.; Shi, M.-M.; Jiang, H.; Hu, X.-L.; Li, W.-Q.; Fu, L.; Chen, H.-Z. Pd/C as a clean and effective heterogeneous catalyst for C–C couplings toward highly pure semiconducting polymers. Macromolecules 2012, 45, 9004–9009. [Google Scholar] [CrossRef]
- Arvela, R.K.; Leadbeater, N.E.; Sangi, M.S.; Williams, V.A.; Granados, P.; Singer, R.D. A reassessment of the transition-metal free Suzuki-type coupling methodology. J. Org. Chem. 2005, 70, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Jawale, D.V.; Gravel, E.; Boudet, C.; Shah, N.; Geertsen, V.; Li, H.; Namboothiri, I.N.N.; Doris, E. Room temperature Suzuki coupling of aryl iodides, bromides and chlorides using a heterogeneous carbon nanotube-palladium nanohybrid catalyst. Catal. Sci. Technol. 2015, 5, 2388–2392. [Google Scholar] [CrossRef] [Green Version]
- Hong, M.C.; Choi, M.C.; Chang, Y.W.; Lee, Y.; Kim, J.; Rhee, H. Palladium nanoparticles on thermoresponsive hydrogels and their application as recyclable Suzuki-Miyaura coupling reaction catalysts in water. Adv. Synth. Catal. 2012, 354, 1257–1263. [Google Scholar] [CrossRef]
- Maegawa, T.; Kitamura, Y.; Sako, S.; Udzu, T.; Sakurai, A.; Tanaka, A.; Kobayashi, Y.; Endo, K.; Bora, U.; Kurita, T.; et al. Heterogeneous Pd/C-catalyzed ligand free, room-temperature Suzuki-Miyaura coupling reactions in aqueous media. Chem Eur. J. 2007, 13, 5937–5943. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.-X.; Gong, W.-J.; Li, H.-X.; Gao, J.; Li, F.-L.; Lang, J.-P. Palladium(II)-catalyzed Suzuki-Miyaura reactions of arylboronic acid with aryl halide in water in the presence of 4-(benzylthio)-N,N,N-trimethylbenzenammonium chloride. Tetrahedron 2014, 70, 3385–3389. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Petersen, T.B.; Abela, A.R. Room-temperature Suzuki-Miyaura couplings in water facilitated by non-ionic amphiphiles. Org. Lett. 2008, 10, 1333–1336. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zeng, Y.; Hu, Q.; Huang, F.; Jin, L.; Mo, W.; Sun, N.; Hu, B.; Shen, Z.; Hu, X.; et al. Efficient catalyst for both Suzuki and Heck cross-coupling reactions: Synthesis and catalytic behaviour of geometry-constrained iminopyridylpalladium chlorides. Adv. Synth. Catal. 2016, 358, 2642–2651. [Google Scholar] [CrossRef]
- Yang, J.; Liu, S.; Zheng, J.-F.; Zhou, J. Room-temperature Suzuki-Miyaura coupling of heteroaryl chlorides and tosylates. Eur. J. Org. Chem. 2012, 6248–6259. [Google Scholar] [CrossRef]
- Najera, C.; Gil-Molto, J.; Karlström, S. Suzuki-Miyaura and related cross-coupling in aqueous solvents catalyzed by di(2-pyridyl)methylamine-palladium dichloride complexes. Adv. Synth. Catal. 2004, 346, 1798–1811. [Google Scholar] [CrossRef]
- Puget, B.; Roblin, J.-P.; Prim, D.; Troin, Y. New 2-(2-pyridyl)piperidines: Synthesis, complexation of palladium and catalytic activity in Suzuki reaction. Tetrahedron Lett. 2008, 49, 1706–1709. [Google Scholar] [CrossRef]
- Terrasson, V.; Prim, D.; Marrot, J. N-Heterocyclic benzhydrylamines as New N,N-Bidentate ligands in palladium complexes: Synthesis, characterization and catalytic activity. Eur. J. Inorg. Chem. 2008, 2739–2745. [Google Scholar] [CrossRef]
- Gunawan, M.-A.; Qiao, C.; Abrunhosa-Thomas, I.; Puget, B.; Roblin, J.-P.; Prim, D.; Troin, Y. Simple pyridylmethylamines: Efficient and robust N,N-ligands for Suzuki-Miyaura coupling reactions. Tetrahedron Lett. 2010, 51, 5392–5394. [Google Scholar] [CrossRef]
- Grach, G.; Pieters, G.; Dinut, A.; Terrasson, V.; Medimagh, R.; Bridoux, A.; Razafimahaleo, V.; Gaucher, A.; Marque, S.; Marrot, J.; et al. N-Heterocyclic pyridylmethylamines: Synthesis, complexation, molecular structure, and application to asymmetric Suzuki-Miyaura and oxidative coupling reactions. Organometallics 2011, 30, 4074–4086. [Google Scholar] [CrossRef]
- Requet, A.; Yalgin, H.; Prim, D. Convenient and rapid strategies towards 6-(hetero)arylpyridylmethylamines. Tetrahedron Lett. 2015, 56, 1378–1382. [Google Scholar] [CrossRef]
- Couty, T.; David, O.; Larmanjat, B.; Marrot, J. Strained azetidinium ylides: New reagents for cyclopropanation. J. Org. Chem. 2007, 72, 1058–1061. [Google Scholar] [CrossRef] [PubMed]
- Ayi, A.I.; Guedj, R. Reaction of hydrogen fluoride in pyridine solution with cis-cyano-2-and cis-amido-2-aziridines. Preparation of β-fluoro-α-amino acids and esters by means of acidic hydrolysis and alcoholysis of β-fluoro-α-amino nitriles and/or β-fluoro-α-amino acid amides. J. Chem. Soc. Perkin Trans. 1983, 2045–2051. [Google Scholar] [CrossRef]
- Keller, L.; Vargas-Sanchez, M.; Prim, D.; Couty, F.; Evano, G.; Marrot, J. Azetidines as ligands in the palladium (II) complexes series. J. Organomet. Chem. 2005, 690, 2306–2311. [Google Scholar] [CrossRef]
- Pieters, G.; Puget, B.; Terrasson, V.; Roblin, J.-P.; Gaucher, A.; Marque, S.; Prim, D.; Troin, Y. On the robustness of methylamines-Pd catalytic systemsin the Suzuki reaction: Compromise examples between synthesis and catalysis. Rev. Chim. (Bucarest) 2010, 61, 825–827. [Google Scholar]
- Besev, M.; Engman, L. Diastereocontrol by a hydroxyl auxiliary in the synthesis of pyrrolidines via radical cyclization. Org. Lett. 2002, 4, 3023–3025. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, N.; Shelby, Q.; Stambuli, J.P.; Hartwig, J.F. Air stable, sterically hindered ferrocenyl dialkylphosphines for palladium-catalyzed C−C, C−N, and C−O bond-forming cross-couplings. J. Org. Chem. 2002, 67, 5553–5566. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, S.; Sudo, T.; Noyori, R.; Itami, K. Direct C−H Arylation of (Hetero)arenes with Aryl Iodides via Rhodium Catalysis. J. Am. Chem. Soc. 2006, 128, 11748–11749. [Google Scholar] [CrossRef] [PubMed]
- Tao, B.; Boykin, D.W. Simple amine/Pd(OAc)2-catalyzed Suzuki coupling reactions of aryl bromides under mild aerobic conditions. J. Org. Chem. 2004, 69, 4330–4335. [Google Scholar] [CrossRef] [PubMed]
- Mino, T.; Shirae, Y.; Saito, T.; Sakamoto, M.; Fujita, T. Palladium-catalyzed Sonogashira and Hiyama reactions using phosphine-free hydrazone ligands. J. Org. Chem. 2006, 71, 9499–9502. [Google Scholar] [CrossRef] [PubMed]
- Mendes Da Silva, J.F.; Perez, A.F.Y.; Pinto de Almeida, N. An efficient and new protocol for phosphine-free Suzuki coupling reaction using palladium-encapsulated and air-stable MIDA boronates in an aqueous medium. RSC Adv. 2014, 4, 28148–28155. [Google Scholar] [CrossRef]
| Entry | Halide Fragment | Boronic Acid Fragment | Halide | Cat. Load. | Conditions | Yield (%) |
|---|---|---|---|---|---|---|
| 1 |  | Cl | 0.1% | r.t., 24 h | 28 | |
| 2 | Cl | 1% | r.t., 18 h | 88 | ||
| 3 |  | Cl | 0.1% | r.t., 24 h | 21 | |
| 4 | Cl | 1% | r.t., 24 h | 69 [29] | ||
| 5 |  | Cl | 0.1% | r.t., 24 h | 21 | |
| 6 | Cl | 1% | r.t., 24 h | 60 | ||
| 7 | Br | 0.01% | 100 °C, 24 h | 89 | ||
| 8 | Br | 0.1% | 100 °C, 6 h | 93 a [30] | ||
| 9 |  | Cl | 1% | r.t., 24 h | 30 | |
| 10 | Br | 0.1% | r.t., 24 h | 43 | ||
| 11 | Br | 1% | r.t., 24 h | 61 | ||
| 12 |  | Cl | 0.1% | r.t., 24 h | 16 | |
| 13 | Cl | 1% | r.t., 24 h | 45 | ||
| 14 | Br | 0.1% | r.t., 24 h | 58 | ||
| 15 |  | Br | 0.1% | r.t., 24 h | 52 | |
| 16 | Br | 1% | r.t., 24 h | 81 [31] | ||
| 17 |  | Cl | 1% | 100°C, 18 h | 32 | |
| 18 | Br | 1% | r.t., 24 h | 39 [32] | ||
| 19 |  | Cl | 1% | 100 °C, 18 h | 25 | |
| 20 | Br | 1% | 100 °C, 18 h | 57 [33] | ||
| 21 |  | Br | 0.1% | r.t., 18 h | 45 | |
| 22 |  | Cl | 1% | r.t., 24 h | 30 | |
| 23 | Cl | 0.1% | 100 °C, 6 h | 89 | ||
| 24 | Br | 0.01% | 100 °C, 24 h | 90 | ||
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boufroura, H.; Large, B.; Bsaibess, T.; Perato, S.; Terrasson, V.; Gaucher, A.; Prim, D. Aziridine- and Azetidine-Pd Catalytic Combinations. Synthesis and Evaluation of the Ligand Ring Size Impact on Suzuki-Miyaura Reaction Issues. Catalysts 2017, 7, 27. https://doi.org/10.3390/catal7010027
Boufroura H, Large B, Bsaibess T, Perato S, Terrasson V, Gaucher A, Prim D. Aziridine- and Azetidine-Pd Catalytic Combinations. Synthesis and Evaluation of the Ligand Ring Size Impact on Suzuki-Miyaura Reaction Issues. Catalysts. 2017; 7(1):27. https://doi.org/10.3390/catal7010027
Chicago/Turabian StyleBoufroura, Hamza, Benjamin Large, Talia Bsaibess, Serge Perato, Vincent Terrasson, Anne Gaucher, and Damien Prim. 2017. "Aziridine- and Azetidine-Pd Catalytic Combinations. Synthesis and Evaluation of the Ligand Ring Size Impact on Suzuki-Miyaura Reaction Issues" Catalysts 7, no. 1: 27. https://doi.org/10.3390/catal7010027







