The Reaction Mechanism of Acetaldehyde Ammoximation to Its Oxime in the TS-1/H2O2 System
Abstract
:1. Introduction
2. Results and Discussion
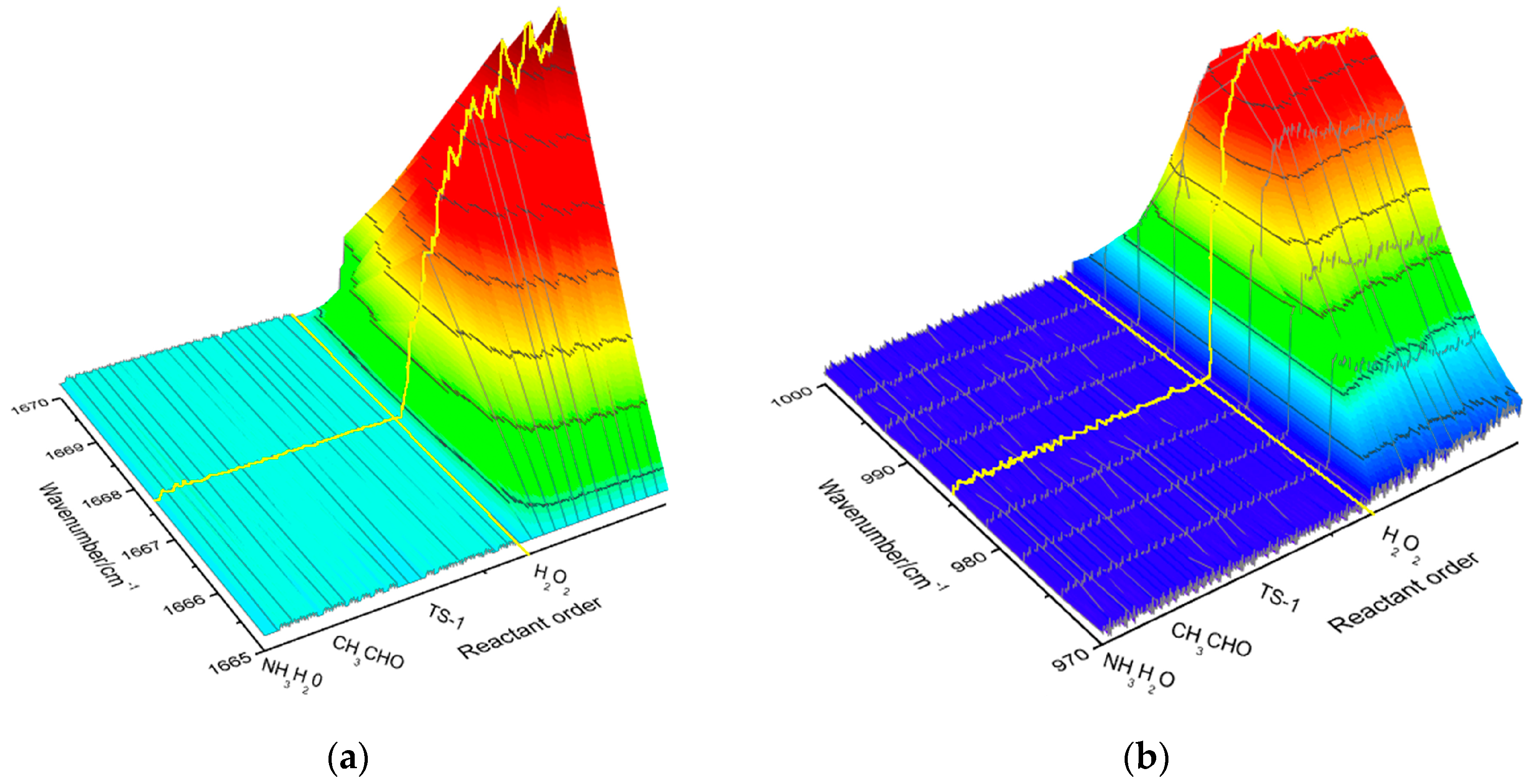
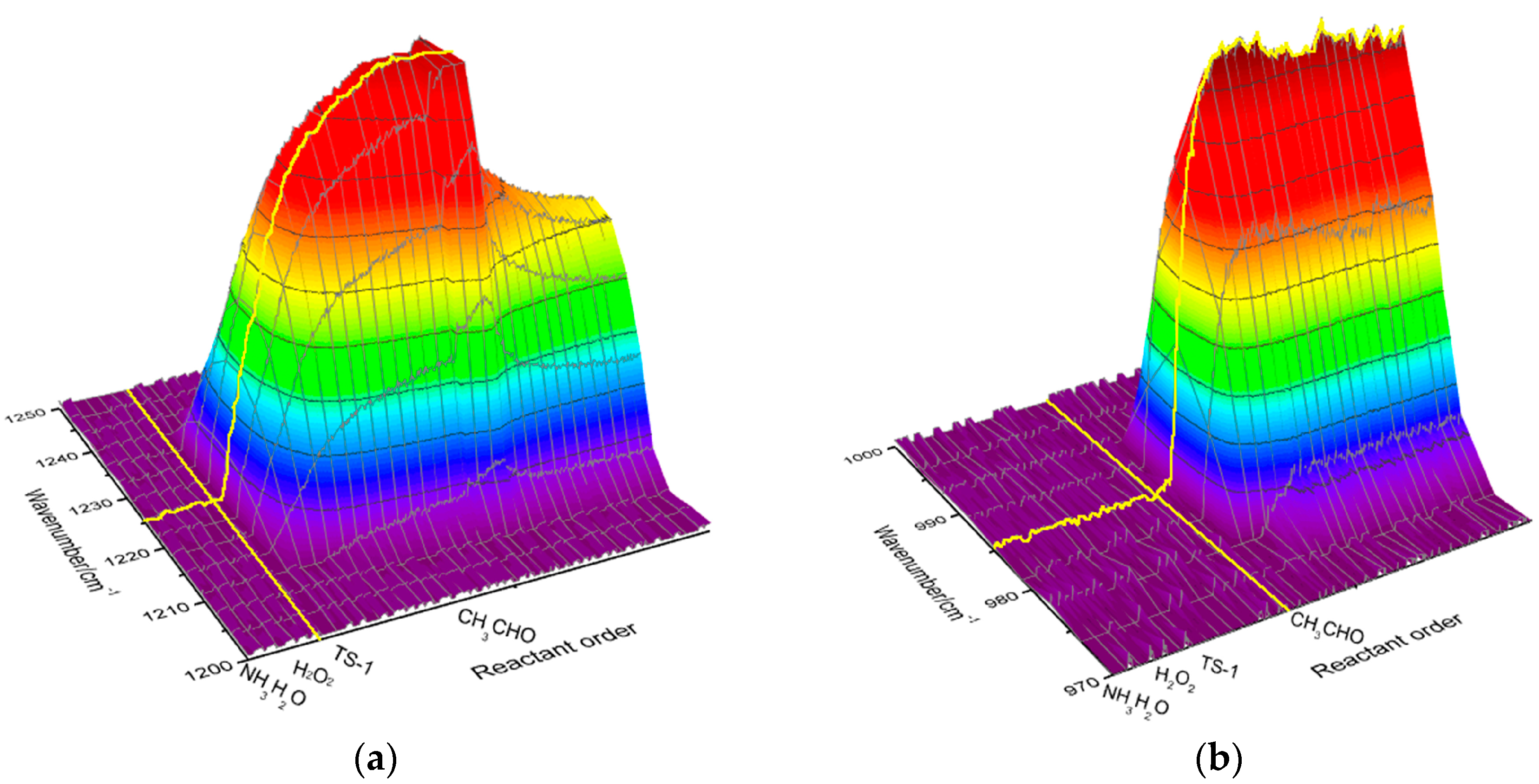
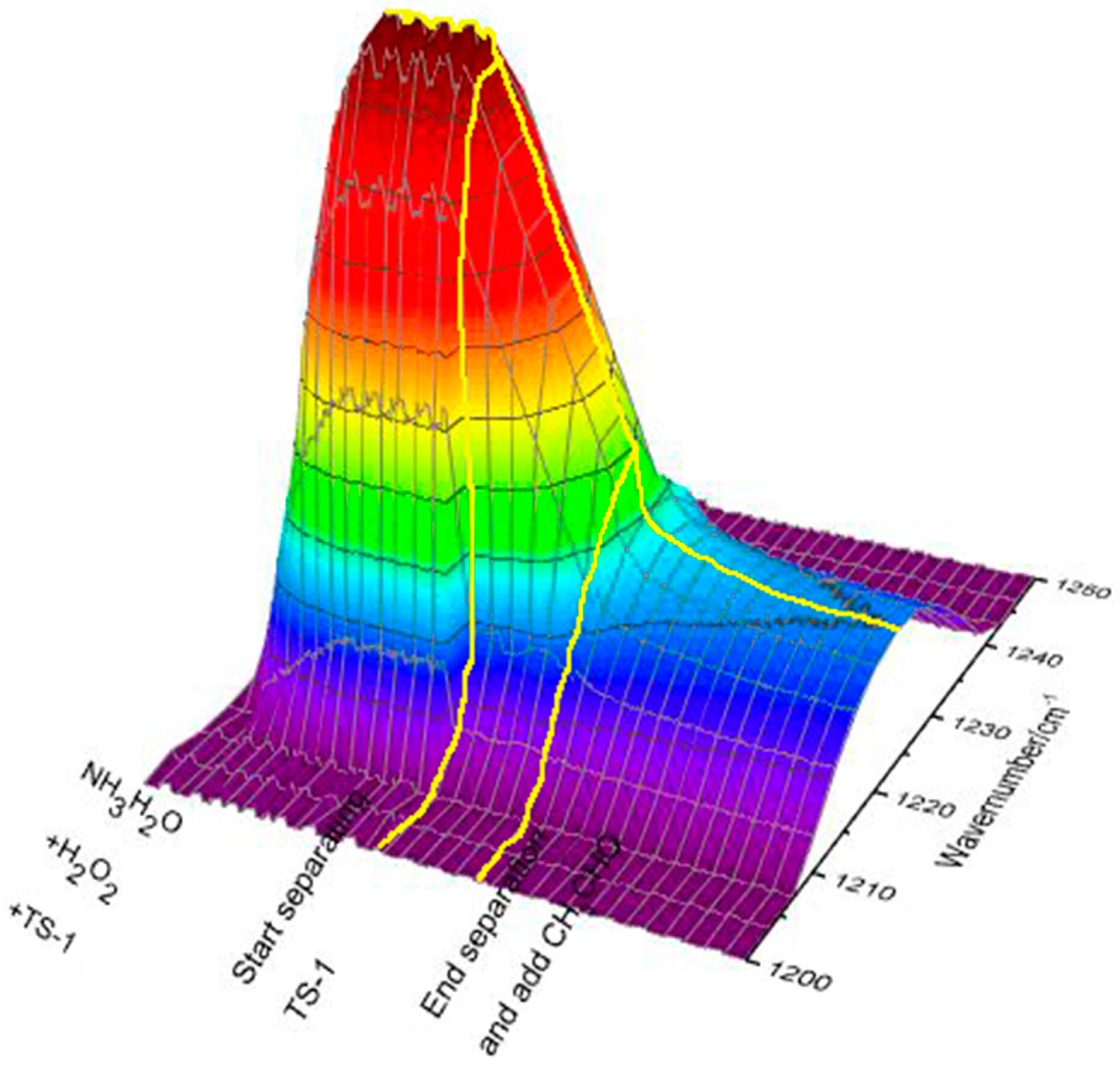
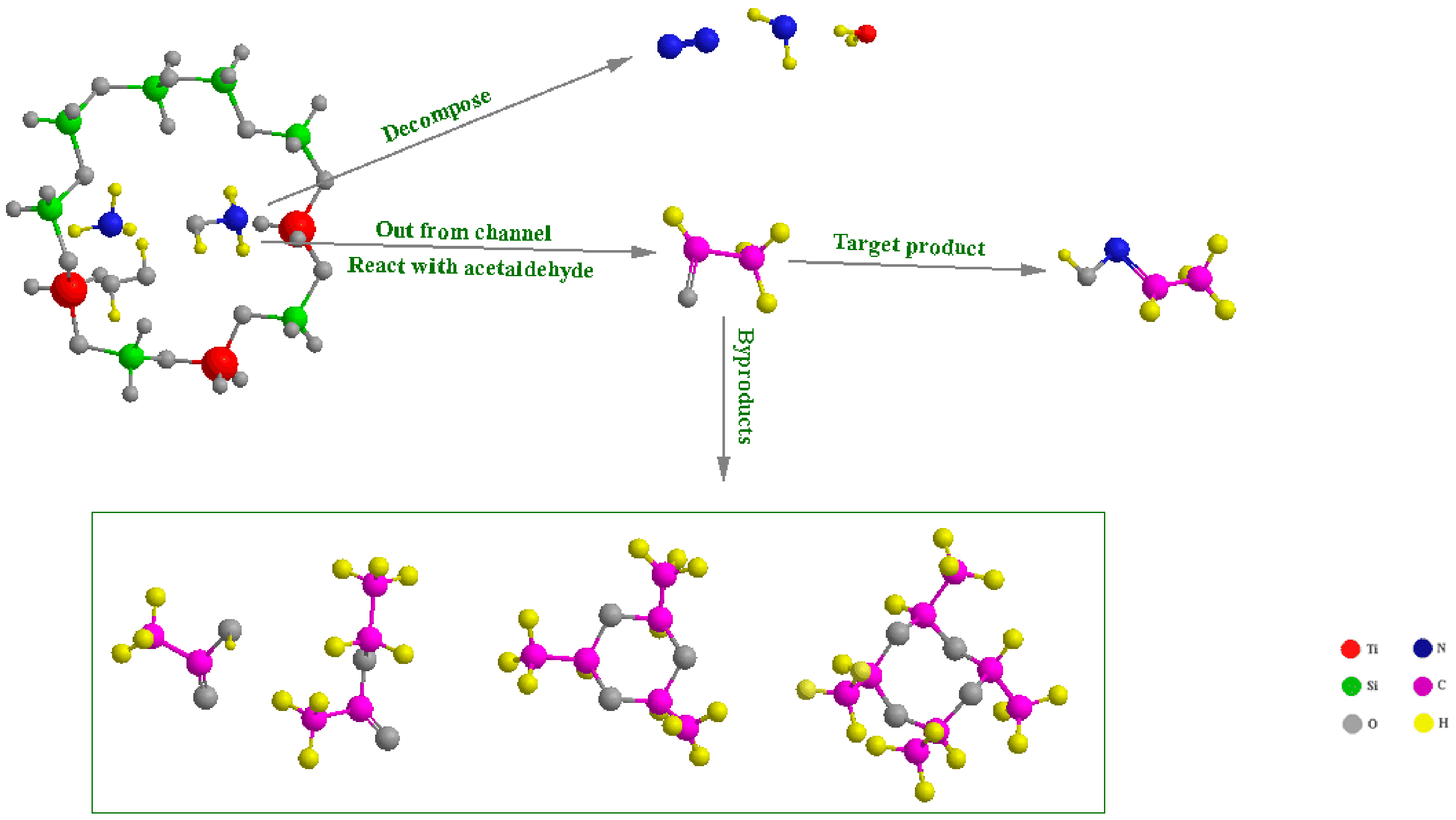
2.1. Analysis of Imine Reaction Mechanism
2.2. Analysis of Hydroxylamine Reaction Mechanism
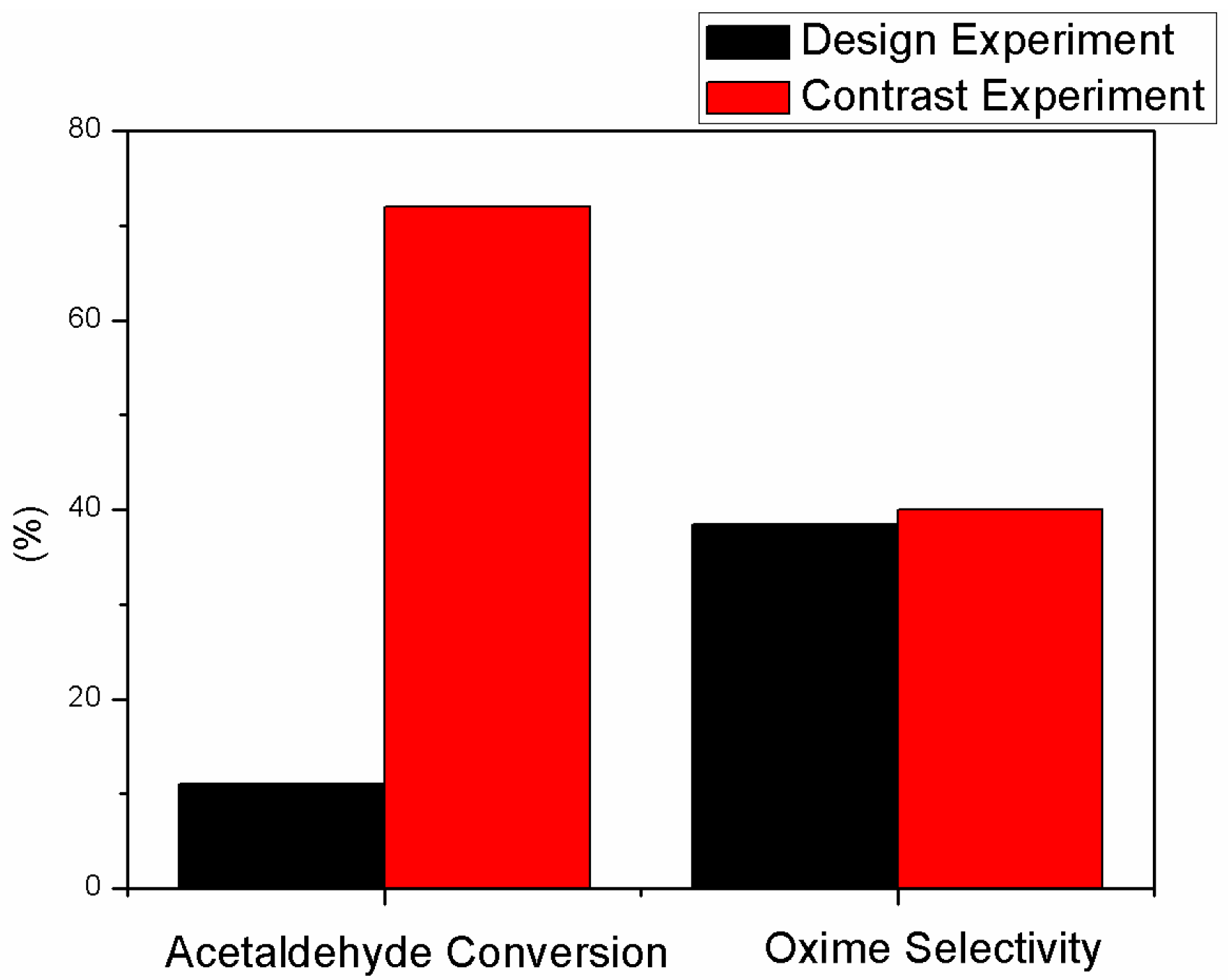
2.3. The Comparative Test and Results
3. Experimental
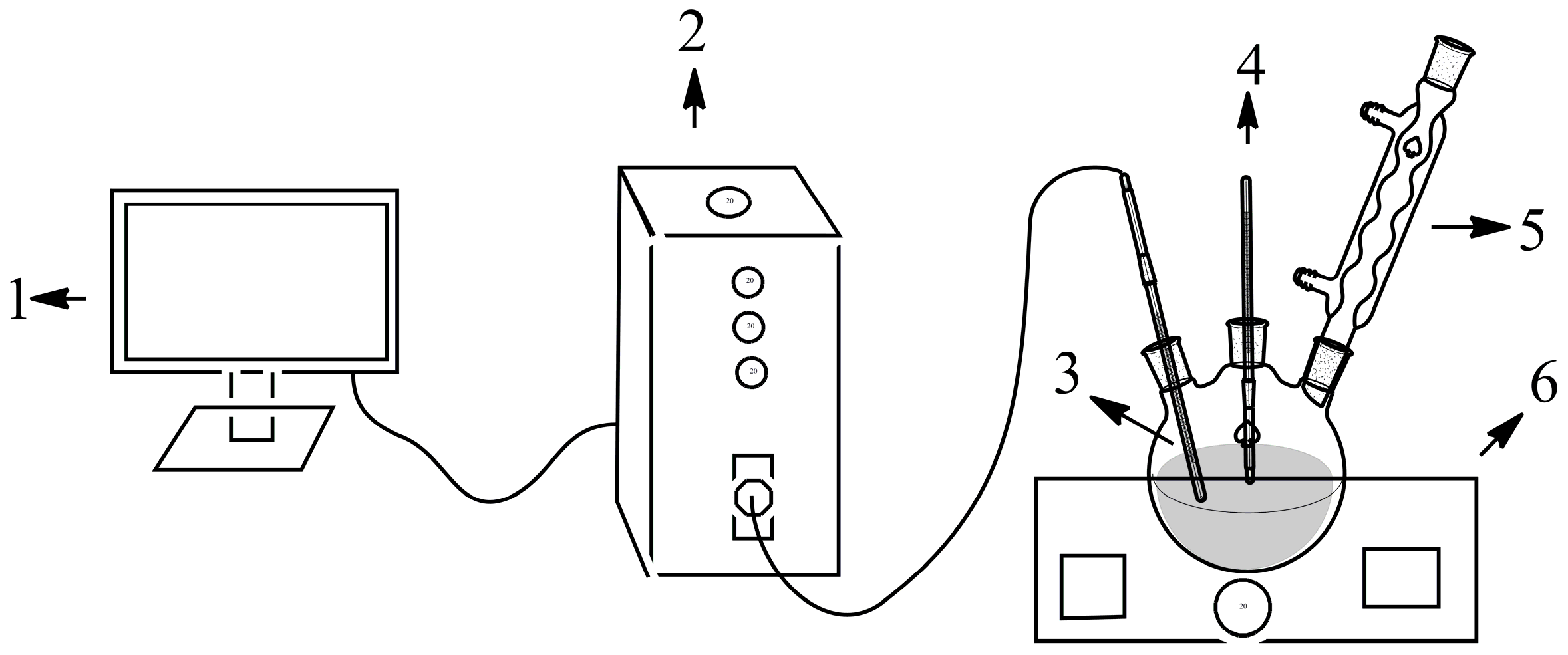
3.1. Materials and Measurements
3.2. Experimental Procedures
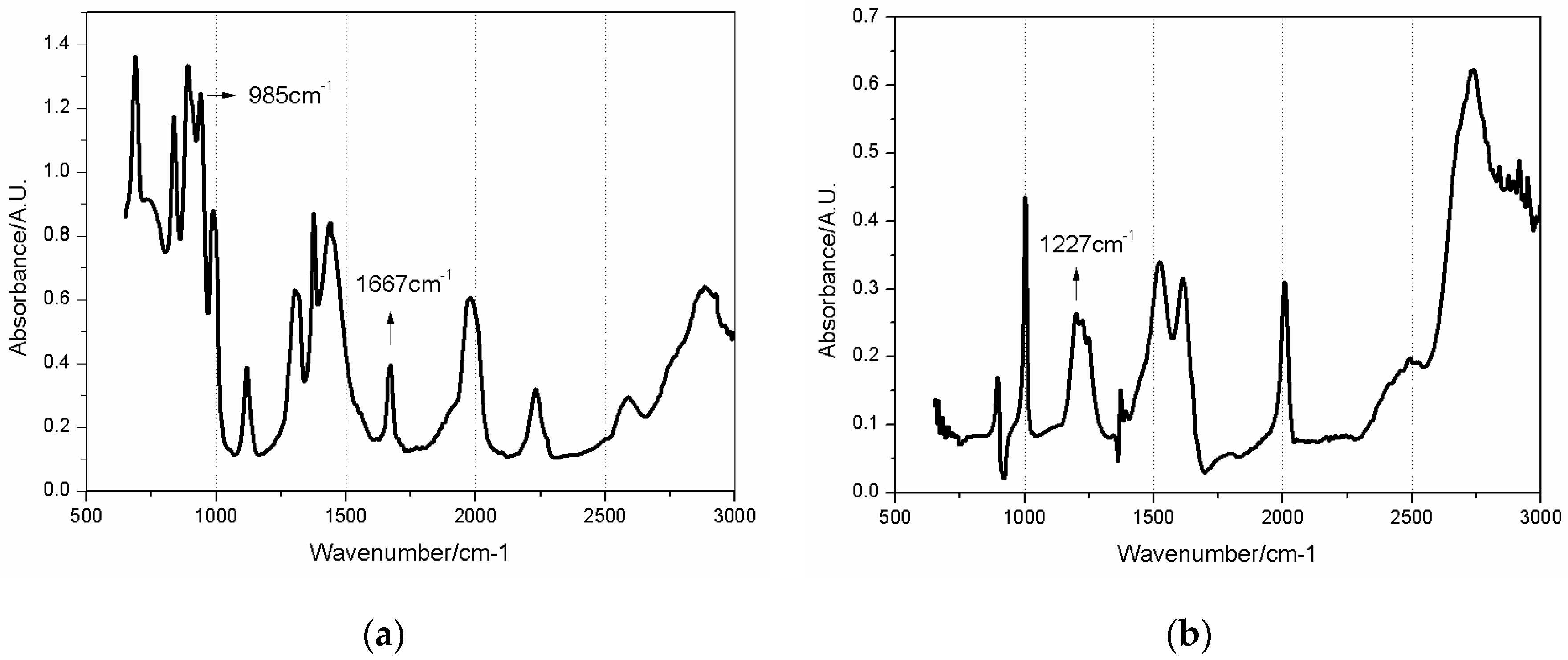
3.3. Spectral Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, S.; Kim, H. An efficient Pd-catalyzed hydration of nitrile with acetaldoxime. Tetrahedron Lett. 2009, 50, 2973–2975. [Google Scholar] [CrossRef]
- Ma, X.; He, Y. Copper (II)-catalyzed hydration of nitriles with the aid of acetaldoxime. Tetrahedron Lett. 2012, 53, 449–452. [Google Scholar] [CrossRef]
- Soledade, M.; Sabine, P. Probing Crucial Metabolic Pathways in Fungal Pathogens of Crucifers: Biotransformation of Indole-3-Acetaldoxime, 4-Hydroxyphenylacetaldoxime, and Their Metabolites. Bioorg. Med. Chem. 2003, 11, 3115–3120. [Google Scholar]
- Shi, H.; Guo, H. Experiment of acetaldehyde oxime being reducer in boiler acid cleaning. Clean. World 2007, 23, 10–12. [Google Scholar]
- Shuo, Z.; Xiu, D. Ammoximation of citronellal with H2O2 catalyzed by TS-1. Chin. J. Catal. 2012, 33, 723–729. [Google Scholar]
- Aurelia, V.; Alina, M. A new route for the synthesis of titanium silicalite-1. Mater. Res. Bull. 2012, 17, 35–41. [Google Scholar]
- Wang, Y.; Zhang, S. The mechanism of catalyst deactivation and by-product formation in acetone ammoximation catalyzed by hollow titanium silicalite. J. Mol. Catal. A Chem. 2014, 385, 1–6. [Google Scholar] [CrossRef]
- Wu, Y. Improve on the synthesis method of acetaldoxime. Chem. Adhes. 2005, 01, 61–62. [Google Scholar]
- Yi, Z.; Xiang, W. Synthesis of titanium silicalite-1 with small crystal size by using mother liquor of titanium silicalite-1 as seeds (II): Influence of synthesis conditions on properties of titaniumsilicalite-1. Microporous Mesoporous Mater. 2012, 162, 105–114. [Google Scholar]
- Qiang, L.; Gang, L. Synthesis of hierarchical TS-1 with convenient separation and the application for the oxidative desulfurization of bulky and small reactants. Fule 2014, 130, 70–75. [Google Scholar]
- Romano, U.; Esposito, A. Selective Oxidation with Ti-Silicalite. Stud. Surf. Sci. Catal. 1990, 55, 33–41. [Google Scholar]
- Liu, X.; Wang, X. Effect of solvent on the propylene epoxidation over TS-1 catalyst. Catal. Today 2004, 93–95, 505–509. [Google Scholar] [CrossRef]
- Kwon, S.; Schweitzer, N. A kinetic study of vapor-phase cyclohexene epoxidation by H2O2 over mesoporous TS-1. J. Catal. 2015, 326, 107–115. [Google Scholar] [CrossRef]
- Chen, R.; Bu, Z. Scouring-ball effect of microsized silica particles on operation stability of the membrane reactor for acetone ammoximation over TS-1. Chem. Eng. J. 2010, 156, 418–422. [Google Scholar] [CrossRef]
- Wang, X.; Meng, B. Direct Hydroxylation of Benzene to Phenol Using Palladium–Titanium Silicalite Zeolite Bifunctional Membrane Reactors. Ind. Eng. Chem. Res. 2014, 53, 5636–5645. [Google Scholar] [CrossRef]
- Nandi, S.; Mukherjee, P. Reaction Modeling and Optimization Using Neural Networks and Genetic Algorithms: Case Study Involving TS-1-Catalyzed Hydroxylation of Benzene. Ind. Eng. Chem. Res. 2002, 41, 2159–2169. [Google Scholar] [CrossRef]
- Tsai, S.; Chao, P. Effects of pore structure of post-treated TS-1 on phenol hydroxylation. Catal. Today 2009, 148, 174–178. [Google Scholar] [CrossRef]
- Liu, G.; Wu, J. Ammoximation of Cyclohexanone to Cyclohexanone Oxime Catalyzed by Titanium Silicalite-1 Zeolite in Three-phase System. Chin. J. Chem. Eng. 2012, 20, 889–894. [Google Scholar] [CrossRef]
- Jiang, H.; She, F. One-step Continuous Phenol Synthesis Technology via Selective Hydroxylation of Benzene over Ultrafine TS-1 in a Submerged Ceramic Membrane Reactor. Chin. J. Chem. Eng. 2014, 22, 1199–1207. [Google Scholar] [CrossRef]
- Li, Z.; Chen, R. Continuous Acetone Ammoximation over TS-1 in a Tubular Membrane Reactor. Ind. Eng. Chem. Res. 2010, 49, 6309–6316. [Google Scholar] [CrossRef]
- Bars, J.; Dakka, J. Ammoximation of cyclohexanone and hydroxyaromatic ketones over titanium molecular sieves. Appl. Catal. A Gen. 1996, 136, 69–80. [Google Scholar] [CrossRef]
- Shin, S.; Chadwick, D. Kinetics of Heterogeneous Catalytic Epoxidation of Propene with Hydrogen Peroxide over Titanium Silicalite (TS-1). Ind. Eng. Chem. Res. 2010, 49, 8125–8134. [Google Scholar] [CrossRef]
- Russo, V.; Tesser, R. Kinetics of Propene Oxide Production via Hydrogen Peroxide with TS-1. Ind. Eng. Chem. Res. 2014, 53, 6274–6287. [Google Scholar] [CrossRef]
- Stare, J.; Neil, J. Mechanistic Aspects of Propene Epoxidation by Hydrogen Peroxide. Catalytic Role of Water Molecules, External Electric Field, and Zeolite Framework of TS-1. J. Chem. Inf. Model. 2009, 49, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Jakkapan, S.; Jumras, L. Mechanisms of the ammonia oxidation by hydrogen peroxide over the perfect and defective Ti species of TS-1 zeolite. Phys. Chem. Chem. Phys. 2013, 15, 18093–18100. [Google Scholar]
- Chang, C.; Hai, Z. Density functional theory studies on hydroxylamine mechanism of cyclohexanone ammoximation on titanium silicalite-1 catalyst. J. Mol. Model. 2013, 19, 2217–2224. [Google Scholar]
- Pozzo, L.; Fornasari, G. TS-1 catalytic mechanism in cyclohexanone oxime production. Catal. Commun. 2002, 3, 369–375. [Google Scholar] [CrossRef]
- Xiang, Z.; Rui, M. In-situ FTIR study on mechanism of cyclohexanone ammoximation to its oxime on TS-1 in liquid phase. J. Hebei Univ. Sci. Technol. 2011, 06, 605–610. [Google Scholar]
- Tvarłzˇková, Z.; Habersberger, K.; Zˇilkova, N.; Jírł, P. Role of surface complexes on titanium-silicate in the ammoximation of cyclohexanone with hydrogen peroxide. Appl. Catal. A Gen. 1991, 79, 105–114. [Google Scholar] [CrossRef]
- Alex, C.; Xi, H. Catalytic Activity of Clay-Based Titanium Silicalite-1 Composite in Cyclohexanone Ammoximation. Ind. Eng. Chem. Res. 2009, 48, 8441–8450. [Google Scholar]
- Liu, H.; Liu, P. Studies on the liquid-phase ammoximation of cyclohexanone over a titanium silicate sieve using on-line ATR–FTIR spectroscopy. Catal. Commun. 2010, 11, 887–891. [Google Scholar] [CrossRef]
- Thangaraj, A.; Sivasanker, S. Catalytic Properties of Crystalline Titanium Silicalites III. Ammoximation of Cyclohexanone. J. Catal. 1991, 131, 394–400. [Google Scholar] [CrossRef]
- Chang, X.; Long, J. Heterogeneous oxidation of cyclohexanone catalyzed by TS-1: Combined experimental and DFT studies. Chin. J. Catal. 2015, 36, 845–854. [Google Scholar]
- Bolis, V.; Bordiga, S. A calorimetric, IR, XANES and EXAFS study of the adsorption of NH3 on Ti-silicalite as a function of the sample pre-treatment. Microporous Mesoporous Mater. 1999, 30, 67–76. [Google Scholar] [CrossRef]
- Zecchina, A.; Bordiga, S. Structural characterization of Ti centres in Ti-silicalite and reaction mechanisms in cyclohexanone ammoximation. Catal. Today 1996, 32, 97–106. [Google Scholar] [CrossRef]
- Yong, Z.; Ya, W. Reaction mechanism of the ammoximation of ketones catalyzed by TS-1. React. Kinet. Catal. Lett. 2006, 87, 25–32. [Google Scholar]
- Le, X.; Jiang, D. Distinctions of hydroxylamine formation and decomposition in cyclohexanone ammoximation over microporous titanosilicates. J. Catal. 2014, 309, 1–10. [Google Scholar] [CrossRef]
- Adamopoulou, T.; Manolis, K. Thermal decomposition of hydroxylamine: Isoperibolic calorimetric measurements at different conditions. J. Hazard. Mater. 2013, 254–255, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Adamopoulou, T.; Papadaki, M. Use of isoperibolic calorimetry for the study of the effect of water concentration, temperature and reactor venting on the rate of hydroxylamine thermal decomposition. J. Loss Prev. Process Ind. 2012, 25, 803–808. [Google Scholar] [CrossRef]
- Liu, L.; Papadaki, M. Isothermal decomposition of hydroxylamine and hydroxylamine nitrate in aqueous solutions in the temperature range 80–160 °C. J. Hazard. Mater. 2009, 165, 573–578. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, C.; Yang, S.; He, G.; Luo, G.; Xu, X.; Jin, H. The Reaction Mechanism of Acetaldehyde Ammoximation to Its Oxime in the TS-1/H2O2 System. Catalysts 2016, 6, 109. https://doi.org/10.3390/catal6070109
Meng C, Yang S, He G, Luo G, Xu X, Jin H. The Reaction Mechanism of Acetaldehyde Ammoximation to Its Oxime in the TS-1/H2O2 System. Catalysts. 2016; 6(7):109. https://doi.org/10.3390/catal6070109
Chicago/Turabian StyleMeng, Chaoqun, Suohe Yang, Guangxiang He, Guohua Luo, Xin Xu, and Haibo Jin. 2016. "The Reaction Mechanism of Acetaldehyde Ammoximation to Its Oxime in the TS-1/H2O2 System" Catalysts 6, no. 7: 109. https://doi.org/10.3390/catal6070109





