Aqueous-Phase Catalytic Chemical Reduction of p-Nitrophenol Employing Soluble Gold Nanoparticles with Different Shapes
Abstract
:1. Introduction
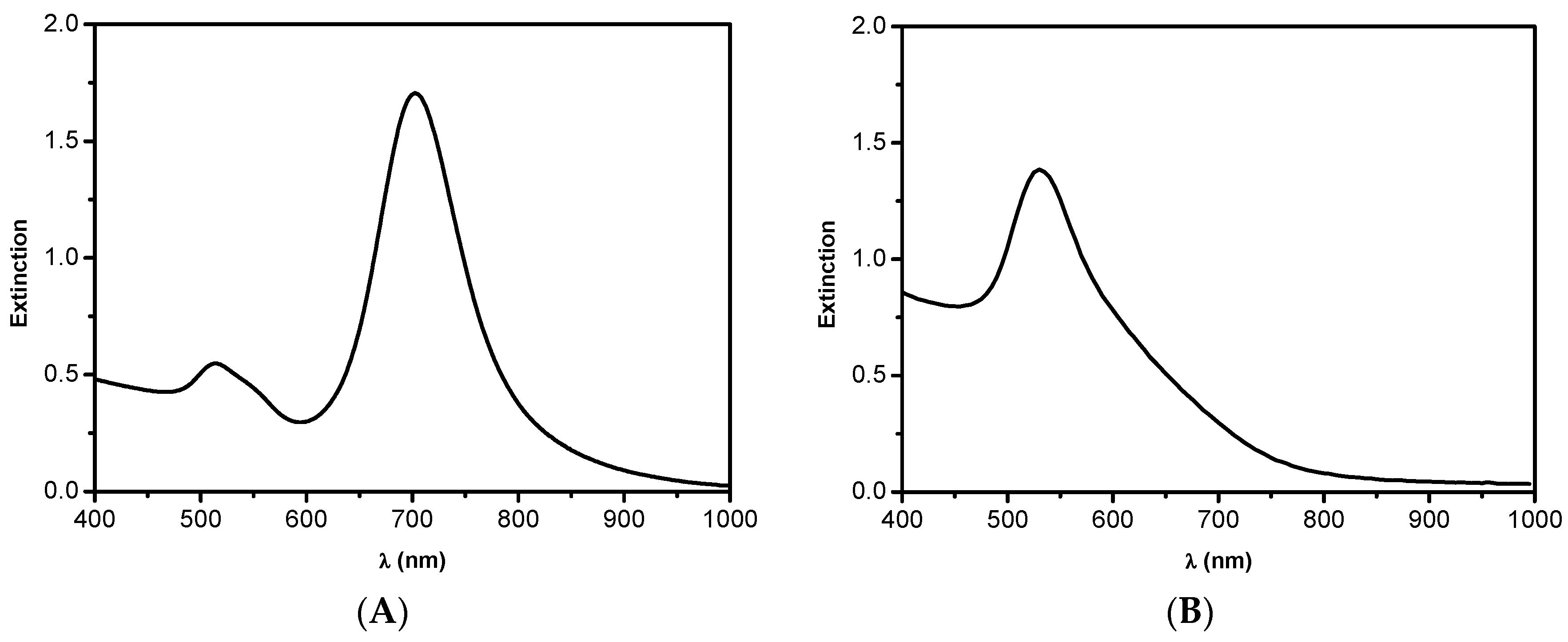
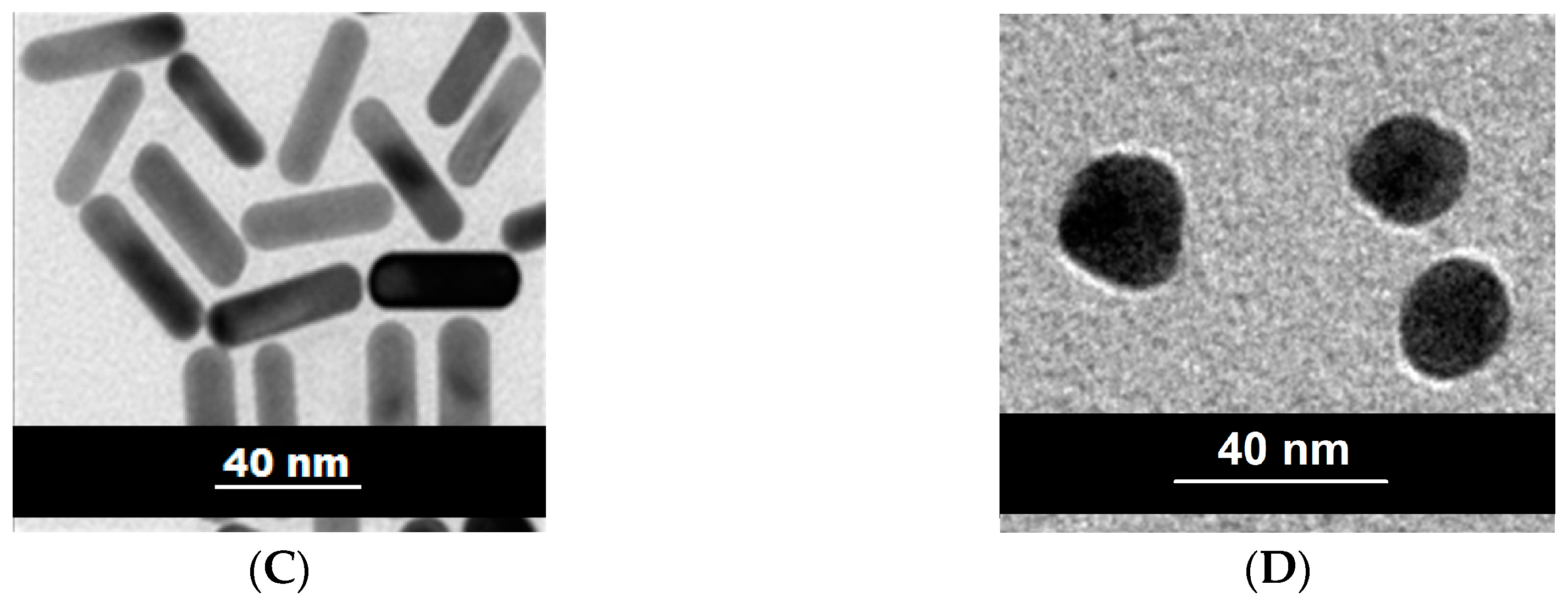
2. Results and Discussion
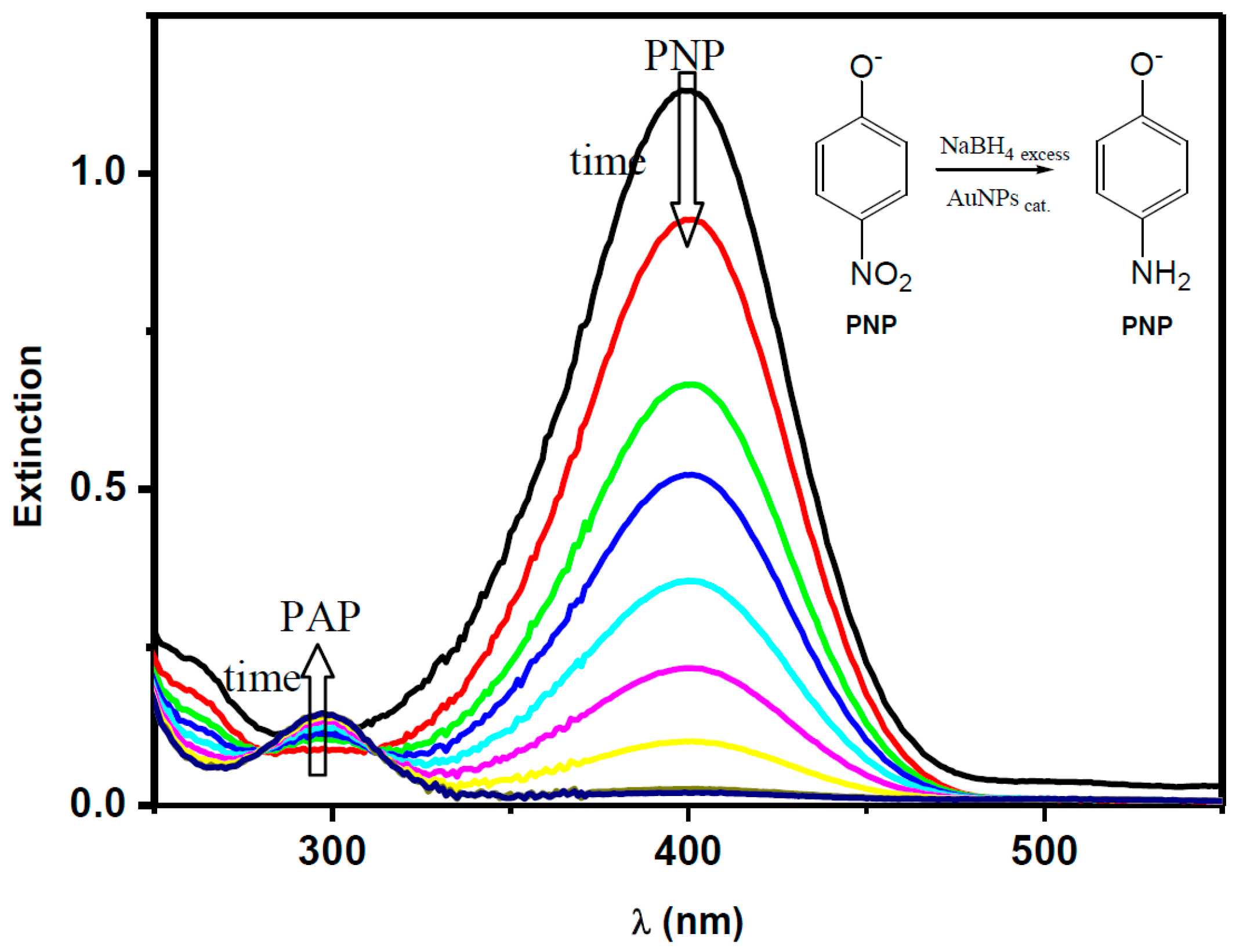
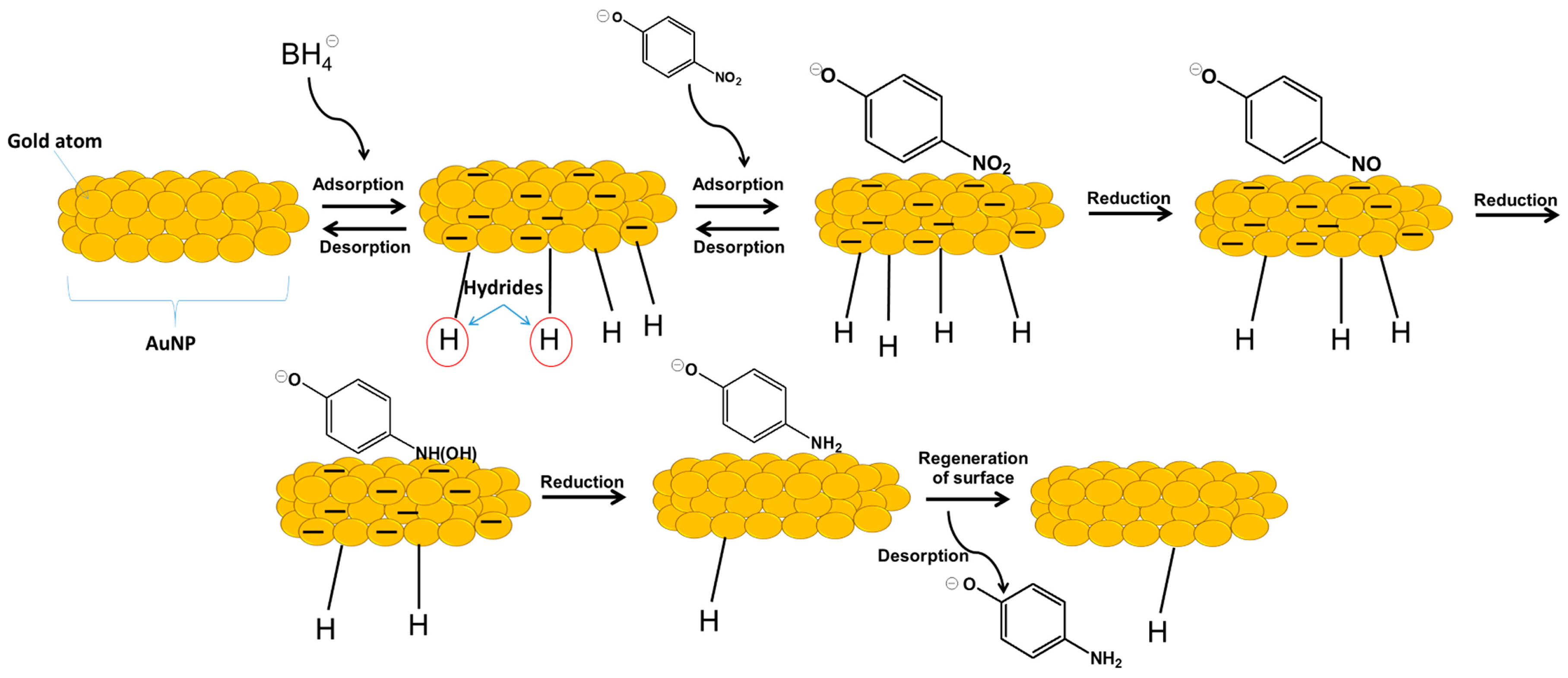
2.1. The Catalytic Activity
2.2. Influence of the NaBH4 Concentration
2.3. The Reaction Temperature
3. Materials and Methods
3.1. General
3.2. Seed-Mediated Synthesis
3.2.1. Seed Solution
3.2.2. Growth Solution
3.2.3. Growth Process
3.3. Reduction Process in the Presence of NaBH4
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Burda, C.; Chen, X.; Narayanan, R.; El-Sayed, M.A. Chemistry and properties of nanocrystals of different shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R.; El-Sayed, M.A. Effect of nanocatalysis in colloidal solution on the tetrahedral and cubic nanoparticle shape: Electron-transfer reaction catalyzed by platinum nanoparticles. J. Phys. Chem. B 2004, 108, 5726–5733. [Google Scholar] [CrossRef]
- Bilé, E.G.; Sassine, R.; Denicourt-Nowicki, A.; Launay, F.; Roucoux, A. New ammonium surfactant-stabilizedrhodium(0) colloidal suspensions: Influence of novel counter-anions on physico-chemical andcatalytic properties. Dalton Trans. 2011, 40, 6524–6531. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, L.; Liu, X.; Cao, M. Hybrids of gold nanoparticles with core-shell hyperbranched polymers: Synthesis, characterization, and their high catalytic activity for reduction of 4-nitrophenol. Catalysts 2016, 6, 3. [Google Scholar] [CrossRef]
- Haruta, M. Catalysis of gold nanoparticles deposited on metal oxides. Cattech 2002, 6, 102–115. [Google Scholar] [CrossRef]
- Rossi, M.; Matarrese, R.; Pina, C.D.; Comotti, M. The catalytic activity of “naked” gold particles. Angew. Chem. Int. Ed. 2004, 43, 5812–5815. [Google Scholar]
- Bond, G.C.; Louis, G.C.C.; Thompson, D.T. Reactions involving hydrogen. In Catalysis by Gold; World Scientific: Singapore, 2006; Volume 6, pp. 244–268. [Google Scholar]
- Hutchings, G.J. Gold catalysis in chemical processing. Catal. Today 2002, 72, 11–17. [Google Scholar] [CrossRef]
- Claus, P.; Brückner, A.; Mohr, C.; Hofmeister, H. Supported gold nanoparticles from quantum dot to mesoscopic size scale: Effect of electronic and structural properties on catalytic hydrogenation of conjugated functional groups. J. Am. Chem. Soc. 2000, 122, 11430–11439. [Google Scholar] [CrossRef]
- Chen, Y.; Qiu, J.; Wang, X.; Xiu, J. Preparation and application of highly dispersed gold nanoparticles supported on silica for catalytic hydrogenation of aromatic nitro compounds. J. Catal. 2006, 242, 227–230. [Google Scholar] [CrossRef]
- Jiang, H.-Y.; Zheng, X.-X. Phosphine-functionalized ionic liquid-stabilized rhodium nanoparticles for selective hydrogenation of aromatic compounds. Appl. Catal. A 2015, 499, 118–123. [Google Scholar] [CrossRef]
- Fountoulaki, S.; Daikopoulou, V.; Gkizis, P.L.; Tamiolakis, I.; Armatas, G.S.; Lykakis, I.N. Mechanistic studies of the reduction of nitroarenes by NaBH4 or hydrosilanes catalyzed by supported gold nanoparticles. ACS Catal. 2014, 4, 3504–3511. [Google Scholar] [CrossRef]
- Layek, K.; Kantam, M.L.; Shirai, M.; Nishio-Hamane, D.; Sasakid, T.; Maheswarana, H. Gold nanoparticles stabilized on nanocrystalline magnesium oxide as an active catalyst for reduction of nitroarenes in aqueous medium at room temperature. Green. Chem. 2012, 14, 3164–3174. [Google Scholar] [CrossRef]
- Tamiolakis, I.; Fountoulaki, S.; Vordos, N.; Lykakisb, I.N.; Armatas, G.S. Mesoporous Au–TiO2 nanoparticle assemblies as efficient catalysts for the chemoselective reduction of nitro compounds. J. Mater. Chem. A 2013, 1, 14311–14319. [Google Scholar] [CrossRef]
- Leung, K.C.-F.; Xuan, S.; Zhu, X.; Wang, D.; Chak, C.-P.; Lee, S.-F.; Ho, W.K.W.; Chung, B.C.T. Gold and iron oxide hybrid nanocomposite materials. Chem. Soc. Rev. 2012, 41, 1911–1928. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lin, C.; Ma, Z.; Yu, H.; Zhou, S. Gold nanoparticles on mesoporous SiO2-coated magnetic Fe3O4 spheres: A magnetically separatable catalyst with good thermal stability. Molecules 2013, 18, 14258–14267. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, I.S.; Park, J. A magnetically separable gold catalyst for chemoselective reduction of nitro compounds. J. Org. Biomol. Chem. 2013, 11, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.; El-Sayed, M.A. Shape-dependent catalytic activity of platinum nanoparticles in colloidal solution. Nano Lett. 2004, 4, 1343–1348. [Google Scholar] [CrossRef]
- Li, J.; Qu, Z.; Qin, Y.; Wang, H. Effect of MnO2 morphology on the catalytic oxidation of toluene over Ag/MnO2 catalysts. Appl. Surf. Sci. 2016, 385, 234–240. [Google Scholar] [CrossRef]
- Fenger, R.; Fertitta, E.; Kirmse, H.; Thünemannc, A.F.; Rademann, K. Size dependent catalysis with CTAB-stabilized gold nanoparticles. Phys. Chem. Chem. Phys. 2012, 14, 9343–9349. [Google Scholar] [CrossRef] [PubMed]
- Hinman, J.G.; Stork, A.J.; Varnell, J.A.; Gewirth, A.A.; Murphy, C.J. Seed mediated growth of gold nanorods: Towards nanorod matryoshkas. Faraday Discuss. 2016, 191, 9–33. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Sikdar, D.; Yap, L.W.; Guo, P.; Premaratne, M.; Li, X.; Cheng, W. Matryoshka-caged gold nanorods: Synthesis, plasmonic properties, and catalytic activity. Nano Res. 2016, 9, 415–423. [Google Scholar] [CrossRef]
- Kundu, S.; Lau, S.; Liang, H. Shape-controlled catalysis by cetyltrimethylammonium bromide terminated gold nanospheres, nanorods, and nanoprisms. J. Phys. Chem. C 2009, 113, 5150–5156. [Google Scholar] [CrossRef]
- Soetan, N.; Zarick, H.F.; Banks, C.; Webb, J.A.; Libson, G.; Coppola, A.; Bardhan, R. Morphology-directed catalysis with branched gold nanoantennas. J. Phys. Chem. C 2016, 120, 10320–10327. [Google Scholar] [CrossRef]
- Sault, A.; Madix, R.; Campbell, C. Adsorption of oxygen and hydrogen on Au(110)-(1 × 2). Surf. Sci. 1986, 169, 347–356. [Google Scholar] [CrossRef]
- Stobinski, L.; Zommer, L.; Dus, R. Molecular hydrogen interactions with discontinuous and continuous thin gold films. Appl. Surf. Sci. 1999, 141, 319–325. [Google Scholar] [CrossRef]
- Shokouhimehr, M. Magnetically separable and sustainable nanostructured catalysts for heterogeneous reduction of nitroaromatics. Catalysts 2015, 5, 534–560. [Google Scholar] [CrossRef]
- Goepel, M.; Al-Naji, M.; With, P.; Wagner, G.; Oeckler, O.; Enke, D.; Gläser, R. Hydrogenation of p-nitrophenol to p-aminophenol as a test reaction for the catalytic activity of supported Pt. Catal. Chem. Eng. Technol. 2014, 37, 551–554. [Google Scholar] [CrossRef]
- Sun, J.; Fu, Y.; He, G.; Sun, X.; Wang, X. Catalytic hydrogenation of nitrophenols and nitrotoluenes over a palladium/graphene nanocomposite. Catal. Sci. Technol. 2014, 4, 1742–1748. [Google Scholar] [CrossRef]
- Johnson, J.A.; Makis, J.J.; Marvin, K.A.; Rodenbusch, S.E.; Stevenson, K.J. Size-dependent hydrogenation of p-nitrophenol with Pd nanoparticles synthesized with poly(amido)amine dendrimer templates. J. Phys. Chem. C 2013, 117, 22644–22651. [Google Scholar] [CrossRef]
- Laidler, K.J. The World of Physical Chemistry; Oxford University Press: New York, NY, USA, 1993. [Google Scholar]
- Murphy, C.J.; Sau, T.K.; Gole, A.M.; Orendorff, C.J.; Gao, J.; Gou, L.; Hunyadi, S.E.; Li, T. Anisotropic metal nanoparticles: Synthesis, assembly, and optical applications. J. Phys. Chem. B 2005, 109, 13857–13870. [Google Scholar] [CrossRef] [PubMed]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
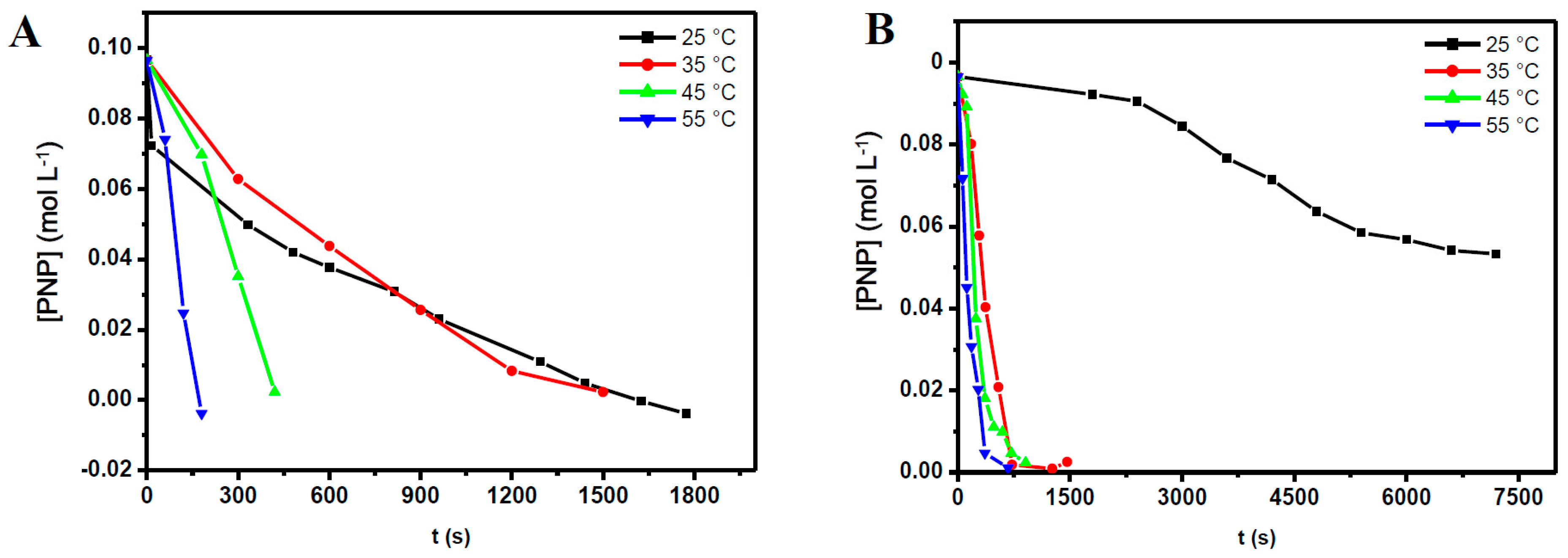
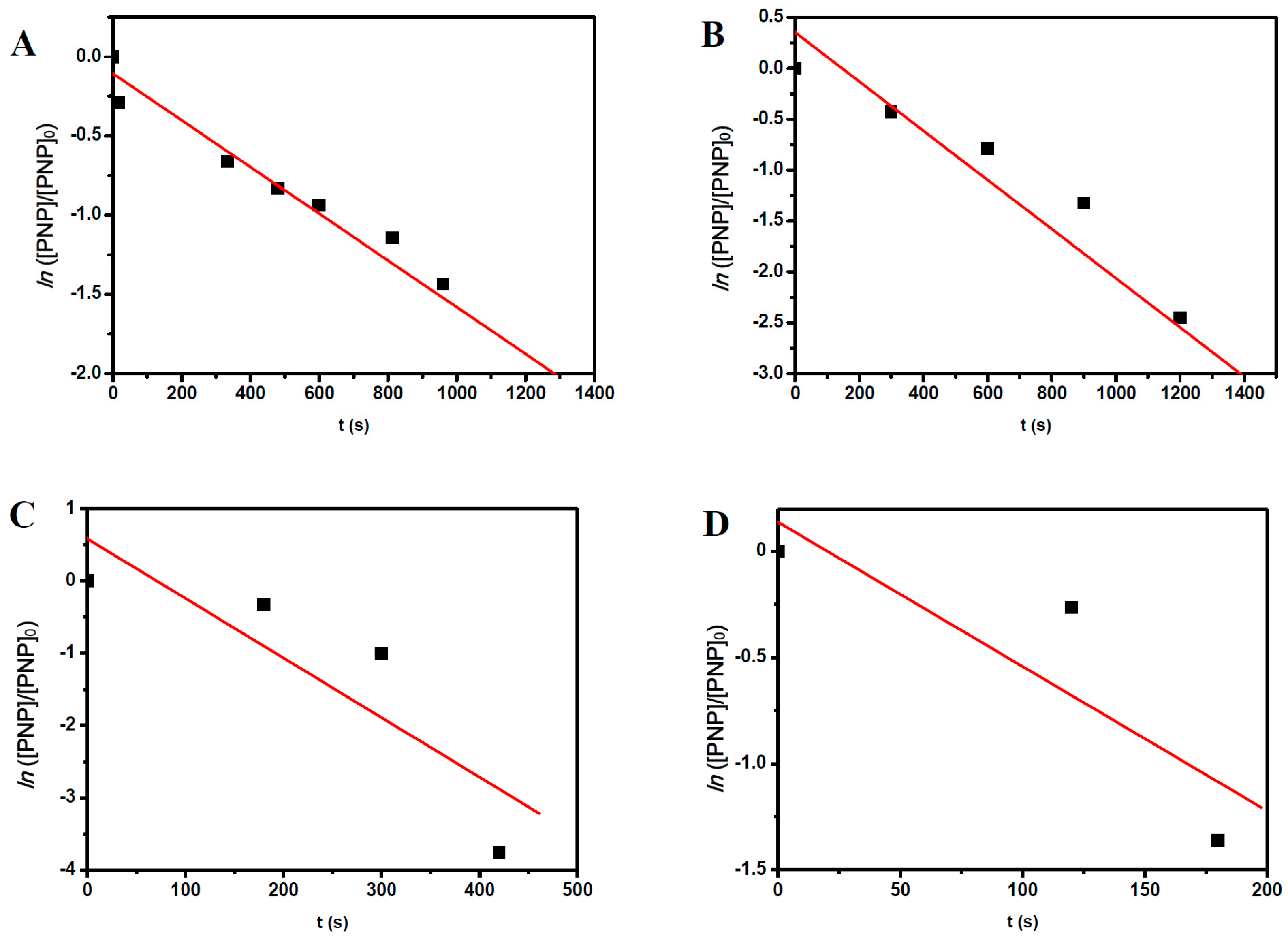
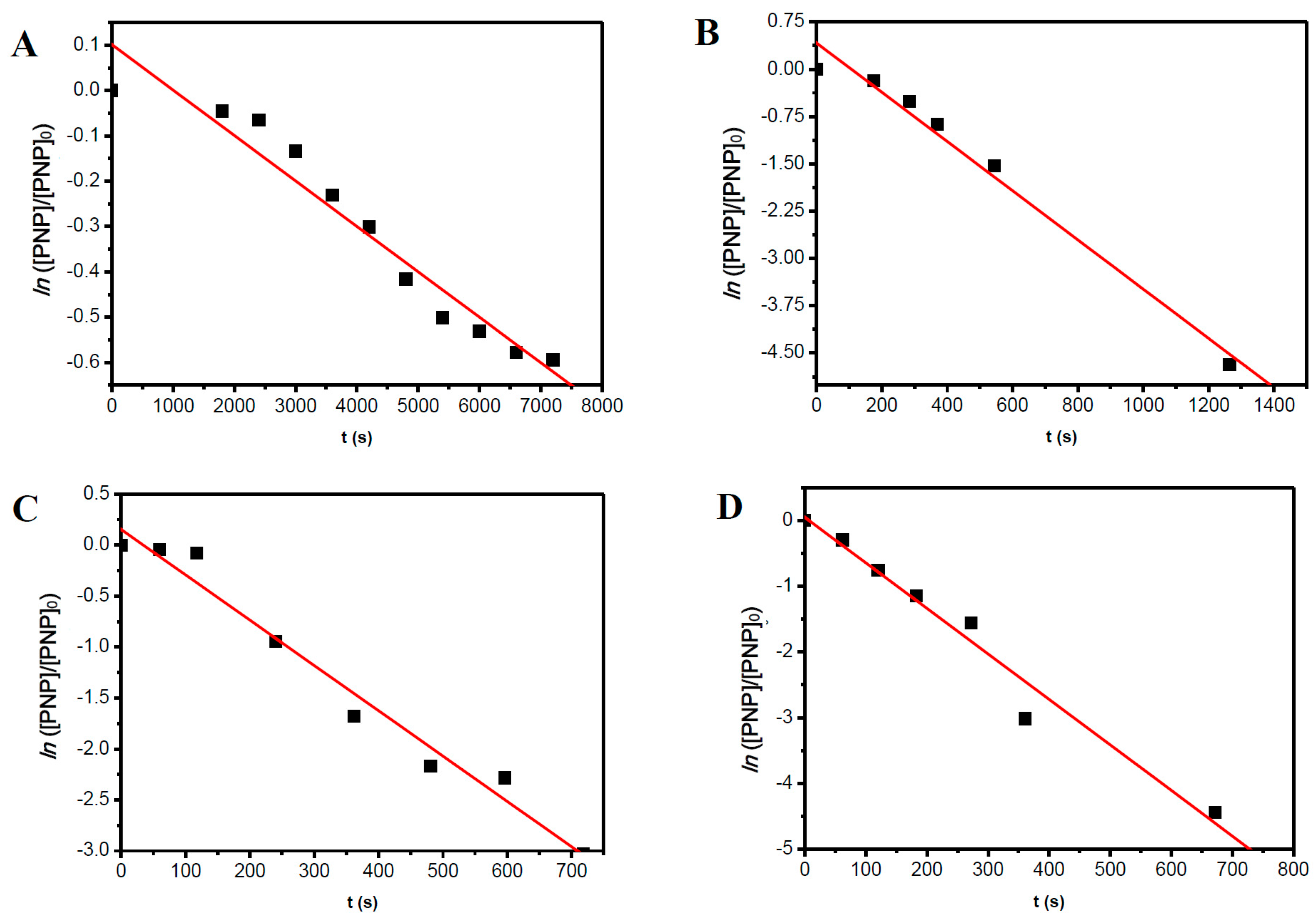
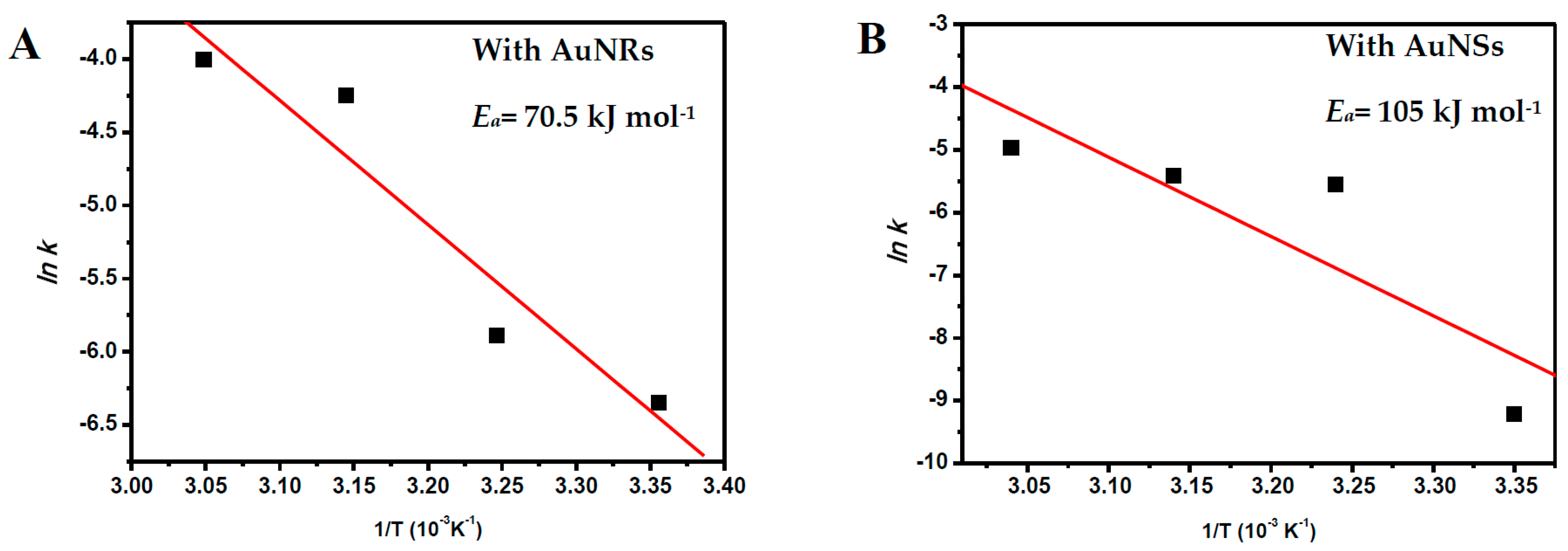
| Entry | [NaBH4] (mol·L−1) | NaBH4/PNP Molar Ratio | Conversion Time (s) | |
|---|---|---|---|---|
| AuNRs | AuNSs | |||
| 1 | 0.10 | 500 | 50 | 266 |
| 2 | 0.05 | 250 | 58 | 340 |
| 3 | 0.025 | 25 | 155 | 555 |
| 4 | 0.0025 | 12.5 | 2314 | 7200 |
| Temperature (°C) | kAuNRs (s−1) | ErrorAuNRs | kAuNSs (s−1) | ErrorAuNSs |
|---|---|---|---|---|
| 25 | 1.0 × 10−3 | ±1.28 × 10−4 | 0.1 × 10−3 | ±8.24 × 10−6 |
| 35 | 3.0 × 10−3 | ±4.06 × 10−4 | 3.9 × 10−3 | ±2.74 × 10−4 |
| 45 | 14 × 10−3 | ±0.005 | 4.4 × 10−3 | ±2.9 × 10−4 |
| 55 | 18 × 10−3 | - | 6.9 × 10−3 | ±5.3 × 10−4 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Oliveira, F.M.; Nascimento, L.R.B.d.A.; Calado, C.M.S.; Meneghetti, M.R.; Da Silva, M.G.A. Aqueous-Phase Catalytic Chemical Reduction of p-Nitrophenol Employing Soluble Gold Nanoparticles with Different Shapes. Catalysts 2016, 6, 215. https://doi.org/10.3390/catal6120215
De Oliveira FM, Nascimento LRBdA, Calado CMS, Meneghetti MR, Da Silva MGA. Aqueous-Phase Catalytic Chemical Reduction of p-Nitrophenol Employing Soluble Gold Nanoparticles with Different Shapes. Catalysts. 2016; 6(12):215. https://doi.org/10.3390/catal6120215
Chicago/Turabian StyleDe Oliveira, Francyelle Moura, Lucas Rafael Bezerra de Araújo Nascimento, Claudia Manuela Santos Calado, Mario Roberto Meneghetti, and Monique Gabriella Angelo Da Silva. 2016. "Aqueous-Phase Catalytic Chemical Reduction of p-Nitrophenol Employing Soluble Gold Nanoparticles with Different Shapes" Catalysts 6, no. 12: 215. https://doi.org/10.3390/catal6120215





