Immobilized Aspergillus niger Lipase with SiO2 Nanoparticles in Sol-Gel Materials
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Molar Ratio of the Gel Precursors
2.2. Choice of Porous Materials
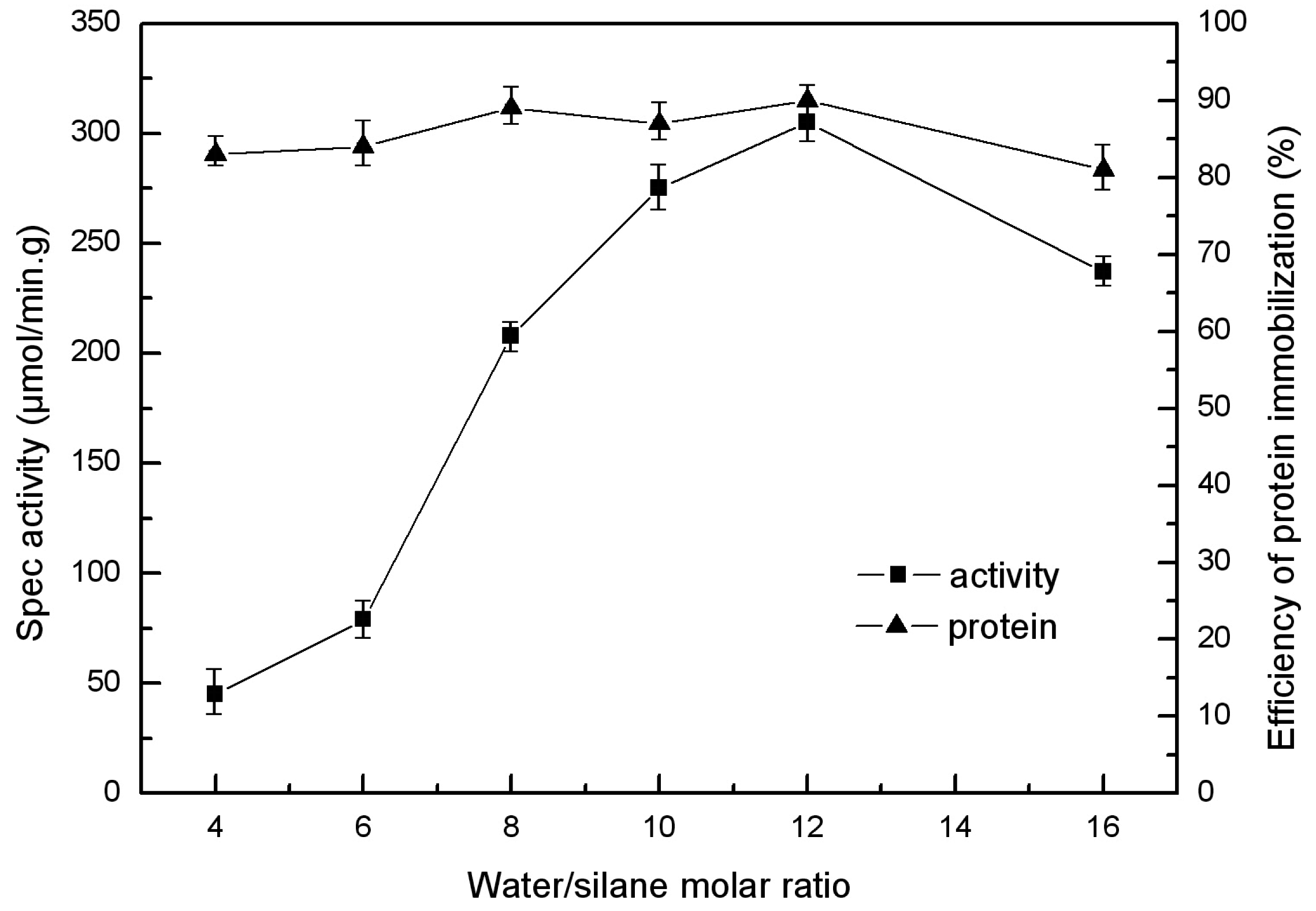
2.3. Molar Ratio of Water to Silane
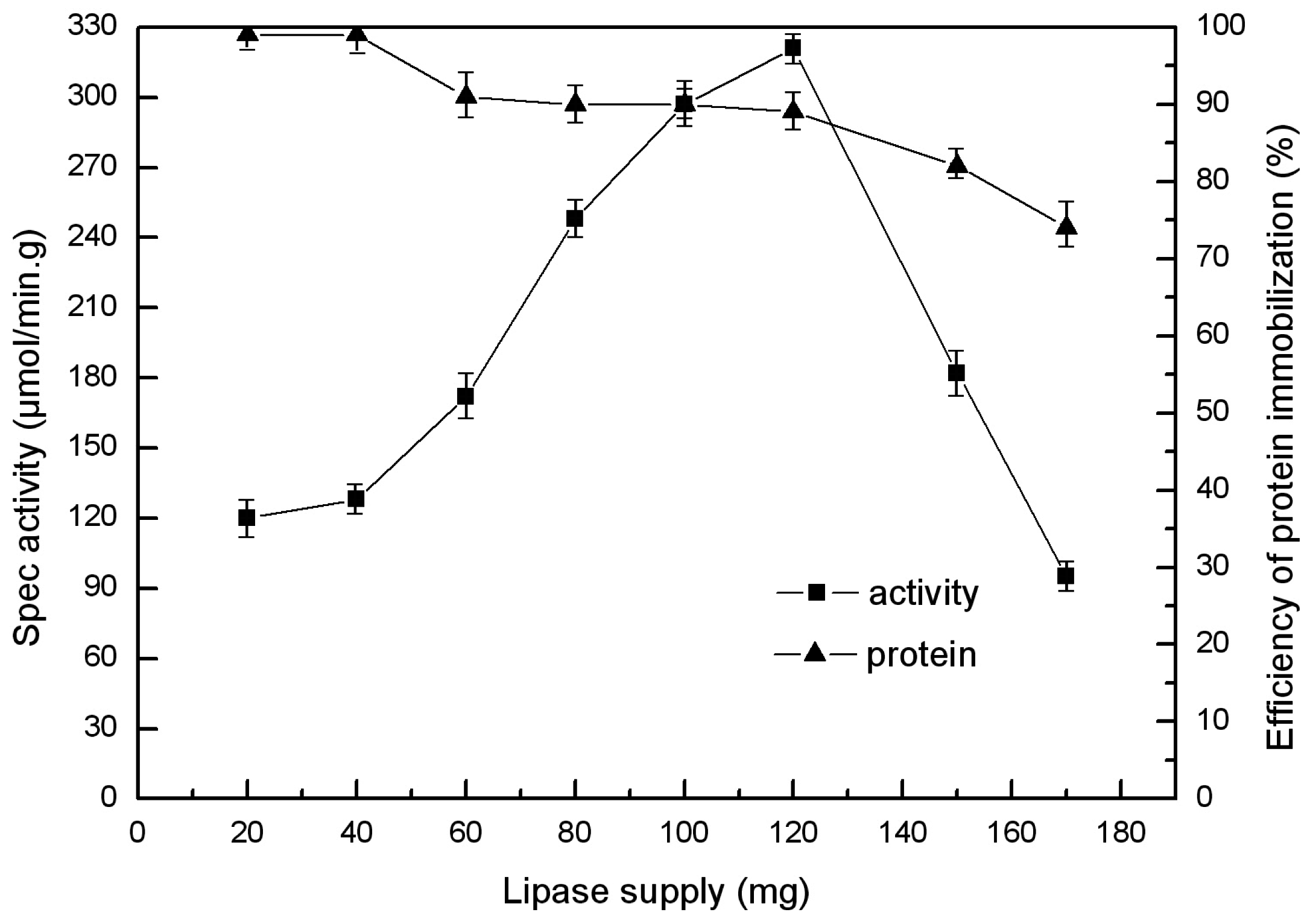
2.4. Choice of Enzyme Supply
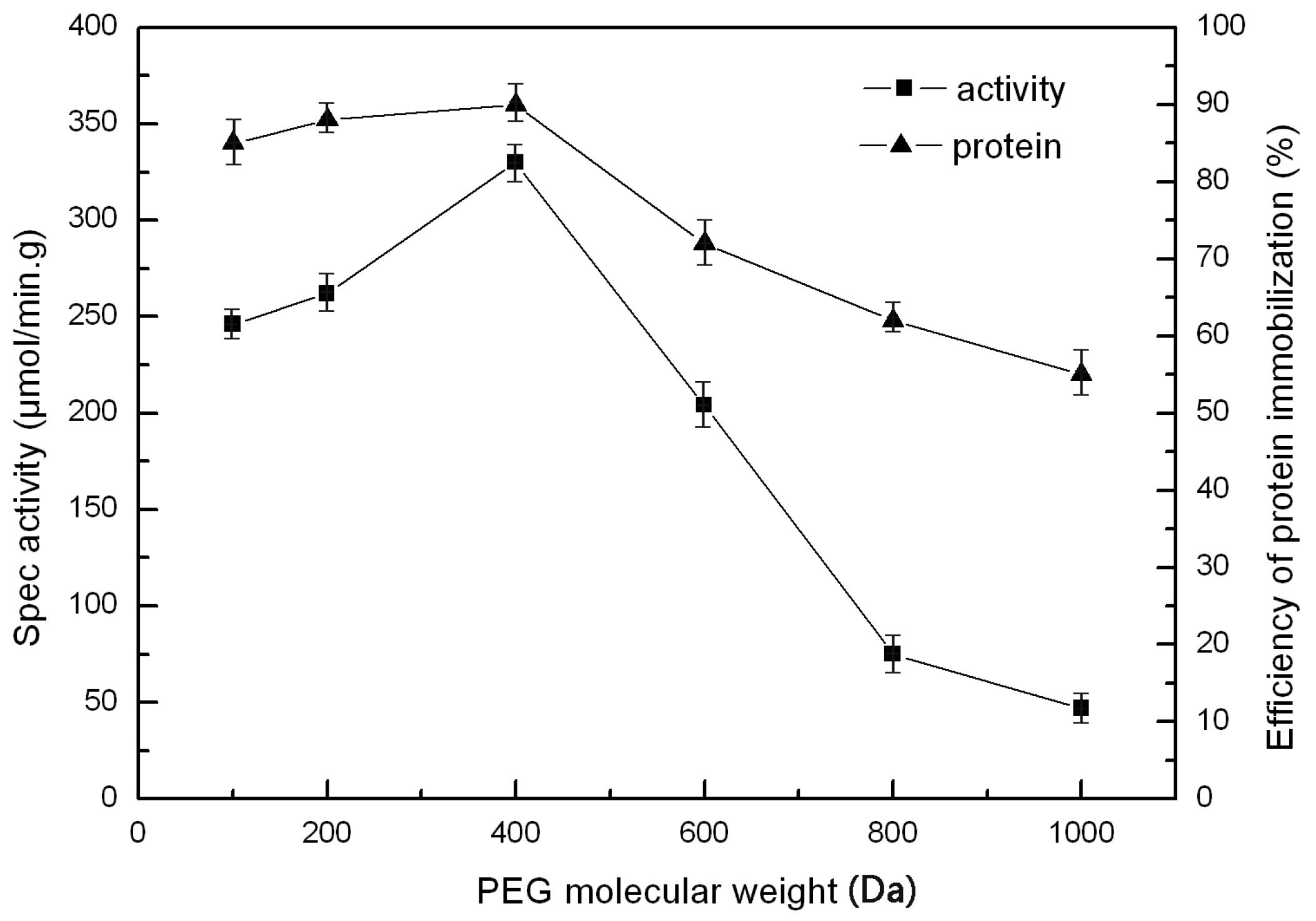
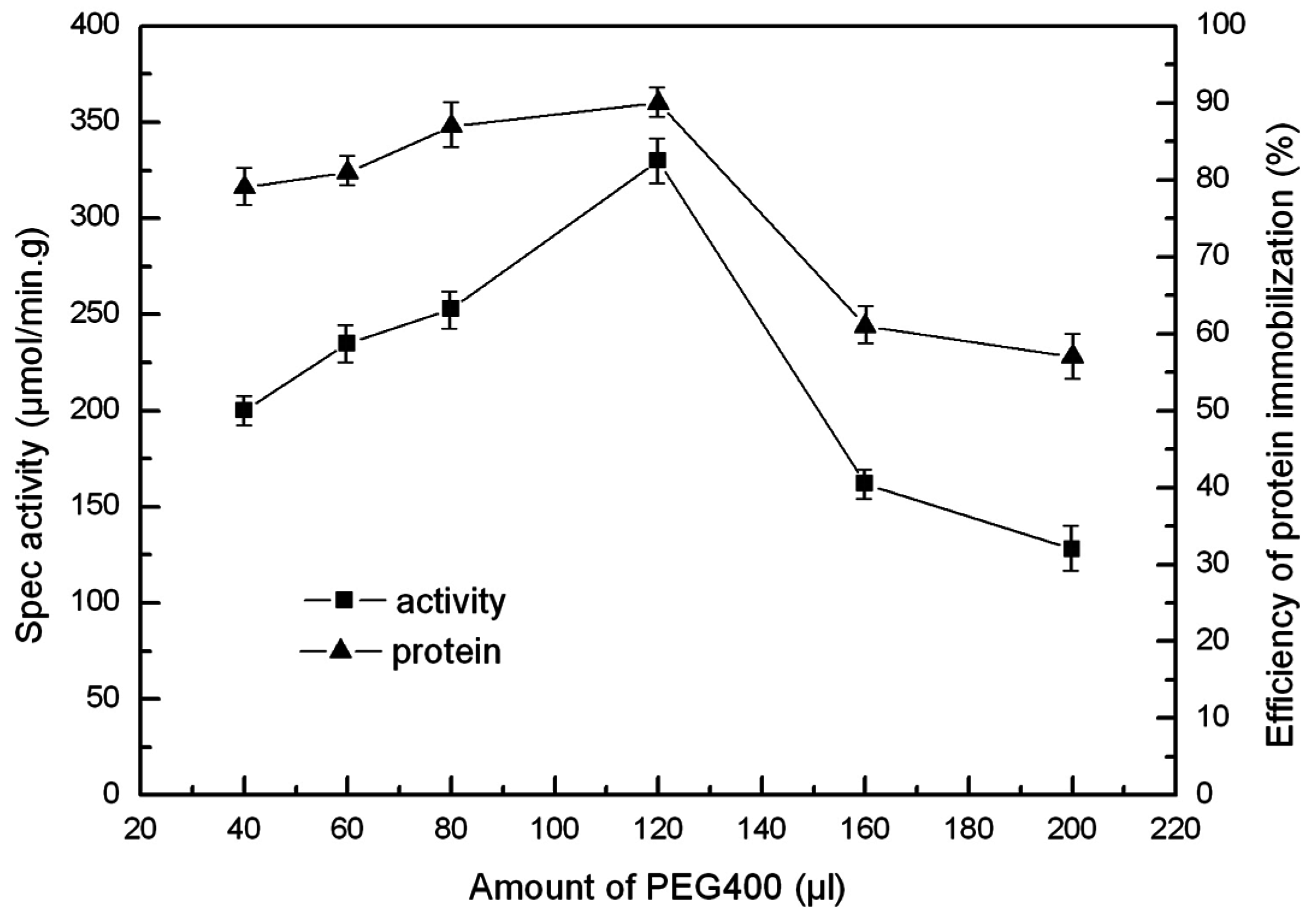
2.5. The Effect of PEG and PVA
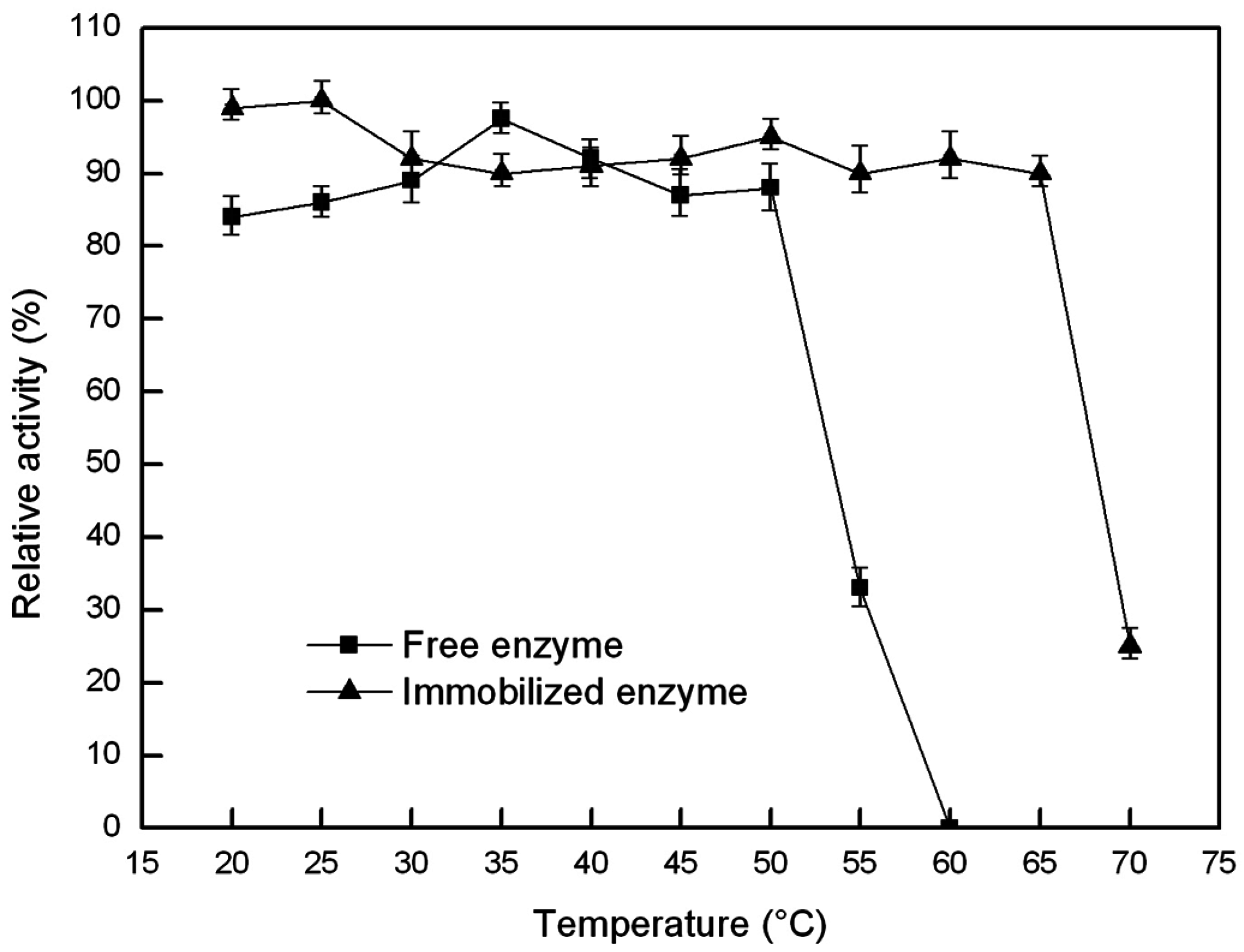
2.6. Stability of the Immobilized Lipase
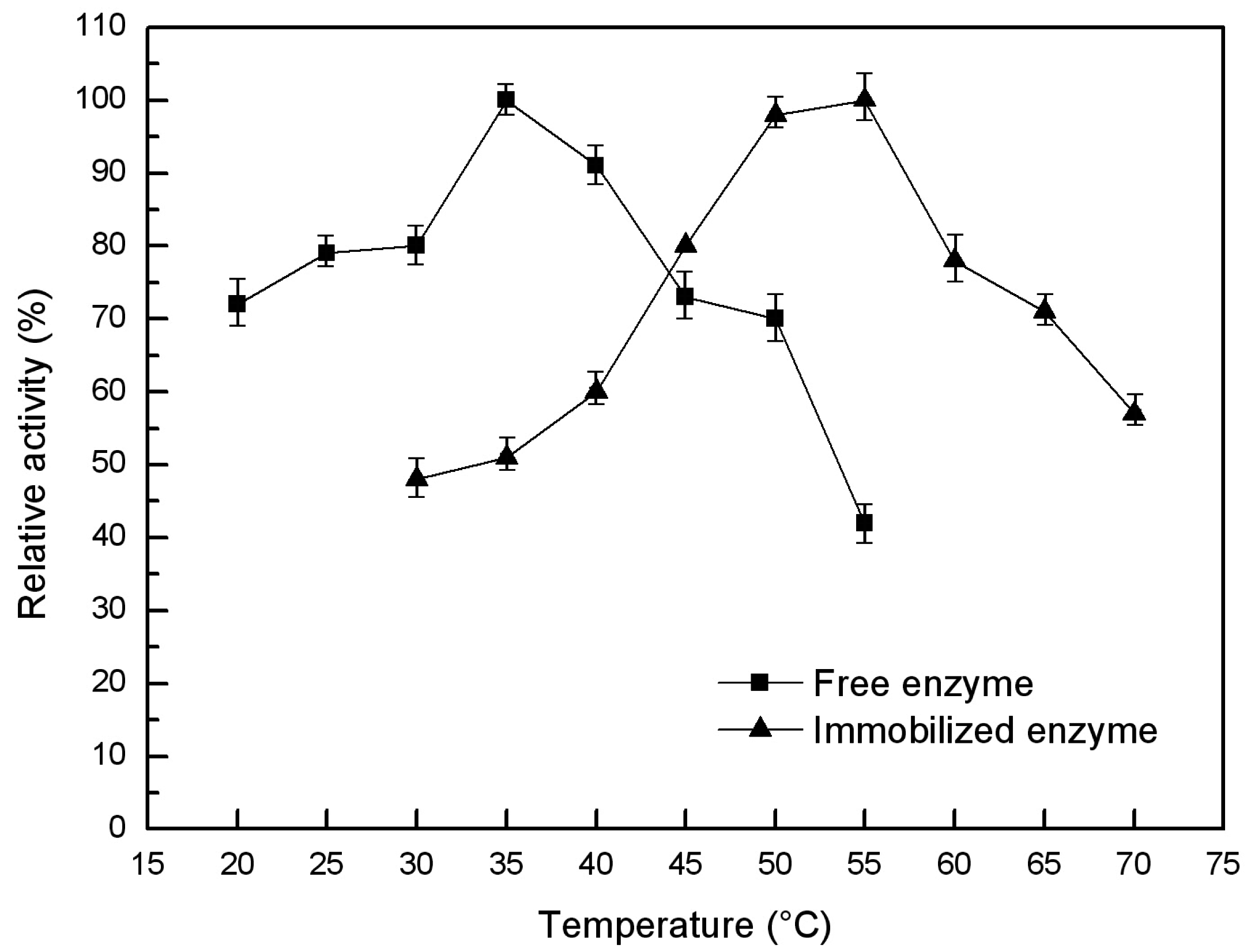
2.6.1. Optimal Temperature and Thermal Stability of the Free and Immobilized Lipase
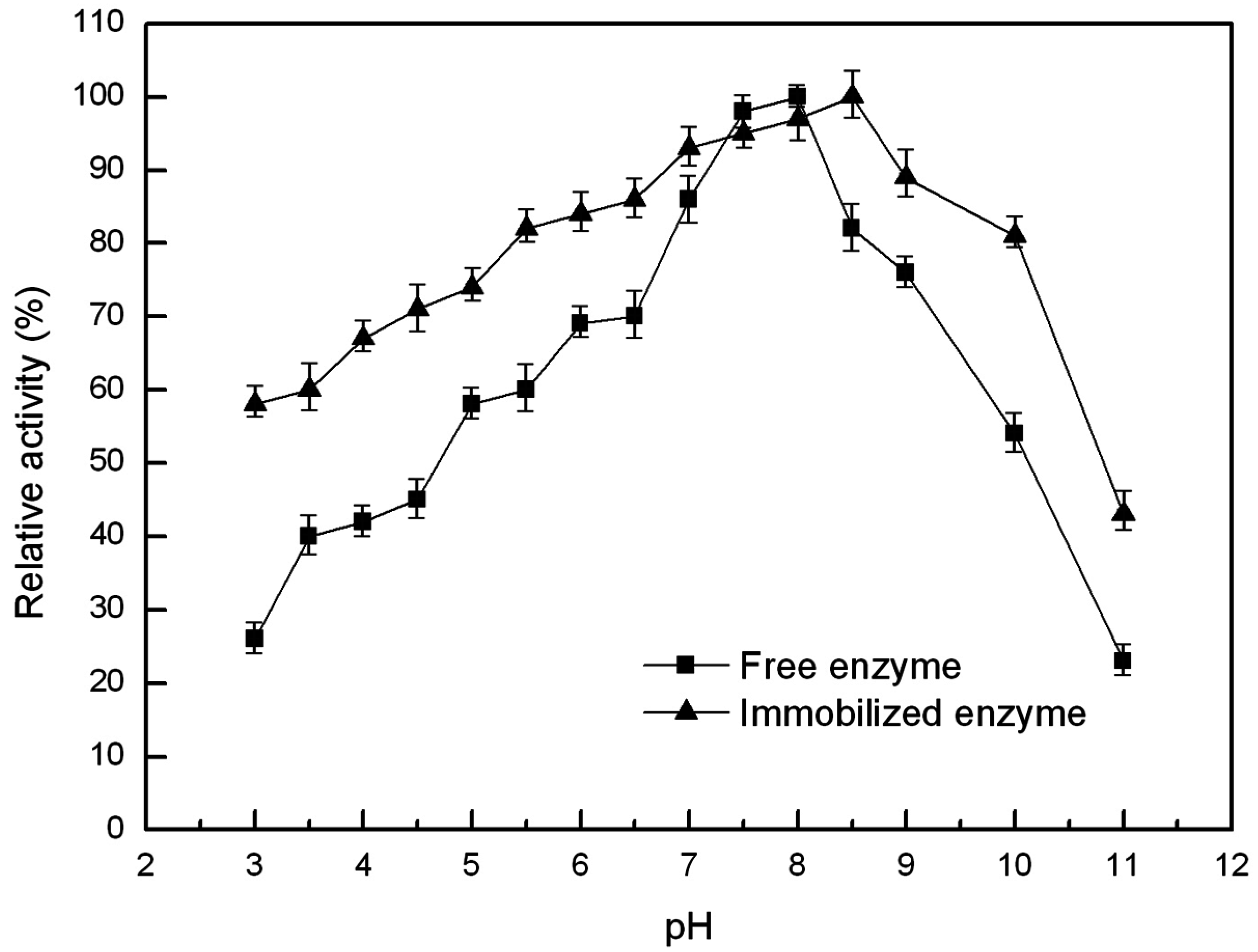
2.6.2. Optimal pH and pH Stability of the Free and Immobilized Lipase
2.7. Comparison of Immobilization Results with Other Related Reports
3. Materials and Methods
3.1. Materials
3.2. Analytical Methods
3.3. Hydrolytic Activity on pNPP
3.4. Protein Determination
3.5. The Effect of Temperature on the Enzyme Activity and Stability
3.6. The Effect of pH on the Enzyme Activity and Stability
3.7. Lipase Immobilization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mahadik, N.D.; Puntambekar, U.S.; Bastawde, K.B.; Khire, J.M.; Gokhale, D.V. Production of acidic lipase by Aspergillus niger in solid state fermentation. Process Biochem. 2002, 38, 715–721. [Google Scholar] [CrossRef]
- Sandana Mala, J.G.; Kamini, N.R.; Puvanakrishnan, R. Strain improvement of Aspergillus niger for enhanced lipase production. Appl. Microbiol. 2001, 47, 181–186. [Google Scholar]
- Pabai, F.; Kermasha, S.; Morin, A. Interesterification of butter fat by partially purified extracellular lipases from Pseudomonas putida, Aspergillus niger and Rhizopus oryzae. World J. Microbiol. Biotechnol. 1995, 11, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Schuster, E.; Dunn-Coleman, N.; Frisvad, J.C.; Van Dijck, P. On the safety of Aspergillus niger. Appl. Microbiol. Biotechnol. 2002, 59, 426–435. [Google Scholar] [PubMed]
- Saisubramanian, N.; Edwinoliver, N.G.; Nandakumar, N.; Kamini, N.R.; Puvanakrishnan, R. Efficacy of lipase from Aspergillus niger as an additive in detergent formulations: A statistical approach. J. Ind. Microbiol. Biotechnol. 2006, 33, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Xu, X. Production of specific structured triacylglycerols by lipase catalyzed reactions. Eur. J. Lipid Sci. Technol. 2000, 102, 287–303. [Google Scholar] [CrossRef]
- Sarıyar, L.; Heperkan, D. The role of Aspergillus flavus and Aspergillus niger in the hydrolysis of hazelnut fat. Int. J. Food Sci. Technol. 2003, 38, 487–492. [Google Scholar] [CrossRef]
- Buisson, P.; Hernandez, C.; Pierre, M.; Pierre, A.C. Encapsulation of lipases in aerogels. J. Non-Cryst. Solids 2001, 285, 295–302. [Google Scholar] [CrossRef]
- Reetz, M.T.; Zonta, A.; Vijayakrishnan, V.; Schimossek, K. Entrapment of lipases in hydrophobic magnetite-containing sol-gel materials: Magnetic separation of heterogeneous biocatalysts. J. Mol. Catal. A: Chem. 1998, 134, 251–258. [Google Scholar] [CrossRef]
- Chen, J.P. Production of ethyl butyrate using gel-entrapped Candida cylindracea lipase. J. Ferment. Bioeng. 1996, 82, 404–407. [Google Scholar] [CrossRef]
- Moreno, J.M.; Sinisterra, J.V. Immobilization of lipase from Candida cylindracea on inorganic supports. J. Mol. Catal. 1994, 93, 357–369. [Google Scholar] [CrossRef]
- Ellaiah, P.; Prabhakar, T.; Ramakrishna, B.; Thaer, T.A.; Adinarayana, K. Production of lipase by immobilized cells of Aspergillus niger. Process Biochem. 2004, 39, 525–528. [Google Scholar] [CrossRef]
- Osuna, Y.; Sandoval, J.; Saade, H.; López, R.G.; Martinez, J.L.; Colunga, E.M.; Fernández, H. Immobilization of Aspergillus niger lipase on chitosan-coated magnetic nanoparticles using two covalent-binding methods. Bioproc. Biosyst. Eng. 2015, 38, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Reetz, M.T.; Zonta, A.; Simpelkamp, J. Efficient immobilization of lipases by entrapment in hydrophobic sol-gel materials. Biotechnol. Bioeng. 1996, 49, 527–534. [Google Scholar] [CrossRef]
- Reetz, M.T. Practical protocols for lipase immobilization via sol-gel techniques. Methods Mol. Bio. 2013, 1051, 241–254. [Google Scholar]
- Tielmann, P.; Kierkels, H.; Zonta, A.; Ilie, A.; Reetz, M.T. Increasing the activity and enantioselectivity of lipases by sol-gel immobilization: Further advancements of practical interest. Nanoscale 2014, 6, 6220–6228. [Google Scholar] [CrossRef] [PubMed]
- Zubiolo, C.; Santos, R.C.A.; Figueiredo, R.T.; Soares, C.M.F.; de Aquino Santana, L.C.L. Morphological and physicochemical aspects of microbial lipase obtained from novel agroindustrial waste encapsulated in a sol-gel matrix. J. Therm. Anal. Calorim. 2015, 120, 1503–1509. [Google Scholar] [CrossRef]
- Souza, R.L.; Faria, E.L.; Figueiredo, R.T.; Fricks, A.T.; Zanin, G.M.; Santos, O.A.; Lima, A.S.; Soares, C.M. Use of polyethylene glycol in the process of sol-gel encapsulation of Burkholderia cepacia lipase. J. Therm. Anal. Calorim. 2014, 117, 301–306. [Google Scholar] [CrossRef]
- De Souza, R.L.; de Faria, E.L.P.; Figueiredo, R.T.; Dos Santos Freitas, L.; Iglesias, M.; Mattedi, S.; Zanin, G.M.; Dos Santos, O.A.A.; Coutinho, J.A.; Lima, Á.S. Protic ionic liquid as additive on lipase immobilization using silica sol-gel. Enzyme Microb. Technol. 2013, 52, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Reetz, M.T.; Tielmann, P.; Wiesenhöfer, W.; Könen, W.; Zonta, A. Second generation sol-gel encapsulated lipases: Robust heterogeneous biocatalysts. Adv. Synth. Catal. 2003, 345, 717–728. [Google Scholar] [CrossRef]
- Chamorro, S.; Sánchez-Montero, J.M.; Alcántara, A.R.; Sinisterra, J.V. Treatment of Candida rugosa lipase with short-chain polar organic solvents enhances its hydrolytic and synthetic activities. Biotechnol. Lett. 1998, 20, 499–505. [Google Scholar] [CrossRef]
- Palomo, J.M.; Muñoz, G.; Fernández-Lorente, G.; Mateo, C.; Fernández-Lafuente, R.; Guisán, J.M. Interfacial adsorption of lipases on very hydrophobic support (octadecyl-Sepabeads): Immobilization, hyperactivation and stabilization of the open form of lipases. J. Mol. Catal. B: Enzym. 2002, 19, 279–286. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A. Biochemical engineering fundamentals; J.E. Bailey and D.F. Ollis, McGraw Hill, New York, NY, 2nd edn., 1986, 984 pages, $47.95. J. Control. Release 1986, 4, 232. [Google Scholar] [CrossRef]
- Mingarro, I.; Abad, C.; Braco, L. Interfacial activation-based molecular bioimprinting of lipolytic enzymes. Proc Natl. Acad. Sci. USA 1995, 92, 3308–3312. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.M.; Chen, I.W. Encapsulation of protein molecules in transparent porous silica matrices via an aqueous colloidal sol-gel process. Acta Mater. 1999, 47, 4535–4544. [Google Scholar] [CrossRef]
- Cygler, M.; Schrag, J.D. Structure and conformational flexibility of Candida rugosa lipase. Biochim. Biophys. Acta 1999, 1441, 205–214. [Google Scholar] [CrossRef]
- El-Batal, A.I.; Farrag, A.A.; Elsayed, M.A.; El-Khawaga, A.M. Biodiesel Production by Aspergillus niger Lipase Immobilized on Barium Ferrite Magnetic Nanoparticles. Bioengineer 2016, 3, 14. [Google Scholar] [CrossRef]
- Zubiolo, C.; Santos, R.C.A.; Carvalho, N.B.; Soares, C.M.F.; Lima, A.S.; de Aquino Santana, L.C.L. Encapsulation in a sol-gel matrix of lipase from Aspergillus niger obtained by bioconversion of a novel agricultural residue. Bioproc. Biosyst. Eng. 2014, 37, 1781–1788. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.L.; Resende, W.C.; Barão, C.E.; Zanin, G.M.; de Castro, H.F.; Santos, O.A.; Fricks, A.T.; Figueiredo, R.T.; Lima, Á.S.; Soares, C.M. Influence of the use of Aliquat 336 in the immobilization procedure in sol-gel of lipase from Bacillus sp. ITP-001. J. Mol. Catal. B: Enzym. 2012, 84, 152–159. [Google Scholar] [CrossRef]
- Macario, A.; Giordano, G.; Frontera, P.; Crea, F.; Setti, L. Hydrolysis of alkyl ester on lipase/silicalite-1 catalyst. Catal. Lett. 2008, 122, 43–52. [Google Scholar] [CrossRef]
- Yilmaz, E.; Sezgin, M. Enhancement of the activity and enantioselectivity of lipase by sol-gel encapsulation immobilization onto β-cyclodextrin-based polymer. Appl. Biochem. Biotechnol. 2012, 166, 1927–1940. [Google Scholar] [CrossRef] [PubMed]
- Hernández-García, S.; García-García, M.I.; García-Carmona, F. Purification, Immobilization and Characterization of Lipase Isoenzyme from Aspergillus niger with C8 Magnetic Particles. Adv. Biosci. Biotechnol. 2014, 5, 633. [Google Scholar] [CrossRef]
- Zdarta, J.; Jesionowski, T. Luffa cylindrica sponges as a thermally and chemically stable support for Aspergillus niger lipase. Biotechnol. Prog. 2016, 32, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Kordel, M.; Hofmann, B.; Schomburg, D.; Schmid, R.D. Extracellular lipase of Pseudomonas sp. strain ATCC 21808: Purification, characterization, crystallization, and preliminary X-ray diffraction data. J. Bacteriol. 1991, 173, 4836–4841. [Google Scholar] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]

| Gel Precursors Molar Ratio | Efficiency of Protein Immobilization (%) | Activity (µmol·min−1·g−1) |
|---|---|---|
| TMOS (tetramethoxysilane) | 90.1 | 6.3 |
| MTMS/TMOS (1:1) | 91.3 | 232.2 |
| MTMS/TMOS (3:1) | 92.1 | 281.2 |
| MTMS/TMOS (5:1) | 90.9 | 315.0 |
| MTMS (methyltreimethoxysilane) | 90.8 | 294.0 |
| Additive (mg) | Efficiency of Protein Immobilization (%) | Activity (µmol·min−1·g−1) |
|---|---|---|
| SiO2 20 | 76.2 | 198.6 |
| SiO2 40 | 83.1 | 227.8 |
| SiO2 50 | 89.8 | 247.0 |
| SiO2 60 | 92.1 | 301.8 |
| SiO2 80 | 91.4 | 224.0 |
| SiO2 100 | 90.3 | 189.1 |
| Celite 20 | 63.7 | 169.0 |
| Celite 40 | 73.4 | 162.9 |
| Celite 50 | 78.9 | 199.2 |
| Celite 60 | 83.2 | 213.7 |
| Celite 80 | 89.1 | 214.9 |
| Celite 100 | 89.4 | 214.3 |
| Controls | 77.5 | 206.7 |
| Additive (mg) | Sample | Efficiency of Protein Immobilization (%) | Activity (µmol·min−1·g−1) |
|---|---|---|---|
| SiO2 | IM1 | 90.8 | 276.7 |
| IM2 | 91.1 | 343.9 | |
| IM3 | 90.6 | 274.1 | |
| None | IM1 | 81.5 | 71.3 |
| IM2 | 84.3 | 198.6 | |
| IM3 | 82.2 | 159.2 |
| Reports | Enzyme | Immobilization Method | Activity Recovery (%) | Published Year |
|---|---|---|---|---|
| 1 | Bacillus sp. ITP-001 lipase | Sol-gel silica matrices encapsulation | 177.5 | 2012 [30] |
| 2 | Rhizomucor miehei lipase | Adsorbed on Silicalite-1 Sol-gel materials | 118.4 | 2008 [31] |
| 3 | Candida rugosa lipase | Sol-gel Encapsulation | 230.1 | 2012 [32] |
| 4 | Aspergillus niger enzyme extraction | Sol-gel encapsulation | 71.4 | 2014 [29] |
| 5 | Aspergillus niger lipase A | Adsorbed on modified magnetic particles | 118.9 | 2014 [33] |
| 6 | Aspergillus niger lipase | Adsorbed Luffa cylindrica sponges | 84.0 | 2016 [34] |
| 7 | Aspergillus niger lipase | Sol-gel encapsulation | 94.0 | This work |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Ke, C.; Huang, Y.; Yan, Y. Immobilized Aspergillus niger Lipase with SiO2 Nanoparticles in Sol-Gel Materials. Catalysts 2016, 6, 149. https://doi.org/10.3390/catal6100149
Xu L, Ke C, Huang Y, Yan Y. Immobilized Aspergillus niger Lipase with SiO2 Nanoparticles in Sol-Gel Materials. Catalysts. 2016; 6(10):149. https://doi.org/10.3390/catal6100149
Chicago/Turabian StyleXu, Li, Caixia Ke, Ying Huang, and Yunjun Yan. 2016. "Immobilized Aspergillus niger Lipase with SiO2 Nanoparticles in Sol-Gel Materials" Catalysts 6, no. 10: 149. https://doi.org/10.3390/catal6100149






