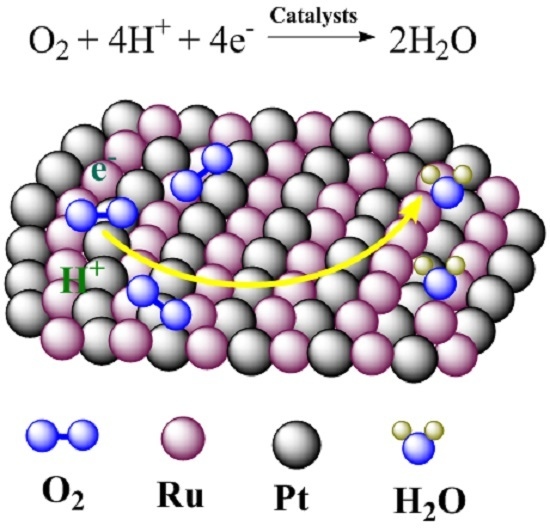
Preparation of Pt–Ru/C as an Oxygen-Reduction Electrocatalyst in Microbial Fuel Cells for Wastewater Treatment
Abstract
:1. Introduction
2. Results and Discussion
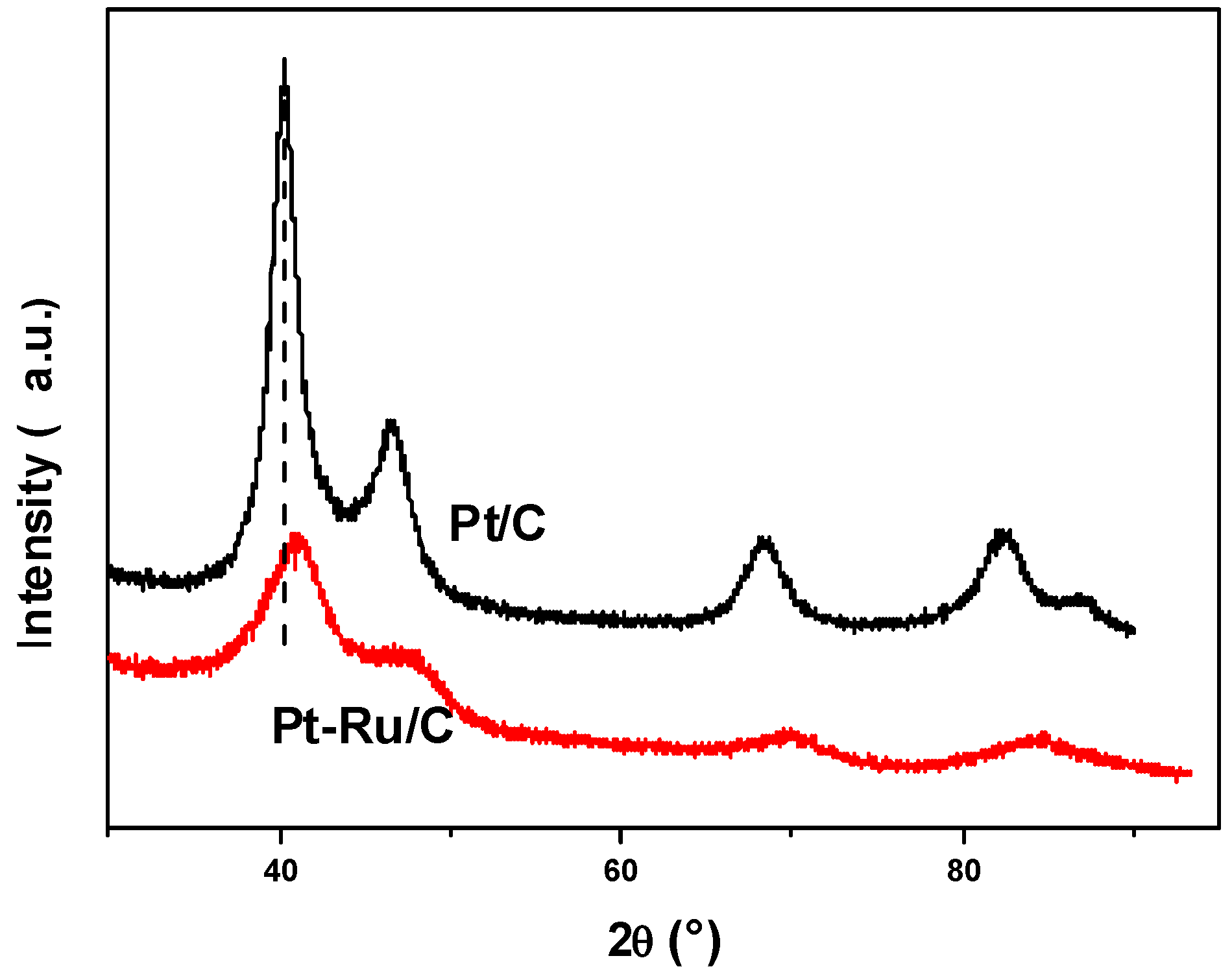
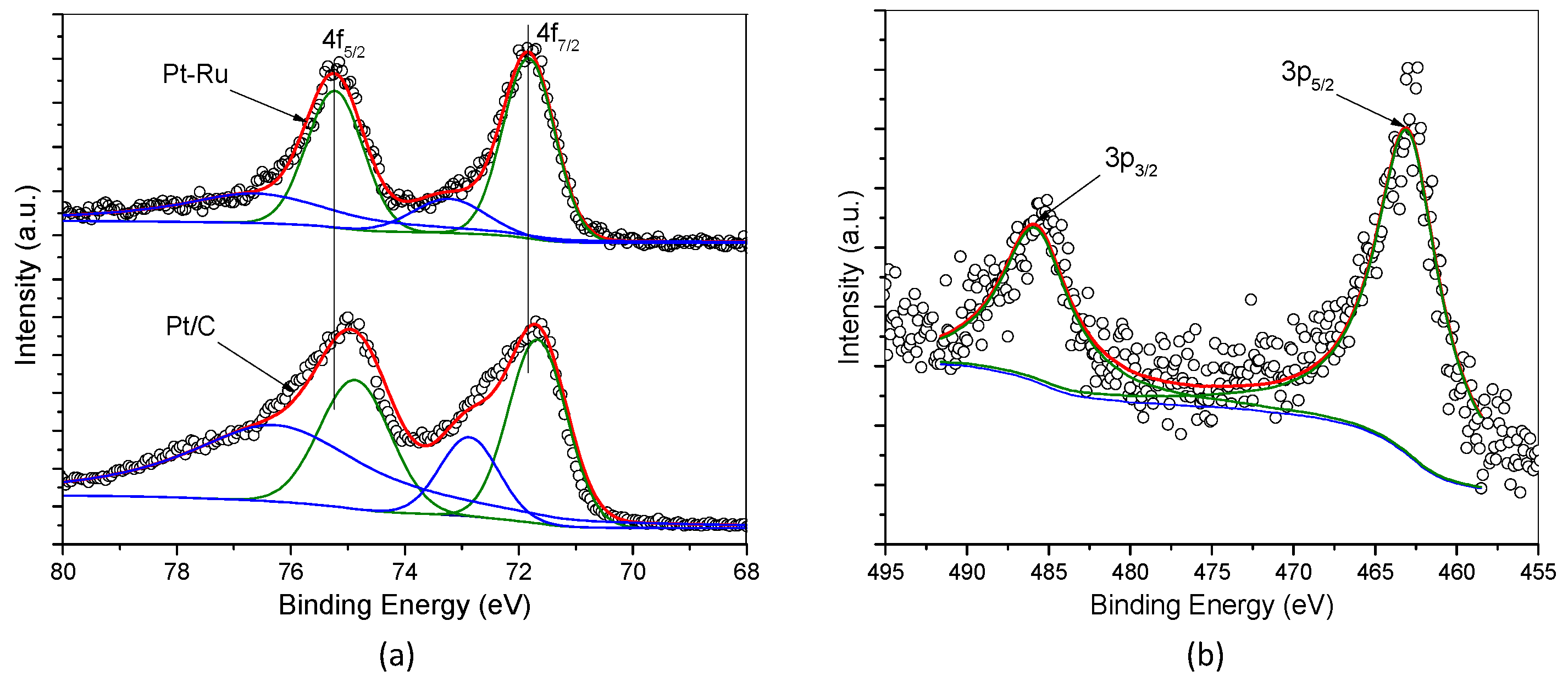
2.1. Physical Characterization
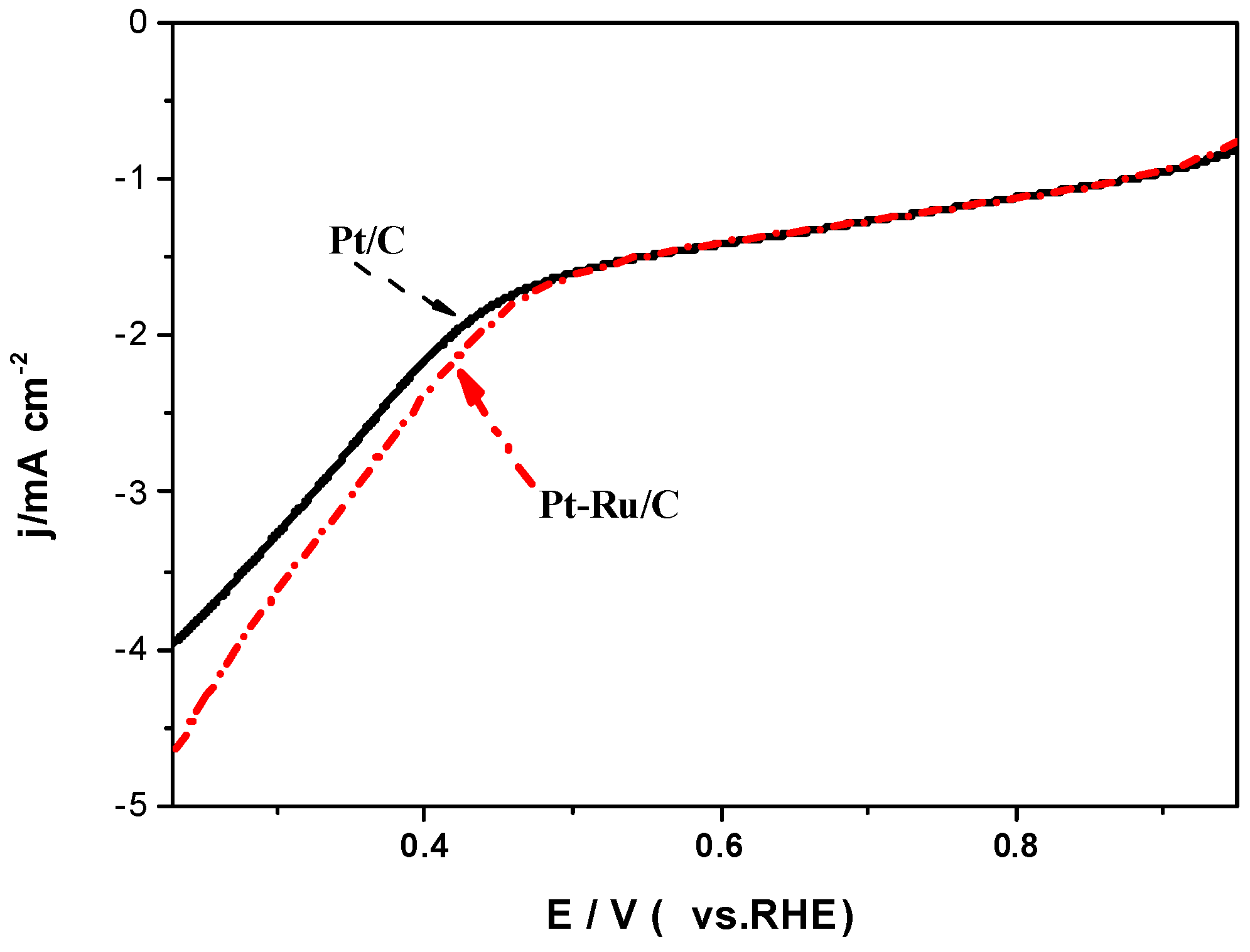
2.2. Electrochemical Activity
3. Experimental
3.1. The Preparation of Catalysts
3.2. Physical Characterization
3.3. Electrochemical Activity
3.4. Microbial Fuel Cell Measurements
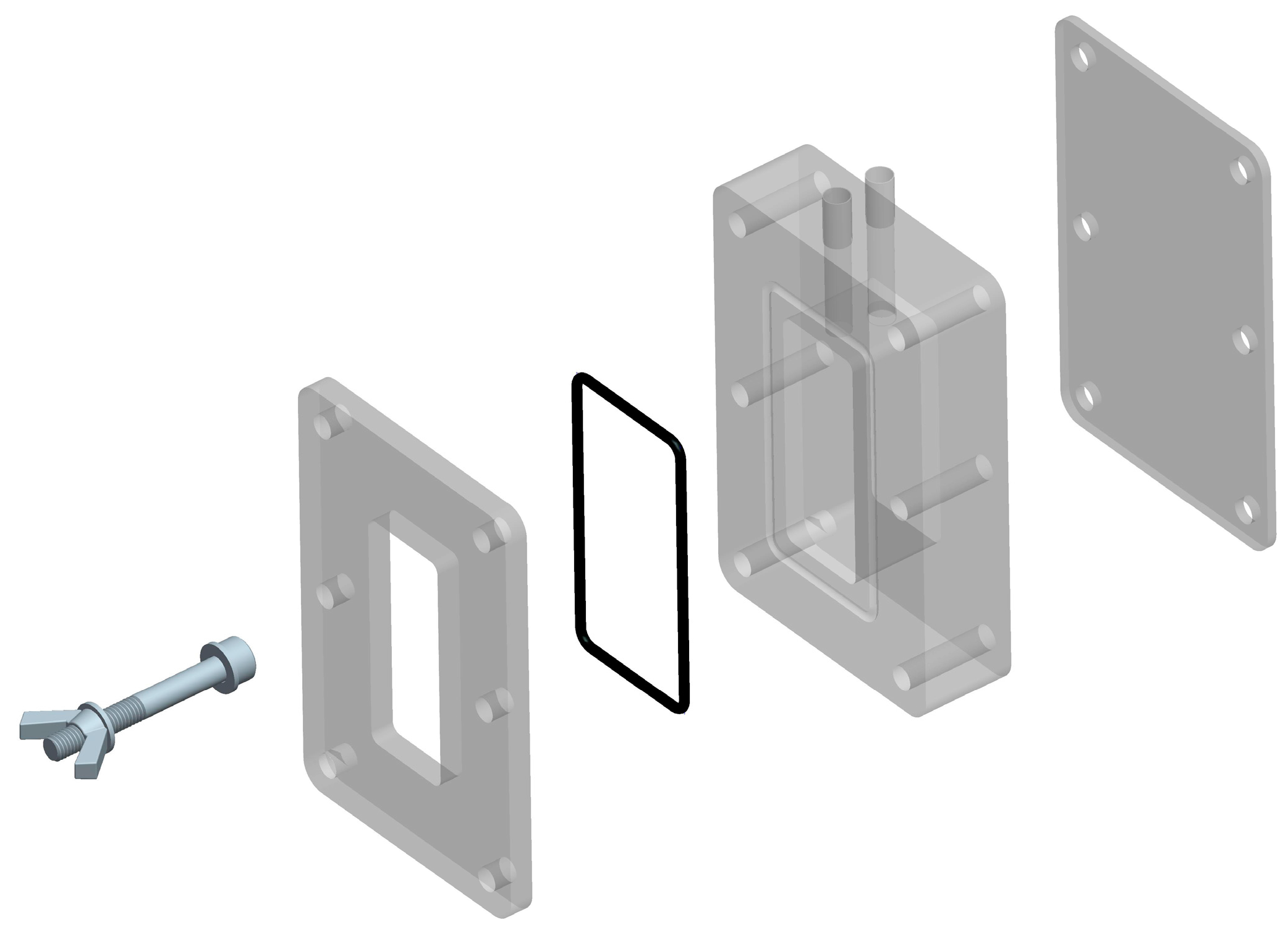
3.4.1. MFC Construction and Electrode Preparation
3.4.2. Enrichment and Operation
3.4.3. Analytical Method
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kannan, M.V.; Gnana Kumar, G. Current status, key challenges and its solutions in the design and development of graphene based orr catalysts for the microbial fuel cell applications. Biosens. Bioelectron. 2016, 77, 1208–1220. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Hou, Y.; Abu-Reesh, I.M.; Chen, J.; He, Z. Oxygen reduction reaction catalysts used in microbial fuel cells for energy-efficient wastewater treatment: A review. Mater. Horiz. 2016, 3, 382–401. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Zardari, P. Electrocatalytical study of carbon supported Pt, Ru and bimetallic Pt–Ru nanoparticles for oxygen reduction reaction in alkaline media. Appl. Surf. Sci. 2015, 345, 223–231. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, M.; Huang, B.; Liu, R.; Zhao, J. Graphene supported Pt–Co alloy nanoparticles as cathode catalyst for microbial fuel cells. Int. J. Electrochem. Sci. 2013, 8, 149–158. [Google Scholar]
- Cetinkaya, A.Y.; Ozdemir, O.K.; Koroglu, E.O.; Hasimoglu, A.; Ozkaya, B. The development of catalytic performance by coating Pt–Ni on CMI7000 membrane as a cathode of a microbial fuel cell. Bioresour. Technol. 2015, 195, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.H.; Li, H.H.; Liu, X.J.; Gao, M.R.; Yu, S.H. Surface composition and lattice ordering-controlled activity and durability of cupt electrocatalysts for oxygen reduction reaction. ACS Catal. 2012, 2, 916–924. [Google Scholar] [CrossRef]
- Maiyalagan, T.; Khan, F.N. Electrochemical oxidation of methanol on Pt/V2O5–C composite catalysts. Catal. Commun. 2009, 10, 433–436. [Google Scholar] [CrossRef]
- Liu, X.; Fu, G.; Chen, Y.; Tang, Y.; She, P.; Lu, T. Pt–Pd–Co trimetallic alloy network nanostructures with superior electrocatalytic activity towards the oxygen reduction reaction. Chem. Eur. J. 2014, 20, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.; Gilman, S. Methanol electro-oxidation on unsupported Pt–Ru alloys at different temperatures. J. Electrochem. Soc. 1996, 143, 1685–1690. [Google Scholar] [CrossRef]
- Sun, H.J.; Ding, L.X.; Chen, Y.; Zhou, Y.M.; Lu, T.H.; Tang, Y.W. Changes of composition and structure of Pt–Ru/C catalyst in the methanol electro-oxidation process. Chin. J. Inorg. Chem. 2010, 26, 25–28. [Google Scholar]
- Chen, Y.; Zhou, Y.; Tang, Y.; Lu, T. Electrocatalytic properties of carbon-supported Pt–Ru catalysts with the high alloying degree for formic acid electrooxidation. J. Power Sources 2010, 195, 4129–4134. [Google Scholar] [CrossRef]
- Paulus, U.; Schmidt, T.; Gasteiger, H.; Behm, R. Oxygen reduction on a high-surface area Pt/vulcan carbon catalyst: A thin-film rotating ring-disk electrode study. J. Electroanal. Chem. 2001, 495, 134–145. [Google Scholar] [CrossRef]
- Mayrhofer, K.; Blizanac, B.; Arenz, M.; Stamenkovic, V.; Ross, P.; Markovic, N. The impact of geometric and surface electronic properties of Pt-catalysts on the particle size effect in electrocatalysis. J. Phys. Chem. B 2005, 109, 14433–14440. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.P.; Lewera, A.; Larsen, R.; Masel, R.I.; Bagus, P.S.; Wieckowski, A. Size effects in electronic and catalytic properties of unsupported palladium nanoparticles in electrooxidation of formic acid. J. Phys. Chem. B 2006, 110, 13393–13398. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lee, J.Y.; Han, M.; Chen, W.; Gan, L.M. Synthesis and characterization of Pt–Ru/C catalysts from microemulsions and emulsions. J. Mater. Chem. 2002, 12, 2453–2458. [Google Scholar] [CrossRef]
- Wagner, C.; Naumkin, A.; Kraut-Vass, A.; Allison, J.; Powell, C.; Rumble, J., Jr. National Institute of Standard & Technology, X-ray Photoelectron Spectroscopy Database, Nist Standard Reference Database 20, Version 3.2 (Web Version). Available online: http://srdata.nist.gov/xps/ (accessed on 6 June 2000).
- Ye, F.; Liu, H.; Hu, W.; Zhong, J.; Chen, Y.; Cao, H.; Yang, J. Heterogeneous Au-Pt nanostructures with enhanced catalytic activity toward oxygen reduction. Dalton Trans. 2012, 41, 2898–2903. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Liu, H.; Ye, F.; Hu, W.; Yang, J. Cage-bell structured Au-Pt nanomaterials with enhanced electrocatalytic activity toward oxygen reduction. Int. J. Hydrog. Energy 2012, 37, 13191–13199. [Google Scholar] [CrossRef]
- Hu, Y.M.; Zhu, A.M.; Zhang, C.L.; Zhang, Q.G.; Liu, Q.L. Microwave-assisted synthesis of double-shell PtRu/TiO2 catalyst towards methanol electro-oxidation. Int. J. Hydrog. Energy 2015, 40, 15652–15662. [Google Scholar] [CrossRef]
- Yang, J.; Chen, X.; Ye, F.; Wang, C.; Zheng, Y.; Yang, J. Core-shell CdSe@ Pt nanocomposites with superior electrocatalytic activity enhanced by lateral strain effect. J. Mater. Chem. 2011, 21, 9088–9094. [Google Scholar] [CrossRef]
- Marković, N.M.; Gasteiger, H.A.; Ross, P.N.; Jiang, X.; Villegas, I.; Weaver, M.J. Electro-oxidation mechanisms of methanol and formic acid on Pt–Ru alloy surfaces. Electrochim. Acta 1995, 40, 91–98. [Google Scholar] [CrossRef]
- Stojmenovic, M.; Momcilovic, M.; Gavrilov, N.; Pasti, I.A.; Mentus, S.; Jokic, B.; Babic, B. Incorporation of Pt, Ru and Pt–Ru nanoparticles into ordered mesoporous carbons for efficient oxygen reduction reaction in alkaline media. Electrochim. Acta 2015, 153, 130–139. [Google Scholar] [CrossRef]
- Zhang, J.; Vukmirovic, M.B.; Sasaki, K.; Nilekar, A.U.; Mavrikakis, M.; Adzic, R.R. Mixed-metal Pt monolayer electrocatalysts for enhanced oxygen reduction kinetics. J. Am. Chem. Soc. 2005, 127, 12480–12481. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Ismail, M.; Kamarudin, S.K.; Saeedfar, K.; Daud, W.R.W.; Hassan, S.H.; Heng, L.Y.; Alam, J.; Oh, S.-E. Carbon nanotube as an alternative cathode support and catalyst for microbial fuel cells. Appl. Energy 2013, 102, 1050–1056. [Google Scholar] [CrossRef]
- Wen, Q.; Wang, S.; Yan, J.; Cong, L.; Chen, Y.; Xi, H. Porous nitrogen-doped carbon nanosheet on graphene as metal-free catalyst for oxygen reduction reaction in air-cathode microbial fuel cells. Bioelectrochemistry 2014, 95, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhou, Y.; Chen, S.; Wang, Z.; Hou, H.; Zhao, F. Cellulose-derived nitrogen and phosphorus dual-doped carbon as high performance oxygen reduction catalyst in microbial fuel cell. J. Power Sources 2015, 273, 1189–1193. [Google Scholar] [CrossRef]
- He, W.; Zhang, X.; Liu, J.; Zhu, X.; Feng, Y.; Logan, B.E. Microbial fuel cells with an integrated spacer and separate anode and cathode modules. Environ. Sci. Water Res. Technol. 2016, 2, 186–195. [Google Scholar] [CrossRef]
- Dong, H.; Yu, H.; Wang, X.; Zhou, Q.; Feng, J. A novel structure of scalable air-cathode without nafion and Pt by rolling activated carbon and PTFE as catalyst layer in microbial fuel cells. Water Res. 2012, 46, 5777–5787. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Chen, Y.; Zhou, Y.; Tang, Y.; Lu, T. Preparation of carbon supported Pd–P catalyst with high content of element phosphorus and its electrocatalytic performance for formic acid oxidation. Electrochem. Commun. 2010, 12, 492–495. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, W.; Wang, H.; Xie, L.; Liang, Y.; Wei, F.; Idrobo, J.-C.; Pennycook, S.J.; Dai, H. An oxygen reduction electrocatalyst based on carbon nanotube-graphene complexes. Nat. Nanotech. 2012, 7, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Watson, V.J.; Nieto Delgado, C.; Logan, B.E. Influence of chemical and physical properties of activated carbon powders on oxygen reduction and microbial fuel cell performance. Environ. Sci. Technol. 2013, 47, 6704–6710. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Sun, Y.; Yuan, Z.; Lü, P.; Kong, X.; Li, L.; Chen, G.; Lu, T. Application of surface-modified carbon powder in microbial fuel cells. Chin. J. Catal. 2014, 35, 770–775. [Google Scholar] [CrossRef]
- Thygesen, A.; Poulsen, F.W.; Angelidaki, I.; Min, B.; Bjerre, A.B. Electricity generation by microbial fuel cells fuelled with wheat straw hydrolysate. Biomass Bioenergy 2011, 35, 4732–4739. [Google Scholar] [CrossRef]
- Clesceri, L.S.; Eaton, A.D.; Greenberg, A.E.; American Public Health Association. Standard Methods for the Examination of Water Wastewater; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Sun, Y.; Lv, P.; Zhen, F.; Cao, X.; Chen, X.; Wang, Z.; Yuan, Z.; Kong, X. Preparation of Pt–Ru/C as an Oxygen-Reduction Electrocatalyst in Microbial Fuel Cells for Wastewater Treatment. Catalysts 2016, 6, 150. https://doi.org/10.3390/catal6100150
Yang G, Sun Y, Lv P, Zhen F, Cao X, Chen X, Wang Z, Yuan Z, Kong X. Preparation of Pt–Ru/C as an Oxygen-Reduction Electrocatalyst in Microbial Fuel Cells for Wastewater Treatment. Catalysts. 2016; 6(10):150. https://doi.org/10.3390/catal6100150
Chicago/Turabian StyleYang, Gaixiu, Yongming Sun, Pengmei Lv, Feng Zhen, Xinyue Cao, Xiaojie Chen, Zhongming Wang, Zhenhong Yuan, and Xiaoying Kong. 2016. "Preparation of Pt–Ru/C as an Oxygen-Reduction Electrocatalyst in Microbial Fuel Cells for Wastewater Treatment" Catalysts 6, no. 10: 150. https://doi.org/10.3390/catal6100150