The Fabrication of Ga2O3/ZSM-5 Hollow Fibers for Efficient Catalytic Conversion of n-Butane into Light Olefins and Aromatics
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
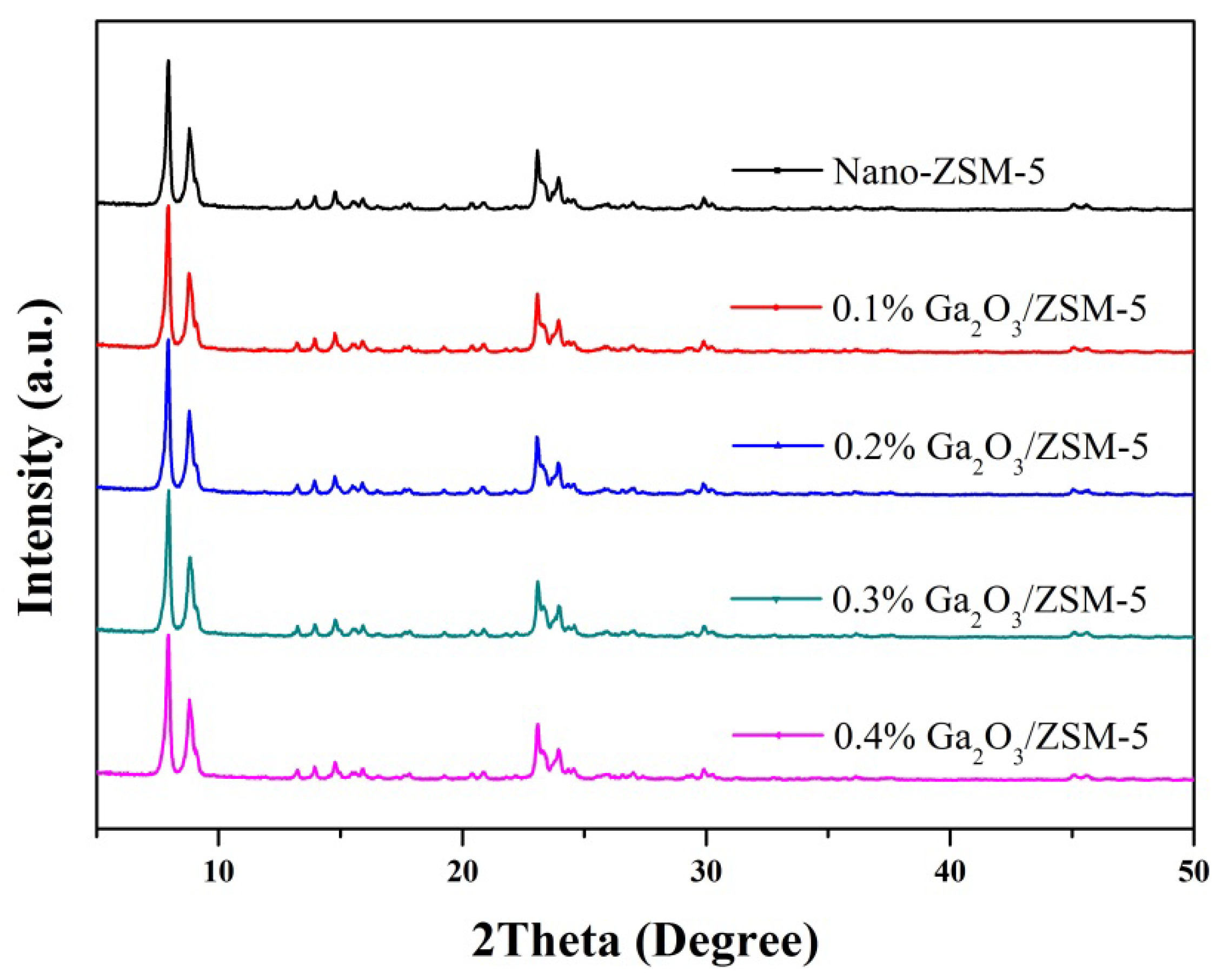
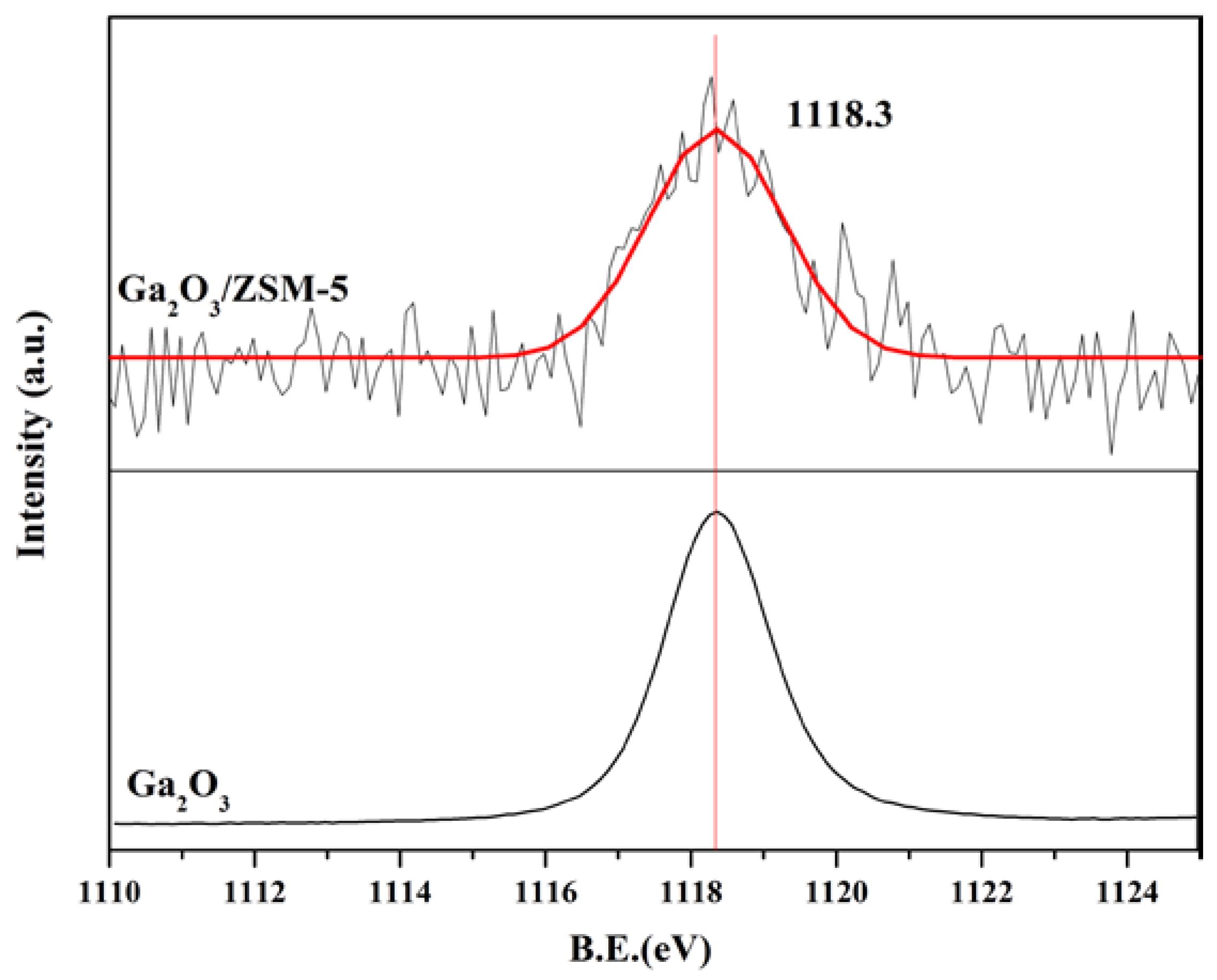
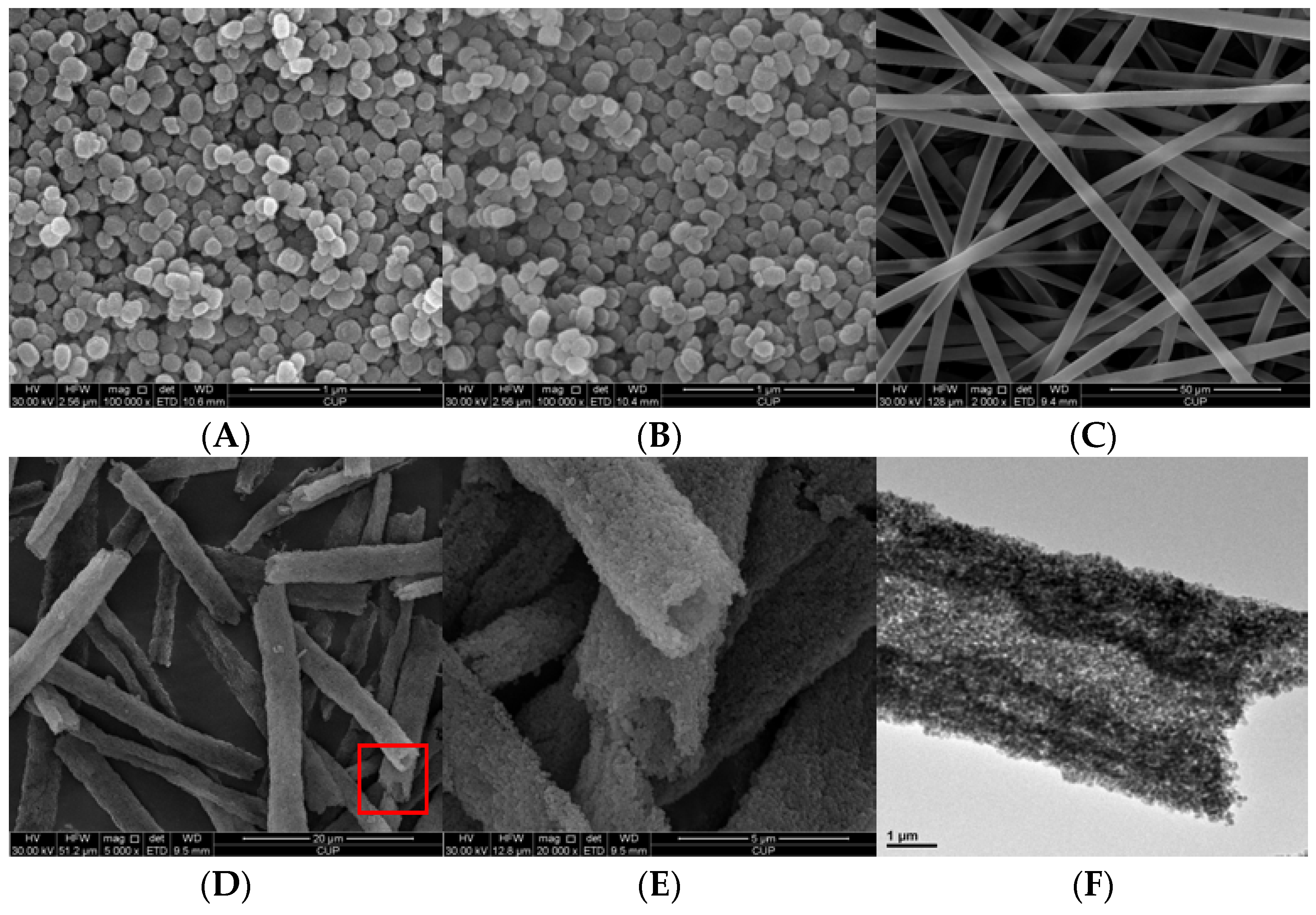
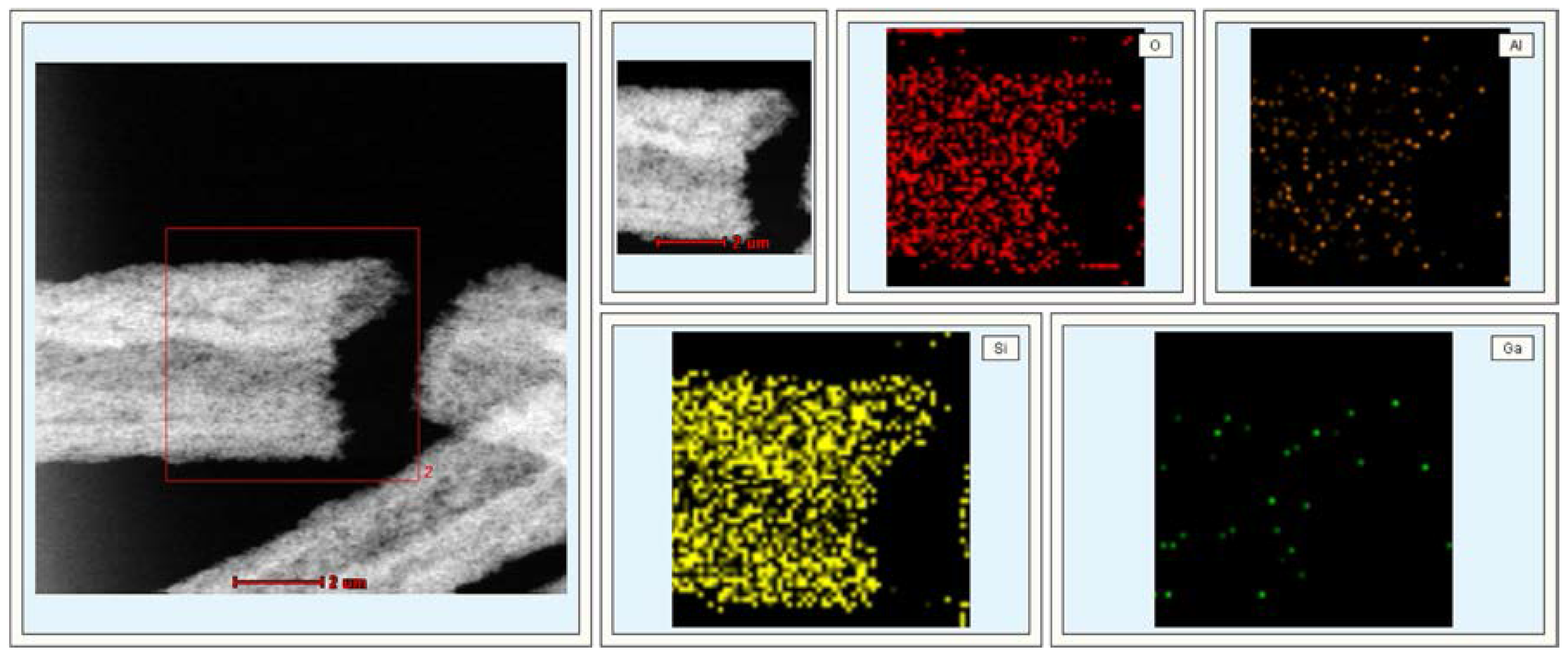
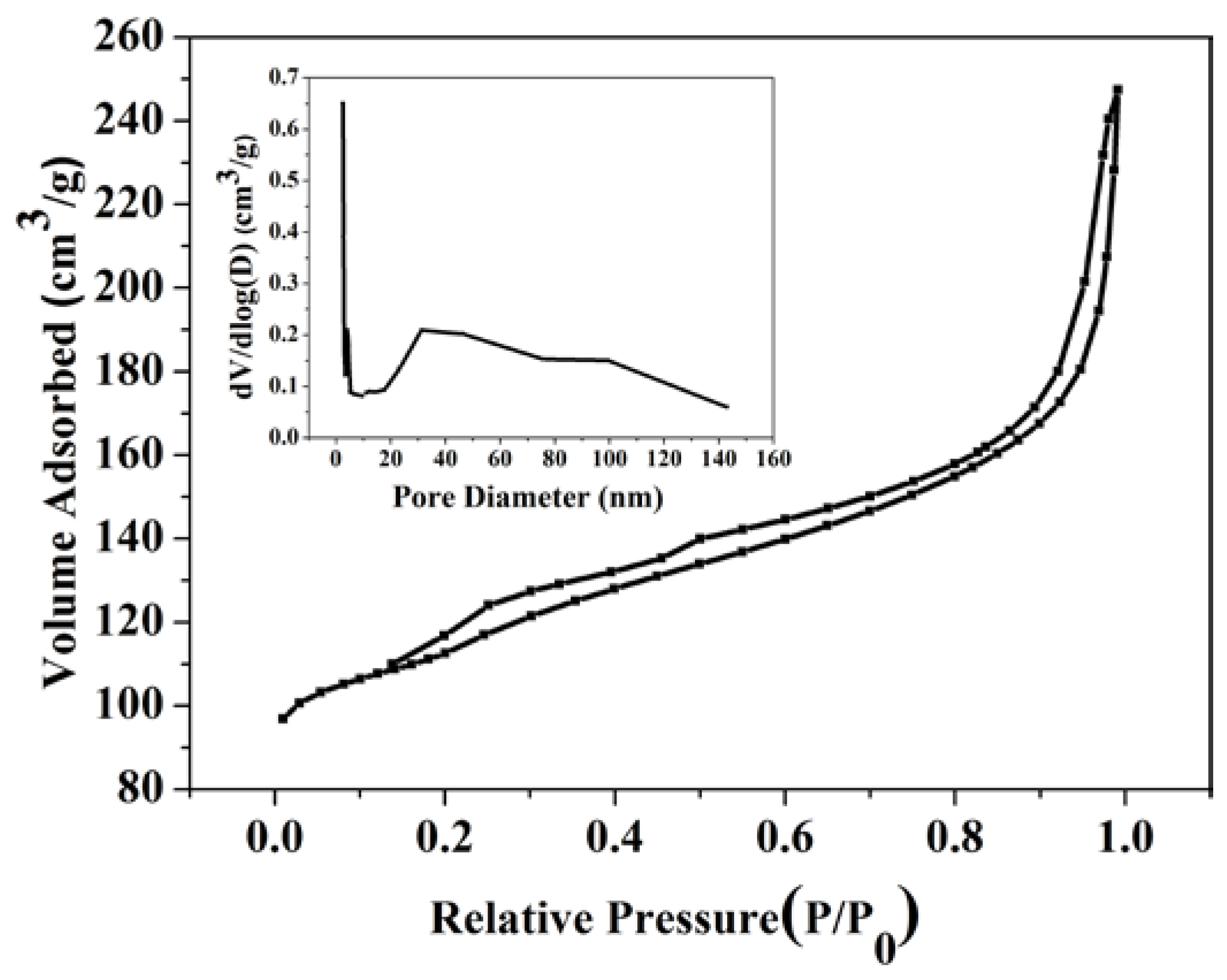
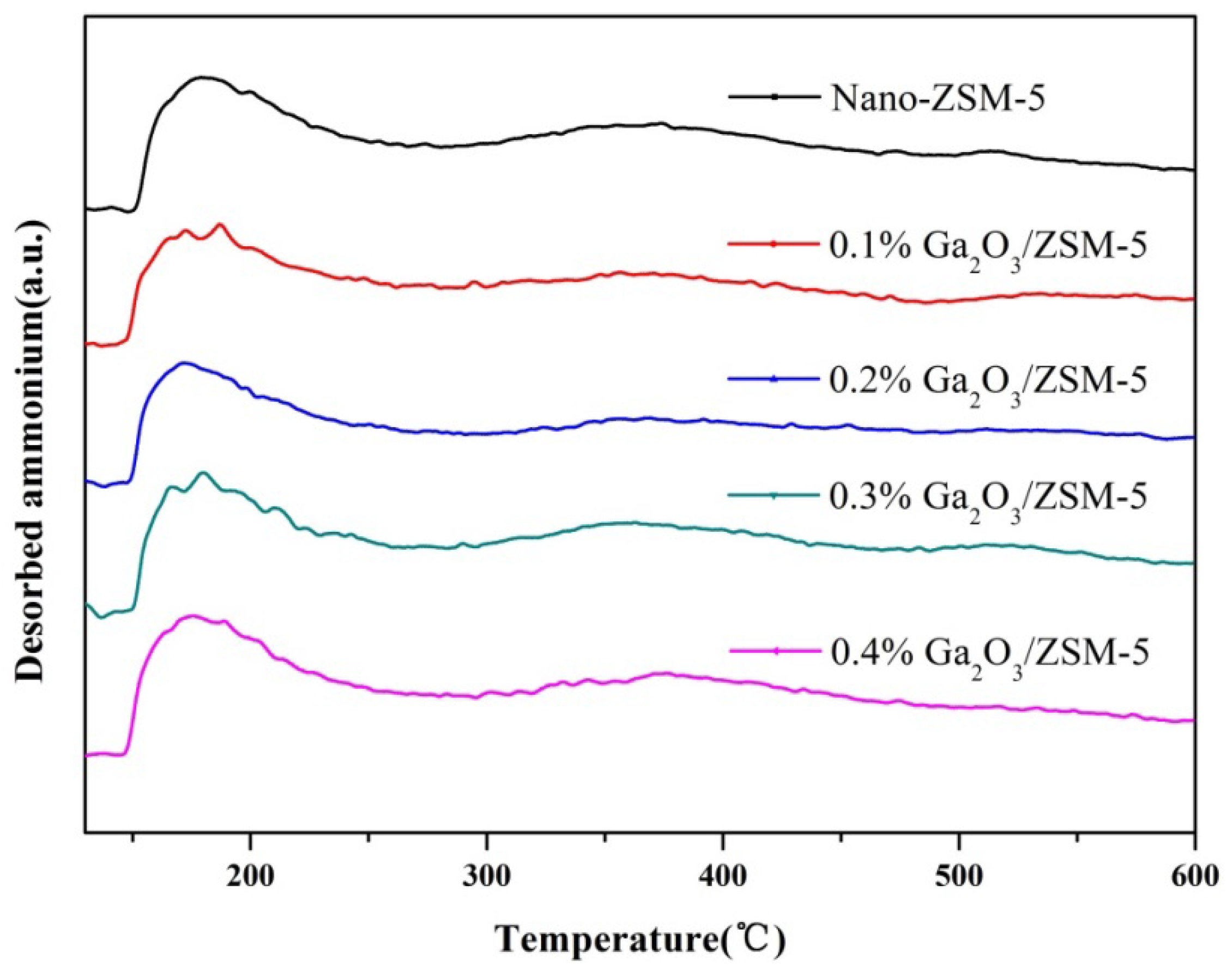
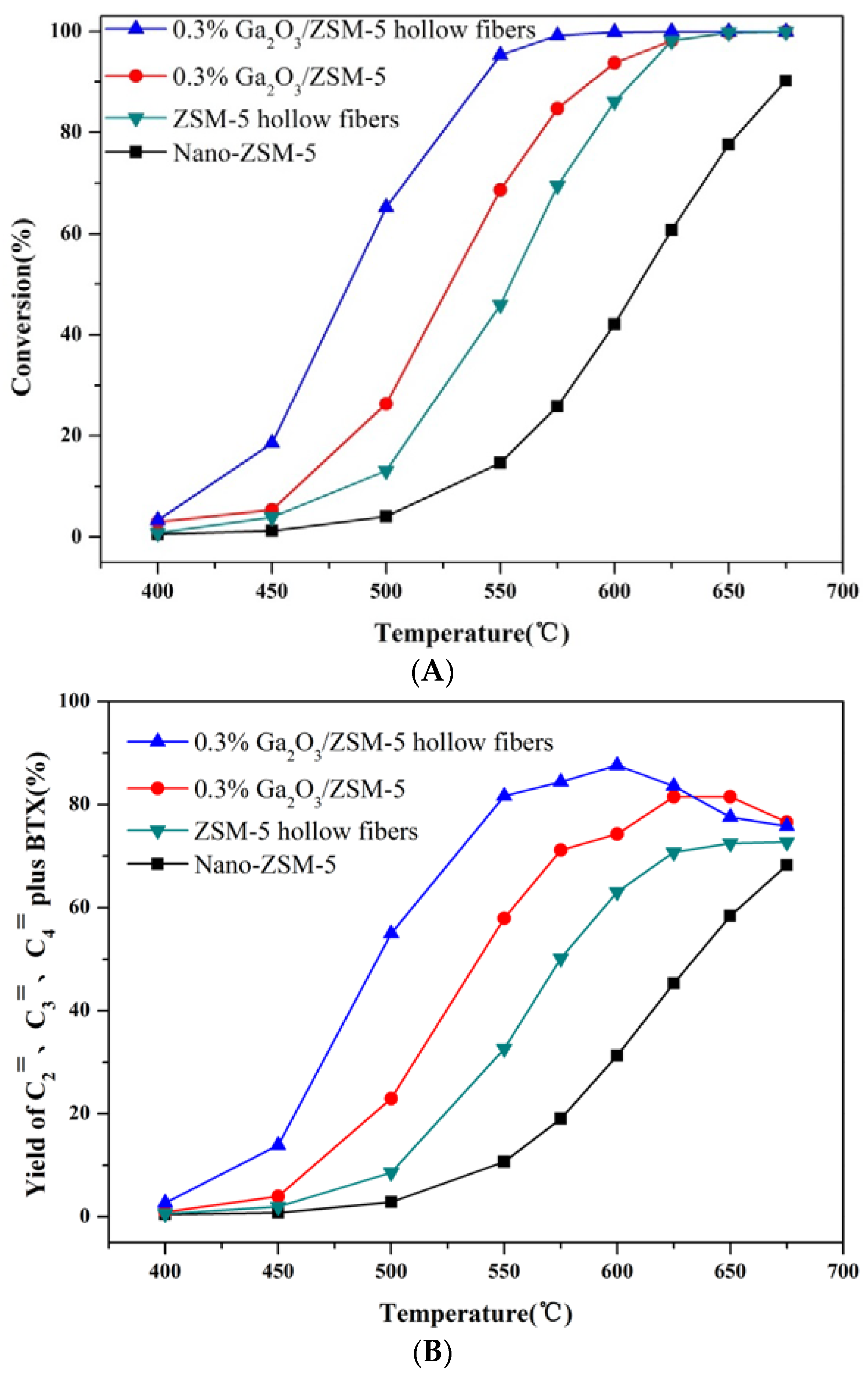
2.2. Catalytic Preformances
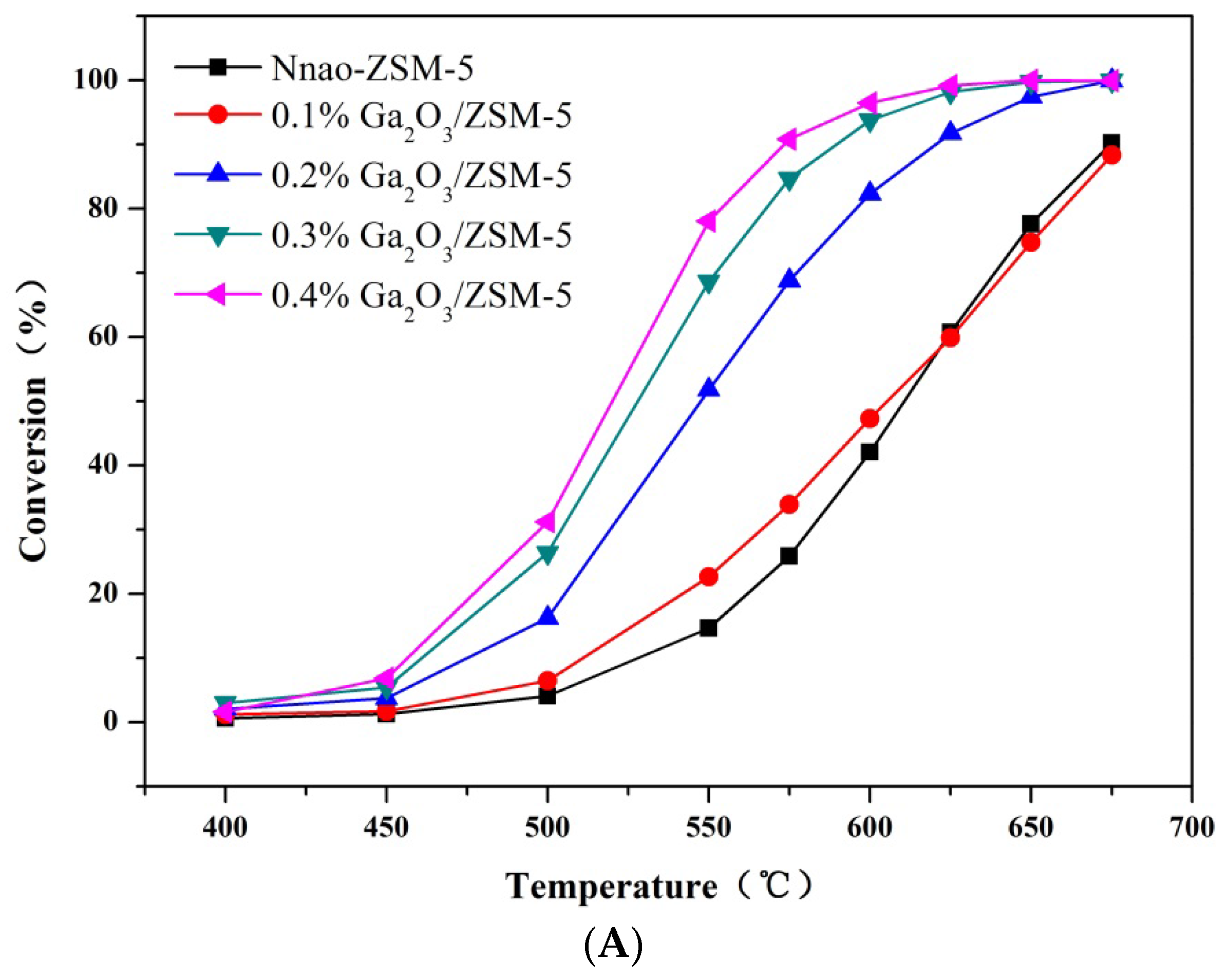
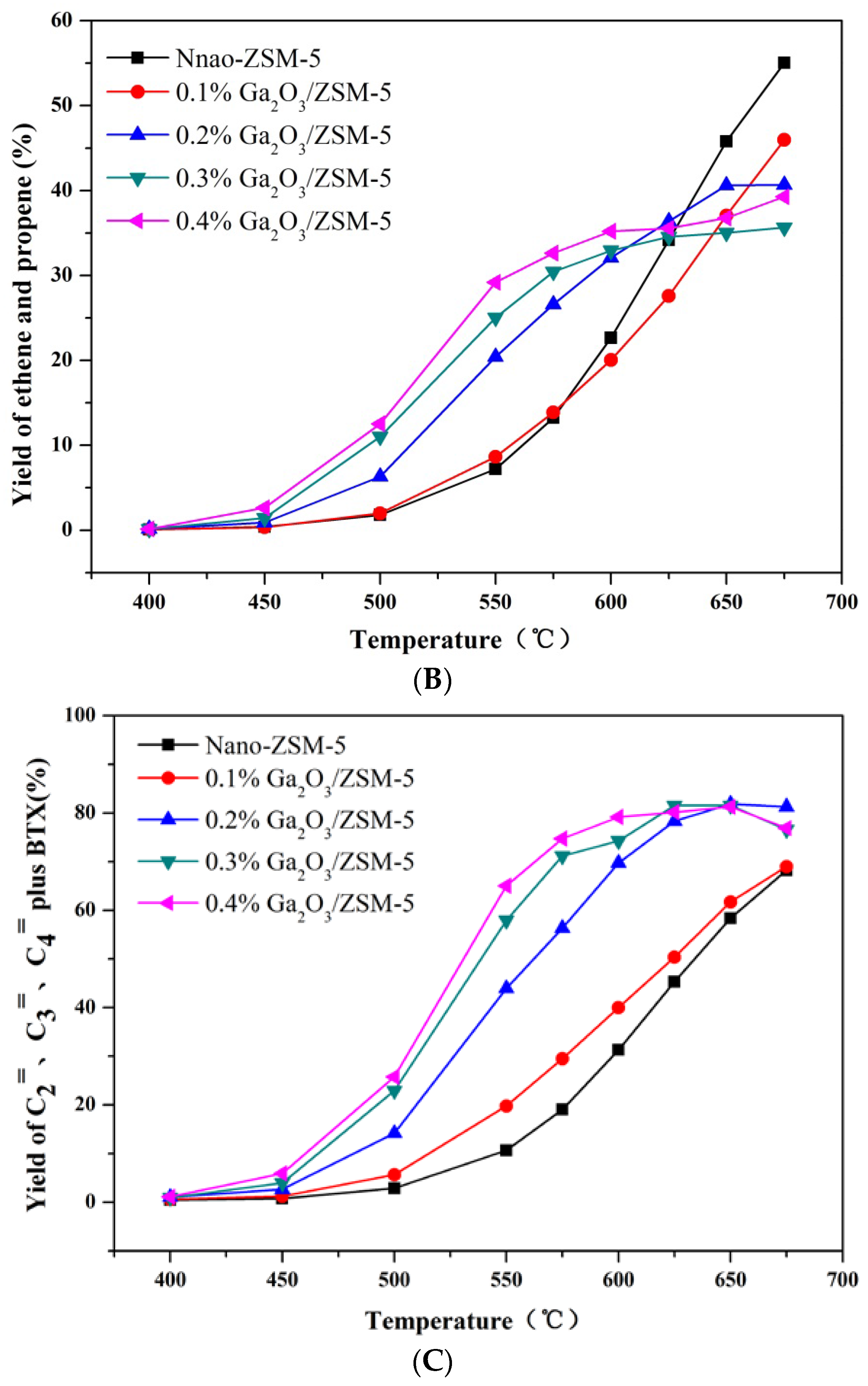
3. Experimental Section
3.1. Catalyst Preparation
3.1.1. ZSM-5 Nanocrystals Preparation
3.1.2. Ga2O3/ZSM-5 Preparation
3.1.3. Hierarchical Ga2O3/ZSM-5 Hollow Fibers Preparation
3.2. Catalyst Characterization
3.3. Reaction Testing
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Maia, A.J.; Oliveira, B.G.; Esteves, P.M.; Louis, B.; Lam, Y.L.; Pereira, M.M. Isobutane and n-butane cracking on Ni-ZSM-5 catalyst: Effect on light olefin formation. Appl. Catal. A 2011, 403, 58–64. [Google Scholar] [CrossRef]
- Meng, X.; Wang, Z.; Zhang, R.; Xu, C.; Liu, Z.; Wang, Y.; Guo, Q. Catalytic conversion of C4 fraction for the production of light olefins and aromatics. Fuel Process. Technol. 2013, 116, 217–221. [Google Scholar] [CrossRef]
- Rahimi, N.; Karimzadeh, R. Catalytic cracking of hydrocarbons over modified ZSM-5 zeolites to produce light olefins: A review. Appl. Catal. A 2011, 398, 1–17. [Google Scholar] [CrossRef]
- Pollesel, P.; Bellussi, G. Industrial applications of zeolite catalysis: Production and uses of light olefins. Stud. Surf. Sci. Catal. 2005, 158, 1201–1212. [Google Scholar]
- Janda, A.; Bell, A.T. Effects of Si/Al ratio on the distribution of framework Al and on the rates of alkane monomolecular cracking and dehydrogenation in H-MFI. J. Am. Chem. Soc. 2013, 135, 19193–19207. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Qiu, C.; Zhuo, Z.; Zhang, D.; Zhao, S.; Wu, H.; Liu, Y.; He, M. Acid strength controlled reaction pathways for the catalytic cracking of 1-butene to propene over ZSM-5. J. Catal. 2014, 309, 136–145. [Google Scholar] [CrossRef]
- Wang, Y.; Yokoi, T.; Namba, S.; Kondo, J.N.; Tatsumi, T. Catalytic cracking of n-hexane for producing propylene on MCM-22 zeolites. Appl. Catal. A 2015, 504, 192–202. [Google Scholar] [CrossRef]
- Pant, K.K.; Kumar, V.A.; Kunzru, D. Potassium-containing calcium aluminate catalysts for pyrolysis of n-heptane. Appl. Catal. A 1997, 162, 193–200. [Google Scholar]
- Jeong, S.M.; Chae, J.H.; Lee, W.-H. Study on the Catalytic Pyrolysis of Naphtha over a KVO3/α-Al2O3 Catalyst for Production of Light Olefins. Ind. Eng. Chem. Res. 2001, 40, 6081–6086. [Google Scholar] [CrossRef]
- Al-Yassir, N.; Le van Mao, R.; Heng, F. Cerium promoted and silica-alumina supported molybdenum oxide in the zeolite-containing hybrid catalyst for the selective deep catalytic cracking of petroleum naphthas. Catal. Lett. 2005, 100, 1–6. [Google Scholar] [CrossRef]
- Na, J.; Liu, G.; Zhou, T.; Ding, G.; Hu, S.; Wang, L. Synthesis and Catalytic Performance of ZSM-5/MCM-41 Zeolites With Varying Mesopore Size by Surfactant-Directed Recrystallization. Catal. Lett. 2013, 143, 267–275. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, G.; Liu, Y.; Di, J.; Wang, Y.; Zhao, Z.; Sun, Q.; Xu, C.; Gao, J.; Duan, A.; et al. Hierarchical macro-meso-microporous ZSM-5 zeolite hollow fibers with highly efficient catalytic cracking capability. Sci. Rep. 2014. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Wei, Y.; Liu, Z.; Li, B.; Qi, Y.; Li, M.; Xie, P.; Meng, S.; He, Y.; Chang, F. A ZSM-5-based Catalyst for Efficient Production of Light Olefins and Aromatics from Fluidized-bed Naphtha Catalytic Cracking. Catal. Lett. 2008, 124, 150–156. [Google Scholar] [CrossRef]
- Zhao, G.; Teng, J.; Xie, Z.; Jin, W.; Yang, W.; Chen, Q.; Tang, Y. Effect of phosphorus on HZSM-5 catalyst for C4-olefin cracking reactions to produce propylene. J. Catal. 2007, 248, 29–37. [Google Scholar] [CrossRef]
- Wakui, K.; Satoh, K.; Sawada, G.; Shiozawa, K.; Matano, K.; Suzuki, K.; Hayakawa, T.; Yoshimura, Y.; Murata, K.; Mizukami, F. Dehydrogenation cracking of n-butane over modified HZSM-5 catalysts. Catal. Lett. 2002, 81, 83–88. [Google Scholar] [CrossRef]
- Lu, J.; Zhao, Z.; Xu, C.; Zhang, P.; Duan, A. FeHZSM-5 molecular sieves-Highly active catalysts for catalytic cracking of isobutane to produce ethylene and propylene. Catal. Commun. 2006, 7, 199–203. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Z.; Xu, C.; Duan, A.; Zhang, L.; Jiang, G. Effects of Light Rare Earth on Acidity and Catalytic Performance of HZSM-5 Zeolite for Catalytic Cracking of Butane to Light Olefins. J. Rare Earths 2007, 25, 321–328. [Google Scholar]
- Jiang, G.; Zhang, L.; Zhao, Z.; Zhou, X.; Duan, A.; Xu, C.; Gao, J. Highly effective P-modified HZSM-5 catalyst for the cracking of C4 alkanes to produce light olefins. Appl. Catal. A 2008, 340, 176–182. [Google Scholar] [CrossRef]
- Jia, C.-J.; Liu, Y.; Schmidt, W.; Lu, A.-H.; Schüth, F. Small-sized HZSM-5 zeolite as highly active catalyst for gas phase dehydration of glycerol to acrolein. J. Catal. 2010, 269, 71–79. [Google Scholar] [CrossRef]
- Zhao, L.; Shen, B.; Gao, J.; Xu, C. Investigation on the mechanism of diffusion in mesopore structured ZSM-5 and improved heavy oil conversion. J. Catal. 2008, 258, 228–234. [Google Scholar] [CrossRef]
- Choi, M.; Na, K.; Kim, J.; Sakamoto, Y.; Terasaki, O.; Ryoo, R. Stable single-unit-cell nanosheets of zeolite MFI as active and long-lived catalysts. Nature 2009, 461, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Martens, J.A.; Blomsma, E.; Jacobs, P.A. Isomerization and Hydrocracking of Heptane over Bimetallic Bifunctional PtPd/H-Beta and PtPd/USY Zeolite Catalysts. J. Catal. 1997, 165, 241–248. [Google Scholar]
- Kim, J.; Kim, W.; Seo, Y.; Kim, J.-C.; Ryoo, R. n-Heptane hydroisomerization over Pt/MFI zeolite nanosheets: Effects of zeolite crystal thickness and platinum location. J. Catal. 2013, 301, 187–197. [Google Scholar] [CrossRef]
- Jackson, S.; Rugmini, S. Dehydrogenation of n-butane over vanadia catalysts supported on θ-alumina. J. Catal. 2007, 251, 59–68. [Google Scholar] [CrossRef]
- Vanlingen, J.; Gijzeman, O.; Weckhuysen, B.; Vanlenthe, J. On the umbrella model for supported vanadium oxide catalysts. J. Catal. 2006, 239, 34–41. [Google Scholar] [CrossRef]
- Cabrera, F.; Ardissone, D.; Gorriz, O.F. Dehydrogenation of propane on chromia/alumina catalysts promoted by tin. Catal. Today 2008, 133–135, 800–804. [Google Scholar] [CrossRef]
- Santhoshkumar, M.; Hammer, N.; Ronning, M.; Holmen, A.; Chen, D.; Walmsley, J.; Oye, G. The nature of active chromium species in Cr-catalysts for dehydrogenation of propane: New insights by a comprehensive spectroscopic study. J. Catal. 2009, 261, 116–128. [Google Scholar] [CrossRef]
- Karamullaoglu, G.; Onen, S.; Dogu, T. Oxidative dehydrogenation of ethane and isobutane with chromium-vanadium-niobium mixed oxide catalys. Chem. Eng. Process. 2002, 41, 337–347. [Google Scholar]
- Nakagawa, K.; Okamura, M.; Ikenaga, N.; Suzuki, N.; Kobayashi, T. Dehydrogenation of ethane over gallium oxide in the presence of carbon dioxide. Chem. Commun. 1998, 9, 1025–1026. [Google Scholar] [CrossRef]
- Sattler, J.J.; Gonzalez-Jimenez, I.D.; Luo, L.; Stears, B.A.; Malek, A.; Barton, D.G.; Kilos, B.A.; Kaminsky, M.P.; Verhoeven, T.W.; Koers, E.J.; et al. Platinum-promoted Ga/Al2O3 as highly active, selective, and stable catalyst for the dehydrogenation of propane. Angew. Chem. Int. Ed. Engl. 2014, 53, 9251–9256. [Google Scholar] [CrossRef] [PubMed]
- Joly, J.F.; Ajot, H.; Merlen, E.; Raatz, F.; Alario, F. Parameters affecting the dispersion of the gallium phase of gallium H-MFI aromatization catalysts. Appl. Catal. A 1991, 79, 249–263. [Google Scholar] [CrossRef]
- Drogone, L.; Moggi, P.; Predieri, G.; Zanoni, R. Niobia and silica-niobia catalysts from sol-gel synthesis: An X-ray photoelectron spectroscopic characterization. Appl. Surf. Sci. 2002, 187, 82–88. [Google Scholar] [CrossRef]
- Sun, Y.; Brown, T.C. Catalytic cracking, dehydrogenation, and aromatization of isobutane over Ga/HZSM-5 and Zn/HZSM-5 at low pressures. Int. J. Chem. Kinet. 2002, 34, 467–480. [Google Scholar] [CrossRef]
- Shin, F.; Tatsuski, Y.; Takayuki, K. Reaction Scheme of Aromatization of Butane over Ga Loaded HZSM-5 Catalyst. J. Jpn. Petrol. Inst. 1998, 41, 37–44. [Google Scholar]
- Qiu, P.; Jack, H.L.; Michael, P.R. Characterization of Ga/ZSM-5 for the catalytic aromatization of dilute ethylene streams. Catal. Lett. 1998, 52, 37–42. [Google Scholar] [CrossRef]
- Mintova, S.; Hölzl, M.; Valtchev, V.; Mihailova, B.; Bouizi, Y.; Bein, T. Closely packed zeolite nanocrystals obtained via transformation of porous amorphous silica. Chem. Mater. 2004, 16, 5452–5459. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.; Jiang, G.; Han, S.; Liu, J.; Zhang, Y.; Liu, Y.; Wang, R.; Zhao, Z.; Xu, C.; Wang, Y.; et al. The Fabrication of Ga2O3/ZSM-5 Hollow Fibers for Efficient Catalytic Conversion of n-Butane into Light Olefins and Aromatics. Catalysts 2016, 6, 13. https://doi.org/10.3390/catal6010013
Han J, Jiang G, Han S, Liu J, Zhang Y, Liu Y, Wang R, Zhao Z, Xu C, Wang Y, et al. The Fabrication of Ga2O3/ZSM-5 Hollow Fibers for Efficient Catalytic Conversion of n-Butane into Light Olefins and Aromatics. Catalysts. 2016; 6(1):13. https://doi.org/10.3390/catal6010013
Chicago/Turabian StyleHan, Jing, Guiyuan Jiang, Shanlei Han, Jia Liu, Yaoyuan Zhang, Yeming Liu, Ruipu Wang, Zhen Zhao, Chunming Xu, Yajun Wang, and et al. 2016. "The Fabrication of Ga2O3/ZSM-5 Hollow Fibers for Efficient Catalytic Conversion of n-Butane into Light Olefins and Aromatics" Catalysts 6, no. 1: 13. https://doi.org/10.3390/catal6010013
APA StyleHan, J., Jiang, G., Han, S., Liu, J., Zhang, Y., Liu, Y., Wang, R., Zhao, Z., Xu, C., Wang, Y., Duan, A., Liu, J., & Wei, Y. (2016). The Fabrication of Ga2O3/ZSM-5 Hollow Fibers for Efficient Catalytic Conversion of n-Butane into Light Olefins and Aromatics. Catalysts, 6(1), 13. https://doi.org/10.3390/catal6010013






