1. Introduction
p-/
m-Xylene, an important intermediate for making polyethylene terephthalate (PET), is industrially derived from the reforming of petroleum naphtha. With depleting stocks of fossil fuels and growing concern on the excessive emission of greenhouse gases, great efforts have been made to develop alternative method of producing sustainable
p-/
m-xylene from biomass-based compounds [
1,
2,
3,
4]. Shiramize and Toste [
1] proposed a method to produce
p-xylene with 2,5-dimethyfuran (derived from lignocellulosic biomass) and acrolein (from glycerol, a side product of biodiesel production) through four step-reactions with an overall yield of 34%. Lyons
et al. [
2] used ethylene (derived from biomass or shale gas) as an alternative feedstock, and synthesized
p-xylene through a very complex process involving trimerizaiton, dehydrogenation, Diels-Alder reaction, and further dehydrogenation. Williams
et al. [
3] suggested a renewable route to
p-xylene from cycloaddition/dehydration of biomass-based dimethylfuran and ethylene with a high selectivity of 75% using Y zeolite as catalyst. Very recently, Cheng
et al. [
4] reported the production of
p-xylene from 2-methylfuran (a product of catalytic fast pyrolysis of lignocellulosic biomass) and propylene using ZSM-5 catalysts. For all of those methods lignocellulosic biomass or its derivatives were used as feedstocks.
Lignin, a residue of pulp/paper industries and lignocellulosics-to-ethanol bioprocess [
5], is a highly cross-linked, oxygenated aromatic polymer consisted of methoxylated phenylpropane units [
6]. In this view, lignin is an abundant, inexpensive and renewable resource of aromatic compounds, such as BTX (benzene, toluene, and xylenes), and may be an attractive alternative resource for producing
p-/
m-xylene. Generally, there are two typical pathways to convert lignin into BTX. Firstly, depolymerization of lignin has been performed to produce amount of phenols (guaiacol, anisole, phenol, cresols,
etc.) [
7,
8,
9], and then catalytic deoxygenation of lignin-derived phenols is used to produce benzene and toluene. For example, phenol was deoxygenated with high selectivity (above 60%) to benzene at 513 K over Ni/HZSM-5 [
10] and at 723 K with CoMoP/MgO catalyst [
11]. Similarly, cresol (
p-cresol or
m-cresol) was also hydrotreated by many researchers at atmospheric pressure over different catalysts to obtain toluene with high selectivity of toluene [
12,
13,
14,
15,
16,
17,
18,
19,
20]. Another pathway to convert lignin into aromatics is catalytic fast pyrolysis producing xylenes with selectivity less than 23%, as reported by Huber
et al. [
21]. Vichaphund
et al. [
22] used metal/HZSM-5 prepared by ion-exchange and impregnation methods producing aromatic compounds from catalytic fast pyrolysis of Jatropha residues. The highest xylenes selectivity is below 30%. Kim
et al. [
23] pyrolyzed native lignin with different HZSM-5 producing xylenes with selectivity less than 10%. Therefore, it is impossible for both the deoxygenation of lignin-derived phenols and fast catalytic pyrolysis of lignin to directly produce
p-/
m-xylene with high selectivity (larger than 10%) [
24].
Side-chain alkylation of toluene with methanol to form styrene and ethylbenzene was studied and the formation of styrene and ethylbenzene was significant on Na(Cs)Y zeolites of exchange degree higher than about 40% [
25]. Gas-phase acylation of phenol with acetic acid to synthesize
o-hydroxyacetophenone was studied on solid acids and zeolites [
26,
27]. The gas-phase alkylation of phenols with methanol was studied on different catalysts (SiO
2-Al
2O
3, HBEA, HZSM5, and HMCM22) [
28,
29]. The main products are cresols and xylenols which are greatly depended on the zeolite pore structure and surface acid properties. The methylation of toluene with methanol is a well-documented reaction to increase the yield of
p-/
m-xylene. This reaction could be catalyzed by various parent zeolites, such as HZSM-5, MOR, MCM-22,
etc., with a high selectivity of
p-xylene up to 90%. However, the maximum conversion of toluene in these processes was generally below 50.0% [
30]. Meanwhile, the alkylation of
m-cresol with methanol was observed to produce xylenols [
31], which also could be converted into xylenes via HDO [
32]. Therefore, if the methanol was co-feeding with hydrogen, the methylation of cresols may also occur, and then
p-/
m-xylene could be produced through the
in situ HDO of xylenols, which is favorable for the high-selective conversion of lignin-based phenols into
p-/
m-xylene.
The objective of this work is to explore and test a new method to improve the p-/m-xylene selectivity through catalytic hydrodeoxygenation and methylation of lignin-based phenols. The hydrodeoxygenation (HDO) of m-cresol and co-catalytic conversion of m-cresol with methanol at different molar ratio over Pt/HZSM-5 were studied to investigate the effect of methanol on co-feeding process. In order to explain the reason why p-/m-xylene selectivity increases, the co-catalytic conversion of m-cresol with methanol under N2, and conversion of the intermediate were carried out. Additionally, we applied this procedure (co-conversion with methanol) to different catalysts and other two phenols (guaiacol and p-cresol).
2. Results and Discussion
In this paper, the HZSM-5 (SiO2/Al2O3 of 25, 57, and 107), desilicated HZSM-5, and HBeta (SiO2/Al2O3 of 25) zeolites were denoted as Z-25, Z-57, Z-107, Z-57D, and B-25, respectively.
In this section, the hydrodeoxygenation of m-cresol was firstly carried out over Pt/Z-57. Then catalytic hydrodeoxygenation and methylation of m-cresol at different molar ratio were studied to investigate the methanol effect on the p-/m-xylene selectivity. Immediately following, co-catalytic conversion under N2 and the intermediate conversion were researched to illustrate the methanol effect on p-/m-xylene selectivity. Based on the discussion above, possible reaction routes for catalytic hydrodeoxygenation and methylation of m-cresol over Pt/Z-57 in the presence of H2 were proposed. Thirdly, in order to validate the reaction routes, catalytic hydrodeoxygenation, and methylation of m-cresol were also conducted over a series of catalysts. Fourthly, the co-conversion with methanol was applied to other kinds of lignin-based phenols to verify its effectiveness.
2.1. Catalytic Hydrodeoxygenation of m-Cresol over Pt/Z-57
Figure 1 presents the conversion of
m-cresol and product distribution with the reaction temperature over Pt/Z-57 catalyst. With the increasing reaction temperature, all the
m-cresol conversion is greater than 95% and slightly changes. However, the product distribution significantly changes. For instance, the toluene selectivity increases from 9.8% at 573 K to 39.8% at 673 K and alters a little at higher temperature, while the selectivity of alkanes (including methylcyclohexane, 1,3-dimethylcyclopentane, and C
3–6) sharply declines from 88.2% at 573 K to 4.4% at 723 K. The
p-/
m-xylene and
o-xylene selectivities have similar variation trend as the toluene selectivity, with separately reaching 16.3% and 4.7% at 673 K and changing a little at higher temperature. Selectivities of benzene and polyalkylated benzenes also raise and reach up to 13.8% and 3.3% at 723 K, respectively. The different selectivity tendencies between aromatic compounds and alkanes could be explained by the fact that at low temperature hydrogenation of toluene and benzene dominates, and H
2 availability on the catalyst surface decreases with the increasing temperature leading to the rapid decrease of alkanes selectivity [
33]. As a result, the aromatic compounds selectivity increases. At the same time, the increase of isomerization, transmethylation, and demethylation activities of
m-cresol over acid sites causes more
o-cresol, xylenols (2,5-xylenol, 2,3-xylenol, and 3,4-xylenol) and phenol (0.9%, 0.0%, and 0.1% at 573 K to 3.3%, 2.1%, and 4.0% at 723 K). The amount of polyalkylated phenols is tiny in the temperature range of 573 K–723 K. Except alkanes, aromatics, and phenolics, other products such as indane, naphthalene, and their derivatives, also exist in small amounts.
Figure 1.
Effect of reaction temperature on (
a) the conversion and deoxygenated product distribution and (
b) oxygenated product distribution from
m-cresol HDO.
![Catalysts 06 00019 i001]()
:
m-cresol conversion,
![Catalysts 06 00019 i002]()
: alkanes,
![Catalysts 06 00019 i003]()
: benzene,
![Catalysts 06 00019 i004]()
: toluene,
![Catalysts 06 00019 i005]()
:
p-/
m-xylene
![Catalysts 06 00019 i006]()
:
o-xylene,
![Catalysts 06 00019 i007]()
: polyalkylated aromatics,
![Catalysts 06 00019 i008]()
: others,
![Catalysts 06 00019 i009]()
: phenol,
![Catalysts 06 00019 i010]()
:
o-cresol;
![Catalysts 06 00019 i011]()
: xylenols,
![Catalysts 06 00019 i012]()
: polyalkylated phenols. (Reaction conditions:
p = 2 MPa,
W/F = 75 g
cat·h/mol
cresol, H
2/cresol = 5, catalyst = 1.0 wt. % Pt/Z-57).
Figure 1.
Effect of reaction temperature on (
a) the conversion and deoxygenated product distribution and (
b) oxygenated product distribution from
m-cresol HDO.
![Catalysts 06 00019 i001]()
:
m-cresol conversion,
![Catalysts 06 00019 i002]()
: alkanes,
![Catalysts 06 00019 i003]()
: benzene,
![Catalysts 06 00019 i004]()
: toluene,
![Catalysts 06 00019 i005]()
:
p-/
m-xylene
![Catalysts 06 00019 i006]()
:
o-xylene,
![Catalysts 06 00019 i007]()
: polyalkylated aromatics,
![Catalysts 06 00019 i008]()
: others,
![Catalysts 06 00019 i009]()
: phenol,
![Catalysts 06 00019 i010]()
:
o-cresol;
![Catalysts 06 00019 i011]()
: xylenols,
![Catalysts 06 00019 i012]()
: polyalkylated phenols. (Reaction conditions:
p = 2 MPa,
W/F = 75 g
cat·h/mol
cresol, H
2/cresol = 5, catalyst = 1.0 wt. % Pt/Z-57).
Generally, the catalytic HDO of oxygen-containing compounds under different conditions using different catalysts is known to proceed through two reaction pathways [
34,
35]. The one path is hydrogenolysis or direct deoxygenation (DDO) of phenols to produce aromatic hydrocarbons. The other one is the coupled ring saturation/rapid dehydration/hydrogenation (HYD) to produce cycloparaffins. The second path involves the intermediate product cyclohexanol or substituted cyclohexanol. In this study, there is undetectable amount of methylcyclohexanol in the products, implying that the DDO reaction route mainly occurs during the
m-cresol HDO process under the tested conditions (
p = 2 MPa,
W/F = 75 g
cat·h/mol
cresol, H
2/cresol = 5). Therefore, alkanes in the products may be generated from the hydrogenation of toluene.
2.2. Catalytic Hydrodeoxygenation and Methylation of m-Cresol over Pt/Z-57
2.2.1. Catalytic hydrodeoxygenation and Methylation at m-Cresol/Methanol Molar Ratio of 1/1
By co-feeding methanol, various methylation reactions of reactants or intermediates may occur as follows: (1) methylation of cresol into xylenols and methylanisole [
31]; (2) methylation of benzene with methanol to give toluene [
36]; (3) methylation of toluene producing xylenes (
o-,
m- and
p-xylene) [
30,
37,
38,
39]; and(4) polyalkylated benzenes and polyalkylated phenols from the methylation of xylenes and xylenols with methanol, respectively.
Catalytic hydrodeoxygenation and methylation of
m-cresol were carried out to examine the possible effect of methanol on the selective conversion into
p-/
m-xylene. The product distribution (at
m-cresol/methanol molar ratio of 1/1) is presented as a function of temperature (see
Figure 2).
Figure 2.
(
a) The
m-cresol conversion and deoxygenated product distribution and (
b) oxygenated product distribution from converting
m-cresol with methanol.
![Catalysts 06 00019 i001]()
:
m-cresol conversion,
![Catalysts 06 00019 i002]()
: alkanes,
![Catalysts 06 00019 i003]()
: benzene,
![Catalysts 06 00019 i004]()
: toluene,
![Catalysts 06 00019 i005]()
:
p-/
m-xylene
![Catalysts 06 00019 i006]()
:
o-xylene,
![Catalysts 06 00019 i007]()
: polyalkylated aromatics,
![Catalysts 06 00019 i008]()
: others,
![Catalysts 06 00019 i009]()
: phenol,
![Catalysts 06 00019 i010]()
:
o-cresol;
![Catalysts 06 00019 i011]()
: xylenols,
![Catalysts 06 00019 i012]()
: polyalkylated phenols,
![Catalysts 06 00019 i013]()
: methylanisole. (Reaction conditions:
m-cresol/methanol molar ratio = 1/1,
p = 2 MPa,
W/F = 75 g
cat·h/mol
cresol, H
2/reagent = 5, catalyst = 1.0 wt. % Pt/Z-57).
Figure 2.
(
a) The
m-cresol conversion and deoxygenated product distribution and (
b) oxygenated product distribution from converting
m-cresol with methanol.
![Catalysts 06 00019 i001]()
:
m-cresol conversion,
![Catalysts 06 00019 i002]()
: alkanes,
![Catalysts 06 00019 i003]()
: benzene,
![Catalysts 06 00019 i004]()
: toluene,
![Catalysts 06 00019 i005]()
:
p-/
m-xylene
![Catalysts 06 00019 i006]()
:
o-xylene,
![Catalysts 06 00019 i007]()
: polyalkylated aromatics,
![Catalysts 06 00019 i008]()
: others,
![Catalysts 06 00019 i009]()
: phenol,
![Catalysts 06 00019 i010]()
:
o-cresol;
![Catalysts 06 00019 i011]()
: xylenols,
![Catalysts 06 00019 i012]()
: polyalkylated phenols,
![Catalysts 06 00019 i013]()
: methylanisole. (Reaction conditions:
m-cresol/methanol molar ratio = 1/1,
p = 2 MPa,
W/F = 75 g
cat·h/mol
cresol, H
2/reagent = 5, catalyst = 1.0 wt. % Pt/Z-57).
At 573 K, the alkanes selectivity of 78.2% drops 11.3% compared with that without methanol (88.2% at 573 K), while further decreases to 3.6% at 723 K. This result implies that the activity of toluene hydrogenation may be suppressed by methanol. Compared with co-feeding methanol, the benzene selectivity decreases (from 0.07% to 0.0% at 573 K and from 13.8% to 11.5% at 723 K), while the toluene selectivity enhances (from 9.8% to 15.1% at 573 K and from 39.9% to 42.9% at 723 K). This can be partly attributed to the methylation of benzene to toluene. The methylation of toluene could be confirmed by the change in the xylenes selectivity, i.e., the p-/m-xylene selectivity (a maximum of 24.0% at 673 K) is about 1.4 times as that without methanol (a maximum of 16.7% at 698 K). Moreover, the deoxygenation of xylenols is also another reaction to produce p-/m-xylene. Similarly, the further methylation of xylenes also takes place which can be demonstrated by the increase of polyalkylated benzenes selectivity after adding methanol (i.e., from 2.8% without methanol to 5.3% with methanol at 673 K).
The decreased phenol and o-cresol selectivities are observed from 0.1% and 0.9% to 0.0% and 0.4% at 573 K, and from 4.1% and 3.3% to 1.1% and 1.6% at 723 K. There are several reasons for this result: (1) phenol and o-cresol converts into cresols and xylenols via an alkylation reaction, respectively; and (2) phenol and o-cresol undergo HDO in the presence of methanol. A small amount of polyalkylated phenols (1.2% at 723 K) are observed compared with negligible amount in the absence of methanol, which may result from the secondary methylation of xylenols.
Above all, the introduction of methanol in the HDO of m-cresol bring several new reactions, including methylation of cresol and toluene, as well as xylenes and other compounds, which obviously improve the selectivity of p-/m-xylene by 44%, possibly through direct methylation of toluene and HDO of xylenols.
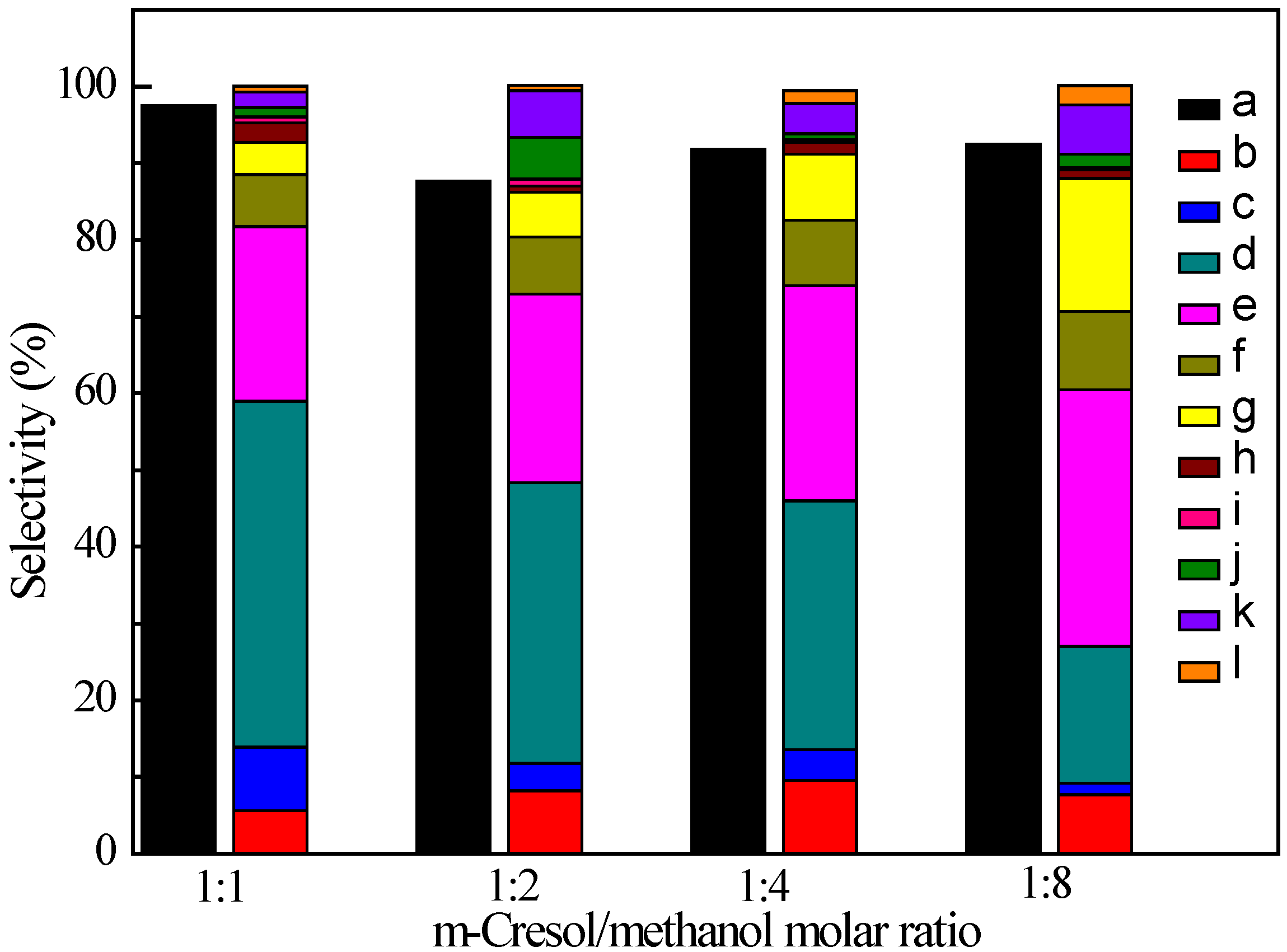
2.2.2. Effect of m-Cresol/Methanol Ratio
Catalytic hydrodeoxygenation and methylation of
m-cresol at different
m-cresol/methanol (C/M) ratio were also experimentally studied towards a further understanding of reaction routes (shown in
Figure 3). At 698 K, the alkanes selectivity is between 5.6% and 9.6% in the investigated C/M ratio range. With the C/M ratio increasing from 1/1 to 1/8, the benzene and toluene selectivities decrease from 8.3% and 45.1% to 1.5% and 17.8%, while the
p-/
m-xylene,
o-xylene, and polyalkylated benzene selectivities increase from 22.8%, 6.8%, and 4.2% to 33.5%, 10.2%, and 17.3%, which also confirm that the further methylation of benzene, toluene, and xylenes produces toluene, xylenes (
p-/
m-xylene and
o-xylene), and polyalkylated benzenes. Especially, when the C/M ratio is greater than or equal to 1/4, the most abundant compound in the product are xylenes. With an increase of methanol at the C/M ratio of 1/8, the
p-/
m-xylene selectivity reaches 33.5%, which is as high as 1.5 times of that at the C/M ratio equal to 1/1, and
ca. 2.0 times as high as that without methanol. The remarkable improvement in the
p-/
m-xylene selectivity may be a result of enhanced methylation reaction rate, as well as the other reactions brought by the methanol, such as methanol to aromatics (MTA) process. Of course, an enhanced methylation reaction rate should be taken as one of the most important pathways as discussed below.
Figure 3.
Effect of the m-cresol/methanol molar ratio on the product distribution. a: m-cresol conversion, b: alkanes, c: benzene, d: toluene, e: p-/m-xylene, f: o-xylene, g: polyalkylated aromatics, h: others, i: phenol, j: o-cresol; k: xylenols, l: polyalkylated phenols. (Reaction conditions: T = 698 K, p = 2 MPa, W/F = 75 gcat·h/molcresol, H2/ reagent = 5, catalyst = 1.0 wt. % Pt/Z-57).
Figure 3.
Effect of the m-cresol/methanol molar ratio on the product distribution. a: m-cresol conversion, b: alkanes, c: benzene, d: toluene, e: p-/m-xylene, f: o-xylene, g: polyalkylated aromatics, h: others, i: phenol, j: o-cresol; k: xylenols, l: polyalkylated phenols. (Reaction conditions: T = 698 K, p = 2 MPa, W/F = 75 gcat·h/molcresol, H2/ reagent = 5, catalyst = 1.0 wt. % Pt/Z-57).
At 698 K, the phenol selectivity is small (less than 1%) in the investigated C/M ratio range. With the increase of the methanol amount in the reactant, the o-cresol and xylenols firstly increase from 1.2% and 2.0% (the C/M ratio of 1/1) to 5.4% and 6.1% (the C/M ratio of 1/2), and then decrease to 0.8% and 3.9% (the C/M ratio of 1/4), and lastly increase to 1.8% and 6.4% (the C/M ratio of 1/8), indicating that there is a complex interaction between the methanol and o-cresol and xylenols needing further investigation.
Therefore, increasing the methanol/
m-cresol molar ratio would further improve the selectivity of
p-/
m-xylene, but also introduce additional reactions, such as the conversion of methanol to hydrocarbons or aromatics [
40]. Obviously, those reactions, to a certain degree, contribute to the considerable improvement of
p-/
m-xylene selectivity, which needs further investigation and clarification.
2.2.3. Co-Catalytic Conversion under N2 and the Intermediate Conversion
To identify the other reactions (MTA, MTH,
etc.) of methanol other than methylation, co-catalytic conversion of
m-cresol with methanol at the C/M ratio of 1/4 was carried out under N
2. Under these conditions, HDO reactions of
m-cresol and xylenols to form toluene and xylenes do not take place [
41]. Thus, the observed alkanes, and aromatics (benzene, toluene,
p-/
m-xylene,
o-xylene, and polyalkylated benzenes) under N
2 (see
Table 1), may be products from MTA and MTH processes. It should be noted in the temperature range of 573 K to 723 K, the
m-cresol conversion under N
2 is less than 51%, while the
m-cresol conversion under H
2 is more than 91%, and the
p-/
m-xylene selectivity (2.6% at 698 K) under N
2 is
ca. 1/11 as much as that with H
2 (28.6% at 723 K), implying that the MTA process, as a minor pathway, only gives a small amount of
p-/
m-xylene in presence of H
2. That is to say, the increased selectivity of
p-/
m-xylene as the increasing C/M ratio is mainly from the toluene methylation and deoxygenation of xylenols. As far as the MTA is concerned, toluene also plays an important role in remarkably enhancing the selectivity of the xylenes (
ca. 8.4 times) as reported by Ilias and Bhan [
42], which may also attribute to high selectivity of
p-/
m-xylene. Similarly, the alkane’s selectivity decreases from 26.8% (with H
2 ) to 1.0% at 648 K (without H
2), confirming that the MTH process indeed produces partial alkanes in the co-catalytic conversion of
m-cresol in spite of further hydrogenation of toluene in the presence of H
2.
Table 1.
Effect of carrier gas on the product distribution from converting m-cresol with methanol.
Table 1.
Effect of carrier gas on the product distribution from converting m-cresol with methanol.
| Reaction Conditions | H2 | N2 |
|---|
| 573 K | 623 K | 648 K | 673 K | 698 K | 723 K | 573 K | 623 K | 648 K | 673 K | 698 K | 723 K |
|---|
| m-Cresol conversion, % | 97.1 | 91.7 | 93.1 | 92.8 | 91.8 | 92.2 | 6.2 | 9.3 | 19.4 | 39.5 | 44.3 | 51.0 |
| Selectivity, % |
| Alkanes | 85.4 | 44.8 | 26.8 | 18.1 | 9.6 | 7.1 | 0.0 | 0.0 | 1.0 | 1.9 | 2.0 | 1.6 |
| Benzene | 0.0 | 0.6 | 1.2 | 2.1 | 4.0 | 5.2 | 0.0 | 0.0 | 0.2 | 0.1 | 0.2 | 0.2 |
| Toluene | 8.5 | 21.7 | 27.9 | 29.8 | 32.4 | 32.1 | 0.0 | 0.8 | 1.0 | 0.8 | 0.7 | 0.7 |
| p-/m-Xylene | 2.1 | 17.0 | 24.2 | 27.3 | 28.0 | 28.6 | 1.4 | 0.9 | 2.1 | 2.4 | 2.6 | 2.3 |
| o-Xylene | 1.0 | 5.1 | 7.2 | 8.3 | 8.5 | 8.9 | 0.0 | 0.0 | 0.4 | 0.5 | 0.5 | 0.5 |
| a PAB | 1.0 | 7.5 | 9.0 | 9.2 | 8.6 | 9.2 | 0.0 | 2.8 | 8.2 | 10.5 | 9.9 | 8.3 |
| Phenol | 0.0 | 0.1 | 0.1 | 0.1 | 0.3 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| o-Cresol | 0.2 | 0.1 | 0.3 | 0.4 | 0.8 | 0.7 | 5.6 | 0.9 | 0.5 | 0.7 | 1.0 | 1.2 |
| Xylenols | 0.9 | 1.9 | 1.9 | 2.5 | 3.9 | 3.5 | 64.2 | 58.0 | 52.3 | 52.6 | 52.8 | 54.3 |
| b PAP | 0.1 | 0.2 | 0.4 | 0.8 | 1.7 | 1.8 | 6.3 | 8.8 | 11.8 | 13.5 | 15.7 | 16.9 |
| c MA | 0.8 | 0.7 | 0.7 | 0.8 | 0.5 | 0.5 | 22.5 | 27.1 | 22.0 | 16.6 | 14.0 | 13.5 |
On the other side, the xylenol’s selectivity under N
2 (54.3% at 723 K) is around 15.5 times compared to that with H
2 (3.5% at 723 K), indicating that the increased
p-/
m-xylene under H
2 may be partially formed from the deoxygenation of xylenols [
32]. Without H
2, the polyalkylated phenols and methylanisole selectivities (16.9% at 723 K and 27.1% at 623 K) raise sharply compared with those with H
2 (1.8% at 723 K and 0.7% at 623 K), illustrating that the reaction rate of xylenols methylation and cresol methylation is much faster in the absence of H
2.
In order to confirm the deoxygenation of xylenols to
p-/
m-xylene, the conversion of 2,4-xylenol was performed under H
2.
Table 2 reports the conversion of 2,4-xylenol and product distribution with the reaction temperature over Pt/Z-57 catalyst. In the investigated temperature range, the 2,4-xylenol conversion is above 97% and the alkanes (including dimethycyclohexane, methylcyclohexane, and pentane) selectivity sharply drops from 95.3% at 573 K to 5.7% at 723 K. With the increasing temperature from 573 K to 723 K, the benzene, toluene,
p-/
m-xylene,
o-xylene, and polyalkylated benzene’s selectivities increase from 0.0%, 0.7%, 2.0%, 0.5%, and 0.2% to 2.7%, 22.5%, 40.0%, 12.2%, and 13.6%, respectively. The total selectivity of the oxygenated product is less than 5.0%. Obviously, it can be concluded that the deoxygenation of 2,4-xylenol could effectively generate
p-/
m-xylene under the experimental conditions.
Table 2.
Product selectivity and conversion for 2,4-xylenol hydrodeoxygenation.
Table 2.
Product selectivity and conversion for 2,4-xylenol hydrodeoxygenation.
| Temperature, K | 2,4-Xylenol Conversion, % | Selectivity, % |
|---|
| Alkanes | Benzene | Toluene | p-/m-Xylene | o-Xylene | a PAB | Cresol | Xylenols | b PAP |
|---|
| 573 | 99.4 | 95.3 | 0.0 | 0.7 | 2.0 | 0.5 | 0.2 | 0.7 | 0.5 | 0.0 |
| 623 | 99.7 | 93.0 | 0.0 | 0.5 | 3.2 | 0.9 | 0.9 | 0.9 | 0.5 | 0.0 |
| 648 | 99.8 | 84.7 | 0.2 | 1.7 | 8.4 | 2.2 | 1.6 | 0.8 | 0.3 | 0.0 |
| 673 | 97.3 | 42.3 | 0.5 | 5.9 | 29.8 | 8.6 | 9.1 | 0.7 | 3.1 | 0.2 |
| 698 | 97.4 | 16.4 | 1.1 | 13.1 | 39.2 | 11.8 | 13.6 | 0.7 | 3.3 | 0.7 |
| 723 | 99.0 | 5.7 | 2.7 | 22.5 | 40.0 | 12.2 | 13.6 | 0.5 | 1.7 | 0.8 |
To sum up, the MTA and MTH of methanol occur in the co-catalytic conversion m-cresol with methanol, but only a small part of p-/m-xylene is produced from the MTA process. Nevertheless, the methylation of toluene and deoxygenation of xylenols are indeed the important pathways of p-/m-xylene formation.
2.2.4. Catalyst Stability
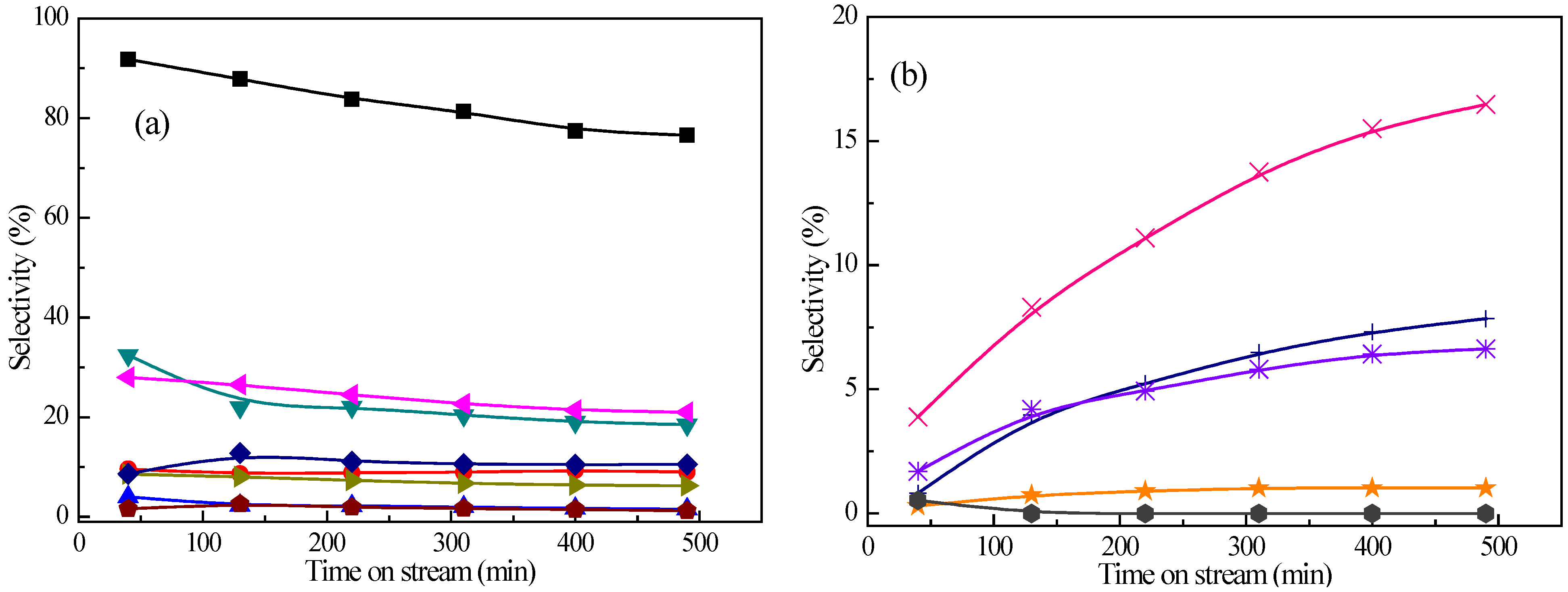
An experiment of co-conversion of
m-cresol with methanol (C/M ratio of 1/4) as a function of time on stream was carried out to test the catalyst stability. The variation of the
m-cresol conversion and product selectivities over Pt/Z-57 at 673 K are plotted in
Figure 4. The catalyst shows deactivation during
ca. 8 h of on-stream reaction on account of the decrease in the conversion from 91.8% to 76.6%. The main products are always
p-/
m-xylene and toluene, and their selectivities decrease from 28.0% and 32.4% to 21.0% and 18.5%. Nevertheless, the selectivities of xylenols,
o-cresol, and polyalkylated phenols increase from 3.9%, 0.8%, and 1.7% to 16.5%, 7.8%, and 6.6%. Thus, it can be seen that the deactivation is attributed to the formation of oxygenated compounds which may lead to the formation of coke on catalysts. The variation of the alkanes,
o-xylene, and polyalkylated benzene’s selectivities is insignificant. Benzene, phenol, methylanisole, and other products are in minor amounts and their selectivities change slightly with increasing the time on stream.
Figure 4.
Effect of time on stream on (
a) the
m-cresol conversion and deoxygenated product distribution and (
b) oxygenated product distribution from converting
m-cresol with methanol.
![Catalysts 06 00019 i001]()
:
m-cresol conversion,
![Catalysts 06 00019 i002]()
: alkanes,
![Catalysts 06 00019 i003]()
: benzene,
![Catalysts 06 00019 i004]()
: toluene,
![Catalysts 06 00019 i005]()
:
p-/
m-xylene
![Catalysts 06 00019 i006]()
:
o-xylene,
![Catalysts 06 00019 i007]()
: polyalkylated aromatics,
![Catalysts 06 00019 i008]()
: others,
![Catalysts 06 00019 i009]()
: phenol,
![Catalysts 06 00019 i010]()
:
o-cresol;
![Catalysts 06 00019 i011]()
: xylenols,
![Catalysts 06 00019 i012]()
: polyalkylated phenols,
![Catalysts 06 00019 i013]()
: methylanisole. (Reaction conditions:
m-cresol/methanol molar ratio = 1/4, catalyst = 1.0 wt. % Pt/Z-57,
W/F = 75 g
cat·h/mol
m-cresol,
T = 673 K, P
H2 = 2 MPa).
Figure 4.
Effect of time on stream on (
a) the
m-cresol conversion and deoxygenated product distribution and (
b) oxygenated product distribution from converting
m-cresol with methanol.
![Catalysts 06 00019 i001]()
:
m-cresol conversion,
![Catalysts 06 00019 i002]()
: alkanes,
![Catalysts 06 00019 i003]()
: benzene,
![Catalysts 06 00019 i004]()
: toluene,
![Catalysts 06 00019 i005]()
:
p-/
m-xylene
![Catalysts 06 00019 i006]()
:
o-xylene,
![Catalysts 06 00019 i007]()
: polyalkylated aromatics,
![Catalysts 06 00019 i008]()
: others,
![Catalysts 06 00019 i009]()
: phenol,
![Catalysts 06 00019 i010]()
:
o-cresol;
![Catalysts 06 00019 i011]()
: xylenols,
![Catalysts 06 00019 i012]()
: polyalkylated phenols,
![Catalysts 06 00019 i013]()
: methylanisole. (Reaction conditions:
m-cresol/methanol molar ratio = 1/4, catalyst = 1.0 wt. % Pt/Z-57,
W/F = 75 g
cat·h/mol
m-cresol,
T = 673 K, P
H2 = 2 MPa).
The TGA measurement was carried out to investigate the deposition of the spent catalyst. The weight change during oxidation with DTG profiles are shown in
Figure 5. The weight loss below 200 °C may be attributed to the removal of water and residual organics absorbed on the catalyst. The weight loss above 200 °C may be attributed to the combustion of coke deposited on catalyst surface [
43]. Obviously, the weight loss (0.8 wt. %) below 200 °C is just 14.5% of the total weight loss (5.5 wt. %), indicating that coke deposition might be one reason for the catalyst deactivation under the experimental conditions.
Figure 5.
TGA profiles of the spent 1.0 wt. % Pt/Z-57 catalysts.
Figure 5.
TGA profiles of the spent 1.0 wt. % Pt/Z-57 catalysts.
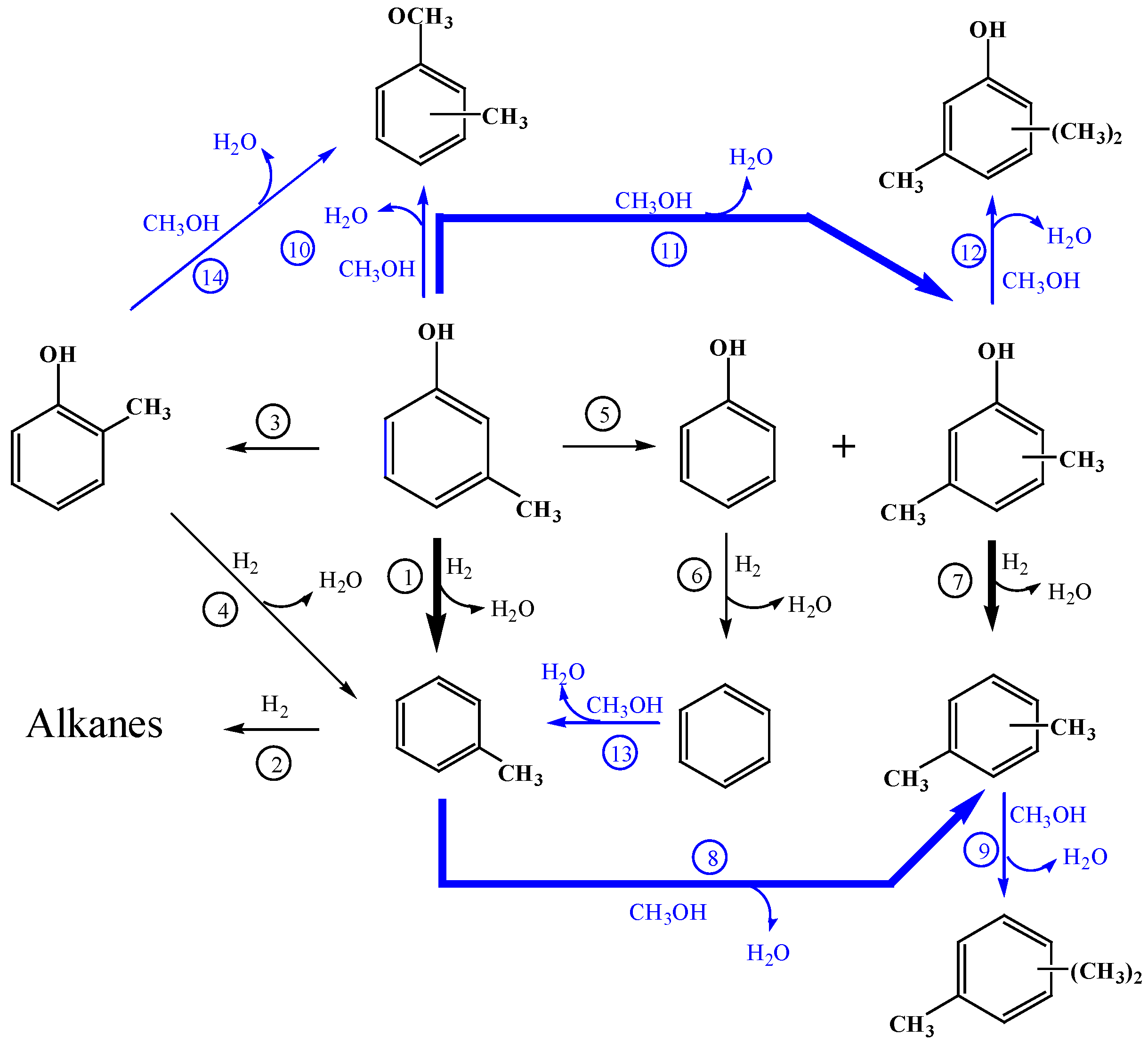
2.2.5. A Possible Reaction Network for Catalytic Hydrodeoxygenation and Methylation of m-Cresol
Based on the discussion above, a possible reaction network for catalytic hydrodeoxygenation and methylation of
m-cresol over Pt/HZSM-5 is shown in
Figure 6. First of all,
m-cresol could be converted into toluene via HDO reaction. Then toluene could be further converted into alkanes and xylenes (
p-/
m-xylene and
o-xylene) through methylation. Of course, the further methylation of xylenes generates polyalkylated benzenes. Two other parallel alkylation reaction pathways of
m-cresol with methanol are described below:
O-alkylation of
m-cresol to methylanisole, and
C-alkylation into xylenols which could be also formed by disproportionation of
m-cresol. The HDO of xylenols generates xylenes accompanied with the formation of polyalkylated phenols as by-products. Phenol could be deoxygenated to benzene which could be converted into toluene through methylation.
m-Cresol could be isomerized to
o-cresol via an isomerization reaction which could be converted into methyanisole and xylenols by methylation, as well as toluene via HDO.
Figure 6.
A possible reaction routes for catalytic hydrodeoxygenation and methylation of m-cresol over Pt/HZSM-5. The blue line represents the reaction involved in methanol.
Figure 6.
A possible reaction routes for catalytic hydrodeoxygenation and methylation of m-cresol over Pt/HZSM-5. The blue line represents the reaction involved in methanol.
2.3. Catalyst Screening
In this section, catalytic hydrodeoxygenation and methylation of
m-cresol (C/M ratio of 1/4) over different catalysts were studied to investigate the effect of the nature of the supporter and metal on the product distribution.
Table 3 shows the conversion of
m-cresol and product distribution at 698 K over seven kinds of catalysts. At 698 K, the abundant compounds are toluene and
p-/
m-xylene on the catalysts except 1.0 wt. % Pt/B-25. For the Pt/HZSM-5 catalysts with parent supporter loading same metal content, the toluene and
p-/
m-xylene selectivities are, respectively, in the range of 32.4% to 36.9% and 27.8% to 28.7%, and the conversion of
m-cresol and product distribution does not exhibit a distinct relation to the SiO
2/Al
2O
3 ratio (acid amount). Nevertheless, for Pt/Z-57 with lower Pt content, the conversion of
m-cresol declines from 91.8% to 83.4%, the alkanes selectivity decreases (from 9.6% to 8.1%), and the total oxygenated compounds (phenol,
o-cresol, xylenols, and polyalkylated phenols) selectivity increases (from 6.7% to 10.0%) with lower Pt loading. This is probably associated with the reducing hydrogenation capacity of the catalyst with low metal loading. The lower selectivities of benzene and toluene, and the higher selectivities of
p-/
m-xylene and polyalkylated benzenes, are also observed over 0.6 wt. % Pt/Z-57 compared to 1.0 wt. % Pt/Z-57. This could be explained that the deoxygenation activity of oxygenated compounds decreases because of the deactivation, and the aromatics’ methylation rate enhances over 0.6 wt. % Pt/Z-57. Over 10.0 wt. % Ni/Z-107, the decrease in the
m-cresol conversion (from 93.7% over 1.0 wt. % Pt/Z-107 to 85.1%), and the selectivities of
p-/
m-xylene and polyalkylated benzenes (from 28.7% and 9.3% over 1.0 wt. % Pt/Z-107 to 22.7% and 6.2%) may be attributed to the coverage of the active sites by high metal loading, while the increase of the alkane’s selectivity (from 8.0% over 1.0 wt. % Pt/Z-107 to 11.4%) is possible relative to the high hydrogenation selectivity over Ni/Z-107. Otherwise, the total oxygenated compounds selectivity on 10.0 wt. % Ni/Z-107 is
ca. two times as much as that on 1.0 wt. % Pt/Z-107, which may result from the catalyst deactivation.
Table 3.
Effect of catalysts on the product distribution from converting m-cresol with methanol.
Table 3.
Effect of catalysts on the product distribution from converting m-cresol with methanol.
| Type of Catalyst | 1.0 wt. % Pt/Z-25 | 0.6 wt. % Pt/Z-57 | 1.0 wt. % Pt/Z-57 | 1.0 wt. % Pt/Z-57D | 1.0 wt. % Pt/Z-107 | 10.0 wt. % Ni/Z-107 | 1.0 wt. % Pt/B-25 |
|---|
| m-Cresol conversion % | 94.5 | 83.4 | 91.8 | 96.7 | 93.7 | 85.1 | 97.2 |
| Selectivity % |
| Alkanes | 7.0 | 8.1 | 9.6 | 10.4 | 8.0 | 11.4 | 4.8 |
| Benzene | 4.2 | 2.6 | 4.0 | 1.3 | 3.3 | 5.5 | 0.4 |
| Toluene | 36.9 | 28.7 | 32.4 | 23.3 | 35.3 | 33.3 | 4.5 |
| p-/m-Xylene | 27.8 | 29.6 | 28.0 | 34.3 | 28.7 | 22.7 | 14.2 |
| o-Xylene | 8.3 | 8.9 | 8.5 | 10.4 | 8.6 | 6.9 | 4.2 |
| a PAB | 7.8 | 10.4 | 8.6 | 16.0 | 9.3 | 6.2 | 45.9 |
| Phenol | 0.3 | 0.4 | 0.3 | 0.2 | 0.3 | 1.3 | 0.3 |
| o-Cresol | 1.6 | 2.1 | 0.8 | 0.6 | 1.4 | 4.8 | 0.9 |
| Xylenols | 3.6 | 6.0 | 3.9 | 2.0 | 3.4 | 6.7 | 8.5 |
| b PAP | 1.2 | 1.5 | 1.7 | 1.0 | 1.0 | 0.8 | 12.5 |
| Oxygenated compounds | 6.5 | 10.0 | 6.7 | 3.8 | 6.1 | 13.6 | 22.1 |
| others | 1.4 | 0.9 | 1.6 | 0.4 | 0.7 | 0.4 | 4.0 |
After introducing mesopores by desilication, the
m-cresol conversion and the alkane’s selectivity increases from 91.8% and 9.6% to 96.7% and 10.4%, while the total oxygenated compounds selectivity is just 56.7% of that over 1.0 wt. % Pt/Z-57, demonstrating that the generation of mesopores and the improved Pt dispersion by desilication are conducive to the
m-cresol transformation, aromatics’ hydrogenation, and deoxygenation of oxygenated compounds. It should be noted that the
p-/
m-xylene selectivity (34.3%) over Pt/Z-57D is the highest among the seven catalysts, and the benzene and toluene selectivities clearly reduce (from 4.0% and 32.4% over Pt/Z-57 to 1.3% and 23.3% over Pt/Z-57D). The reason for this may be correlated with the increase of the aromatics methylation rate in the generated mesopores thanks to appropriate aperture size and the decrease in the effective diffusion length by desilication [
44]. For Pt/B-25, the
m-cresol conversion (97.2%) is the largest among the seven catalysts. Meanwhile, the selectivities of larger molecules (such as polyalkylated benzenes, xylenols, and polyalkylated phenols) are also the maximal. This is related to the 12-membered ring channels’ convenience to further alkylation of
p-/
m-xylene, cresols, and xylenols, which causes the consumption of
p-/
m-xylene and the increase of larger molecules.
As a consequence, the nature of the supporter and metal of a catalyst has significant influence on the catalytic hydrodeoxygenation and methylation of m-cresol. The optimal catalyst should have suitable pore structure, metal loading, and acid amount. Among the investigated catalysts, 1.0 wt. % Pt/Z-57D is the best with highest p-/m-xylene selectivity of 34.3% in the experimental condition.
2.4. Catalytic Hydrodeoxygenation and Methylation of Other Phenols
Catalytic hydrodeoxygenation and methylation of other lignin-derived model compounds (guaiacol and
p-cresol) were carried out over the optimum catalyst (Pt/Z-57D) at the phenolic compound/methanol ratio of 1/4 at 673 K. As shown in
Table 4, the reactivity of the tested phenolics decreases in the order guaiacol (100.0%) >
m-cresol (95.3%) >
p-cresol (93.0%). The selectivities of alkanes (33.8% > 27.3% > 17.5%) and benzene (6.6% > 1.3% > 1.1%) are in the same order as that of the reactivity, while the order of the selectivities of toluene (14.4% < 18.0% < 23.1%) and
p-/
m-xylene (21.3% < 22.2% < 30.0%) are opposite. The higher selectivities of alkylated compounds (toluene,
p-/
m-xylene, polyalkylated benzenes, and xylenols) from cresol conversion than those from guaiacol conversion may be attributed to the methyl group directly connecting with the aromatic ring in the cresol molecule. The different product distribution from
p-cresol conversion with methanol and
m-cresol conversion with methanol may relate to the electronic effect caused by the different position of the methyl group.
Table 4.
Effect of phenols on the product distribution.
Table 4.
Effect of phenols on the product distribution.
| Reactant | Guaiacol | p-Cresol | m-Cresol |
|---|
| Conversion, % | 100.0 | 93.0 | 95.3 |
| Selectivity, % |
| Alkanes | 33.8 | 27.3 | 17.5 |
| Benzene | 6.6 | 1.3 | 1.1 |
| Toluene | 14.4 | 18.0 | 23.1 |
| p-/m-Xylene | 21.3 | 22.2 | 30.0 |
| o-Xylene | 6.2 | 6.7 | 9.0 |
| a PAB | 11.2 | 14.4 | 13.6 |
| Phenol | 0.6 | 0.6 | 0.2 |
| o-Cresol | 1.6 | 1.4 | 0.9 |
| Xylenols | 2.0 | 5.0 | 2.6 |
| b PAP | 1.4 | 2.4 | 1.4 |
As a result, although the conversion and product distribution have some difference from various kinds of phenolics with methanol, catalytic hydrodeoxygenation and methylation of lignin-derived model compounds can effectively generate p-/m-xylene. This result could be as a reference for direct conversion of lignin-derived model compounds (lignin phenols) with methanol.
3. Experimental Section
3.1. Materials
m-Cresol (purity 99.7%, Aladdin, Shanghai, China), p-cresol (purity 99.0%, Aladdin, Shanghai, China), guaiacol (purity 99.0%, J&K, Tianjin, China), 2,4-xylenol (purity 99.0%, J&K, Tianjin, China), and methanol (purity 99.0%, J&K, Tianjin, China) were used as received. The HZSM-5 (SiO2/Al2O3 of 25, 57 and 107) and HBeta (SiO2/Al2O3 of 25) zeolites were purchased from Shanghai Novel Chemical Technology Co. Ltd. (Shanghai, China) and H2PtCl6·6H2O as the active component precursor was obtained from Tianjin Guangfu Fine Chemical Research Institute (Tianjin, China). Other compounds (cyclohexane, toluene, o-xylene, m-xylene, p-xylene, phenol, 2,5-xylenol, 2,6-xyelnol, and 3,4-xylenol, GCS specifications) for identifying and calibrating products were also purchased from Tianjin Guangfu Fine Chemical Research Institute (Tianjin, China). Hydrogen (purity 99.96%) was obtained from Tianjin Six-Party Industrial Gases Co., Ltd. (Tianjin, China).
3.2. Catalyst Preparation and Characterization
The desilicated HZSM-5 sample was prepared by heating the parent HZSM-5 (SiO2/Al2O3 of 57) in a 0.5 M NaOH solution (10 mL per gram of zeolite) at 333 K under stirring for 60 min. Pt/HZSM-5 was prepared by impregnating HZSM-5 zeolite with aqueous solutions of H2PtCl6·6H2O. The prepared sample was dried at 393 K for 12 h, and then calcined at 773 K for 4 h in air. After calcination, catalysts were ground to a fine powder and pressed into pellets. These pellets were then crushed and sieved to give particles sized 250–400 μm (40–60 mesh). Ni/HZSM-5 and Pt/HBeta were prepared as the same method as Pt/HZSM-5. In this paper, the HZSM-5 (SiO2/Al2O3 of 25, 57 and 107), desilicated HZSM-5 and HBeta (SiO2/Al2O3 of 25) zeolites were denoted as Z-25, Z-57, Z-107, Z-57D, and B-25, respectively.
The BET (Brunauer-Emmett-Teller) surface area and pore volume of catalysts were determined by using N
2 isothermal (77 K) adsorption on Micromeritics Tristar 3000 physisorption analyzer (Atlanta, GA, USA). The SiO
2/Al
2O
3 ratio of the fresh catalysts was determined using an X-ray Fluorescence spectrometer (Philips Magix PW 2403 XRF, Amsterdam, Holland). NH
3-temperature programmed desorption (NH
3-TPD) and CO pulse chemisorptions were performed on an Altamira-300 temperature programming unit equipped with a thermal conductivity detector (TCD). The chemical composition, textural properties, calculated dispersion, and NH
3 uptake of the parent and desilicated materials are exhibited in
Table 5.
The coke deposited on the spent catalysts was quantified by thermogravimetry (TGA-Q500, TA, New Castle, DE, USA). The TG experiments were carried out by raising the sample temperature from room temperature up to 1073 K at a rate of 10 K/min in a mixture flow of N2 balance gas (40 mL/min) and air sample gas (40 mL/min).
Table 5.
Chemical composition, textural properties, NH3 uptake, and calculated dispersion of the catalysts.
Table 5.
Chemical composition, textural properties, NH3 uptake, and calculated dispersion of the catalysts.
| Catalysts | a Metal Content, wt. % | b SiO2/Al2O3 | c SBET, m2/g | d Vo, cm3/g | e Vm, cm3/g | NH3 Uptake (μmol/g) | f Calculated Dispersion, % |
|---|
| Pt/Z-25 | 1.0 | 29.2 | 334 | 0.11 | 0.10 | 959.2 | 14.1 |
| Pt/Z-57 | 0.6 | - | 368 | 0.11 | 0.04 | 627.6 | 13.7 |
| Pt/Z-57 | 1.0 | 50.6 | 400 | 0.12 | 0.08 | 717.6 | 10.4 |
| Pt/Z-57D | 1.0 | 38.6 | 342 | 0.10 | 0.12 | 513.0 | 17.2 |
| Pt/Z-107 | 1.0 | 100.4 | 342 | 0.11 | 0.10 | 413.8 | 18.0 |
| Ni/Z-107 | 10.0 | - | 264 | 0.10 | 0.05 | 472.3 | 0.6 |
| Pt/B-25 | 1.0 | 21.6 | 503 | 0.18 | 0.18 | 938.8 | 7.0 |
3.3. Experiment Procedure
Experiments were carried out in a continuous-flow tubular fixed-bed reactor made of stainless steel (i.d. = 10 mm, l = 1000 mm), which was described in detail elsewhere [
45]. In a typical run, 3 g of catalyst diluted with SiC was packed into the stainless steel reactor, and then reduced at 723 K for 4 h in a flow of H
2 (120 mL/min) controlled by a mass flow controller. Reactants (
m-cresol or a mixture of
m-cresol (
p-cresol or guaiacol) with methanol,
W/Fm-cresol = 75 g
cat·h/mol
m-cresol) were feed through a HPLC pump. The pressure of H
2 was 2 MPa and the molar ratio of H
2/reagent kept constant at five. The catalyst bed temperature was measured by a K-type thermocouple and maintained using a tube furnace equipped with a temperature controller. The reaction temperature arrived at a heating rate of 5 K/min from room temperature and in this study it changed from 573 K to 723 K. A water-cooled condenser was used to separate condensable from volatile products. The gas product stream was collected in a gas bag. The liquid product was sampled for analysis with an off-line GC. Each experiment was repeated three times, and the results were deviated
ca. 4%.
A Bruker 456-GC (Karlsruhe, Germany) equipped with BR-5ms column (30 m × 0.25 mm × 0.25 μm) and a flame ionization detector (FID) was used to quantify the liquid products. The GC oven temperature was programmed as follow: maintained 318 K for 2 min, and ramped from 318 K to 523 K with a heating rate of 10 K/min, and finally kept 523 K for 5 min. Standards of known concentration containing observed reactants and products, i.e., a mixture of m-cresol, cylclohexane, toluene, p-xylene, phenol, and 2,4-dimethyl phenol, were used to calibrate the conversion and yield from FID area. Identification of products was carried out by liquid product injections to an Agilent 7890A gas chromatograph (GC) equipped with HP-5ms column (30 m × 0.25 mm × 0.25 μm) and a 5975C mass selective detector (MSD), with temperature starting at 323 K for 5 min, and then 5 K/min up to 443 K and, lastly, dwelling at 443 K for 2 min. Each sample was analyzed at least twice, and the deviation error of analysis was about ±1%.
The W/F (catalyst weight/molar feed flow rate) values reported were found by dividing the total weight of catalyst in the reactor bed by the molar flow rate of m-cresol (or other kinds of lignin-based phenols) injected during a given experiment. Product selectivity is defined as the mass fraction of the material in the total product.
 : m-cresol conversion,
: m-cresol conversion,  : alkanes,
: alkanes,  : benzene,
: benzene,  : toluene,
: toluene,  : p-/m-xylene
: p-/m-xylene  : o-xylene,
: o-xylene,  : polyalkylated aromatics,
: polyalkylated aromatics,  : others,
: others,  : phenol,
: phenol,  : o-cresol;
: o-cresol;  : xylenols,
: xylenols,  : polyalkylated phenols. (Reaction conditions: p = 2 MPa, W/F = 75 gcat·h/molcresol, H2/cresol = 5, catalyst = 1.0 wt. % Pt/Z-57).
: polyalkylated phenols. (Reaction conditions: p = 2 MPa, W/F = 75 gcat·h/molcresol, H2/cresol = 5, catalyst = 1.0 wt. % Pt/Z-57).
 : m-cresol conversion,
: m-cresol conversion,  : alkanes,
: alkanes,  : benzene,
: benzene,  : toluene,
: toluene,  : p-/m-xylene
: p-/m-xylene  : o-xylene,
: o-xylene,  : polyalkylated aromatics,
: polyalkylated aromatics,  : others,
: others,  : phenol,
: phenol,  : o-cresol;
: o-cresol;  : xylenols,
: xylenols,  : polyalkylated phenols. (Reaction conditions: p = 2 MPa, W/F = 75 gcat·h/molcresol, H2/cresol = 5, catalyst = 1.0 wt. % Pt/Z-57).
: polyalkylated phenols. (Reaction conditions: p = 2 MPa, W/F = 75 gcat·h/molcresol, H2/cresol = 5, catalyst = 1.0 wt. % Pt/Z-57).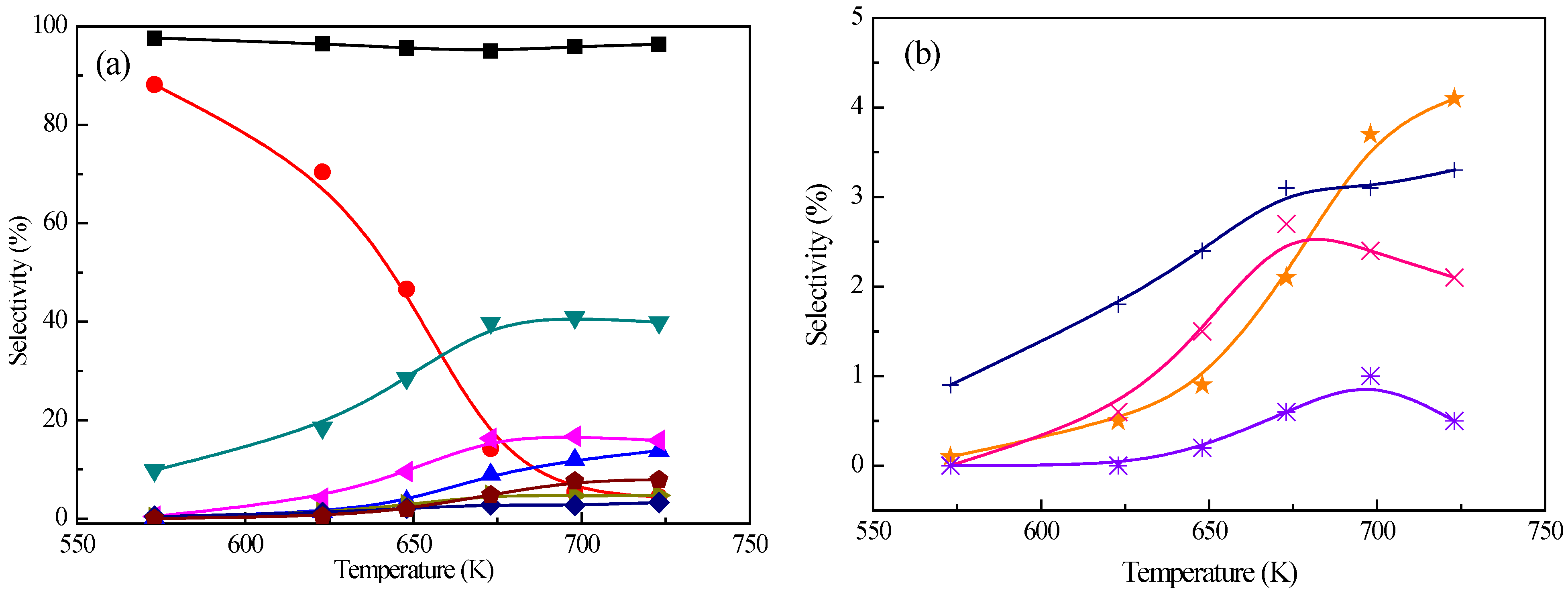
 : m-cresol conversion,
: m-cresol conversion,  : alkanes,
: alkanes,  : benzene,
: benzene,  : toluene,
: toluene,  : p-/m-xylene
: p-/m-xylene  : o-xylene,
: o-xylene,  : polyalkylated aromatics,
: polyalkylated aromatics,  : others,
: others,  : phenol,
: phenol,  : o-cresol;
: o-cresol;  : xylenols,
: xylenols,  : polyalkylated phenols,
: polyalkylated phenols,  : methylanisole. (Reaction conditions: m-cresol/methanol molar ratio = 1/1, p = 2 MPa, W/F = 75 gcat·h/molcresol, H2/reagent = 5, catalyst = 1.0 wt. % Pt/Z-57).
: methylanisole. (Reaction conditions: m-cresol/methanol molar ratio = 1/1, p = 2 MPa, W/F = 75 gcat·h/molcresol, H2/reagent = 5, catalyst = 1.0 wt. % Pt/Z-57).
 : m-cresol conversion,
: m-cresol conversion,  : alkanes,
: alkanes,  : benzene,
: benzene,  : toluene,
: toluene,  : p-/m-xylene
: p-/m-xylene  : o-xylene,
: o-xylene,  : polyalkylated aromatics,
: polyalkylated aromatics,  : others,
: others,  : phenol,
: phenol,  : o-cresol;
: o-cresol;  : xylenols,
: xylenols,  : polyalkylated phenols,
: polyalkylated phenols,  : methylanisole. (Reaction conditions: m-cresol/methanol molar ratio = 1/1, p = 2 MPa, W/F = 75 gcat·h/molcresol, H2/reagent = 5, catalyst = 1.0 wt. % Pt/Z-57).
: methylanisole. (Reaction conditions: m-cresol/methanol molar ratio = 1/1, p = 2 MPa, W/F = 75 gcat·h/molcresol, H2/reagent = 5, catalyst = 1.0 wt. % Pt/Z-57).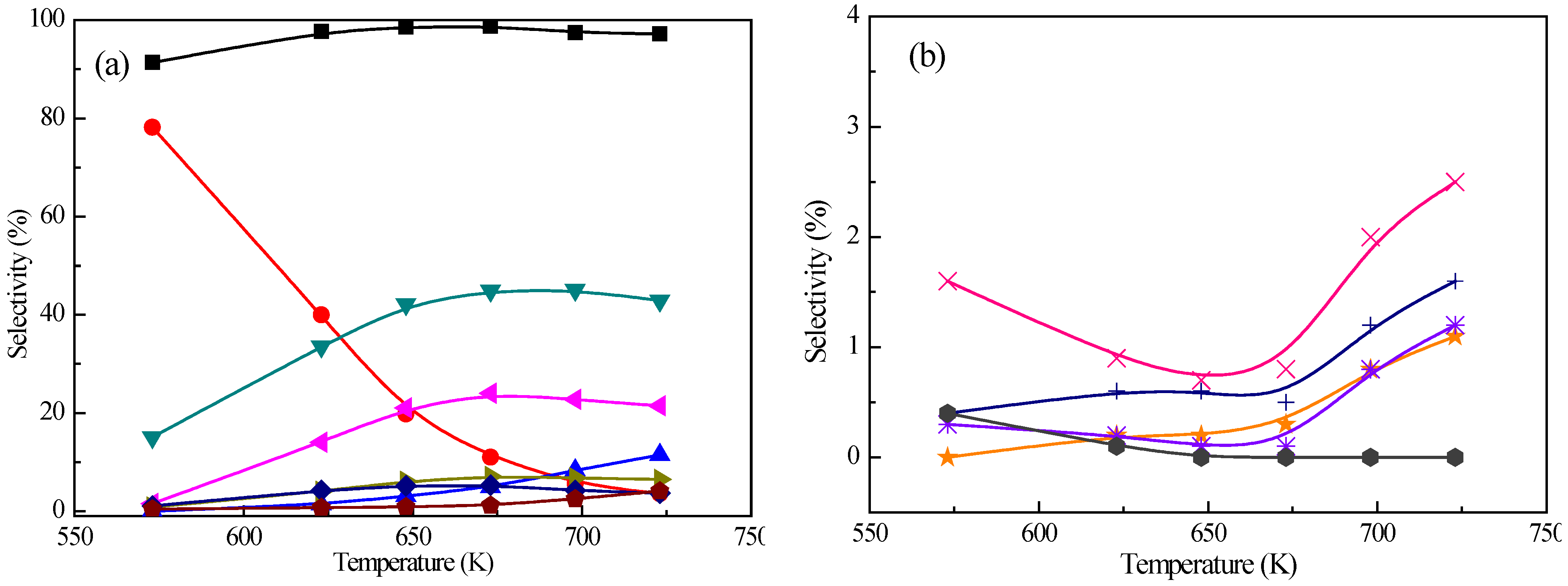
 : m-cresol conversion,
: m-cresol conversion,  : alkanes,
: alkanes,  : benzene,
: benzene,  : toluene,
: toluene,  : p-/m-xylene
: p-/m-xylene  : o-xylene,
: o-xylene,  : polyalkylated aromatics,
: polyalkylated aromatics,  : others,
: others,  : phenol,
: phenol,  : o-cresol;
: o-cresol;  : xylenols,
: xylenols,  : polyalkylated phenols,
: polyalkylated phenols,  : methylanisole. (Reaction conditions: m-cresol/methanol molar ratio = 1/4, catalyst = 1.0 wt. % Pt/Z-57, W/F = 75 gcat·h/molm-cresol, T = 673 K, PH2 = 2 MPa).
: methylanisole. (Reaction conditions: m-cresol/methanol molar ratio = 1/4, catalyst = 1.0 wt. % Pt/Z-57, W/F = 75 gcat·h/molm-cresol, T = 673 K, PH2 = 2 MPa).
 : m-cresol conversion,
: m-cresol conversion,  : alkanes,
: alkanes,  : benzene,
: benzene,  : toluene,
: toluene,  : p-/m-xylene
: p-/m-xylene  : o-xylene,
: o-xylene,  : polyalkylated aromatics,
: polyalkylated aromatics,  : others,
: others,  : phenol,
: phenol,  : o-cresol;
: o-cresol;  : xylenols,
: xylenols,  : polyalkylated phenols,
: polyalkylated phenols,  : methylanisole. (Reaction conditions: m-cresol/methanol molar ratio = 1/4, catalyst = 1.0 wt. % Pt/Z-57, W/F = 75 gcat·h/molm-cresol, T = 673 K, PH2 = 2 MPa).
: methylanisole. (Reaction conditions: m-cresol/methanol molar ratio = 1/4, catalyst = 1.0 wt. % Pt/Z-57, W/F = 75 gcat·h/molm-cresol, T = 673 K, PH2 = 2 MPa).