1. Introduction
The heterogeneously catalyzed ammoxidation is a selective oxidation of olefins and methyl aromatics (or hetero aromatics), respectively, that is carried out in the presence of ammonia, leading to commercially valuable nitriles [
1,
2]. Outstanding examples are the reaction of propene to acrylonitrile and the conversion of
m- and
p-xylene to isoterephthalic and terephthalic dinitrile, respectively [
3,
4]. The production of acrylonitrile has global importance; about seven million tons were produced worldwide in 2015 with
ca. 2.5% annual consumption growth rate for the next five years. To be brief, the reaction follows a Mars-van Krevelen mechanism,
i.e., nucleophilic oxygen is introduced into an activated allyl or benzyl species forming an oxygen-containing intermediate [
5,
6]. Such an intermediate interacts with ammonia adsorbed on the catalyst surface, forming an imine that is further dehydrated to a nitrile [
7]. As mentioned above, olefins or methyl aromatics and hetero aromatics can be used as feed; in this regard, the ammoxidation of 2-methylpyrazine (MP) belongs to this group of reactions. It allows the manufacture of 2-cyanopyrazine (CP) in a single step and is an industrially important reaction as well. In detail, CP is an intermediate compound to synthesize pyrazinamide (PyA) that is globally used as an effective anti-tubercular drug [
8].
A variety of transition metal–containing catalyst systems, on supports or bulk materials as well, have been applied for ammoxidation reactions in general [
1,
3], MP in particular, e.g., [
1,
9,
10]. For instance, vanadium phosphate-based solids [
11,
12], FePO
4 [
13], MoVPO [
14], hetero polyacids [
15,
16], V
2O
5/TiO
2 [
9,
10], VMoO
x [
17], AuNPs [
18] or SbVMn mixed oxides [
19,
20] are described catalyst systems for the title reaction. The conversion is generally carried out in the temperature range of 360–420 °C under ambient pressure. Recently, we have studied the properties of various metal vanadates (e.g., AlVO
4, FeVO
4, CrVO
4, NbVO
5, LaVO
4, and BiVO
4) as a new class of catalytic active materials. Some of these solids revealed improved yields for CP [
21,
22]. Besides CP, pyrazine (Py), pyrazinamide (PyA), CO and CO
2 were observed as by-products. Pyrazine formation is supposed to occur from the demethylation of MP that might happen as a consecutive step after methyl group activation; the hetero aromatic molecule is activated by hydride abstraction, leading to a methylene-like species as we found out recently by
in situ- FTIR spectroscopy of the ammoxidation of toluene to benzonitrile [
7,
23]. Here, a benzaldehyde-like intermediate was formed which might react toward an imine in the presence of ammonia; the nitrile is formed afterwards by oxidative dehydration. Otherwise, benzene was also seen in this case as a result of demethylation. Pyrazinamide formation is expected from the hydrolysis of CP. CO and CO
2 result from unavoidable total oxidation which also generates water (
Scheme 1).
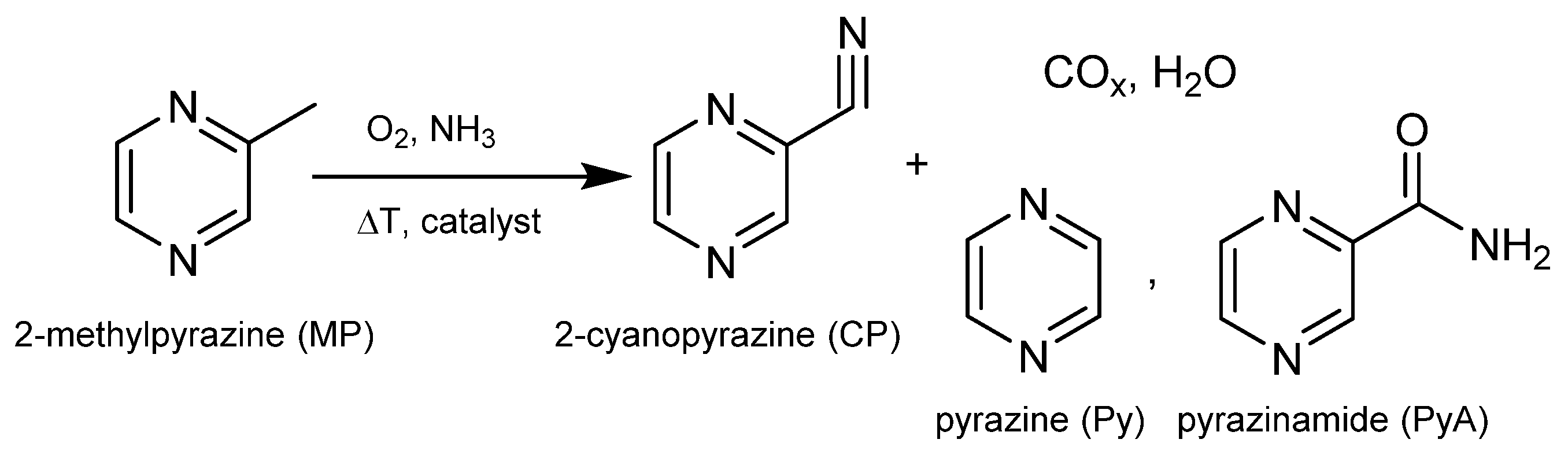
Scheme 1.
Reaction scheme for ammoxidation of MP leading to CP, other hetero aromatic by-products and products of total oxidation as well.
Scheme 1.
Reaction scheme for ammoxidation of MP leading to CP, other hetero aromatic by-products and products of total oxidation as well.
It has been observed previously that the nature of metal in metal vanadate has shown clear influence on the catalytic performance [
21,
24]. Very recently, the near-surface region and the outermost surface layer of such vanadates were studied by X-ray Photoelectron Spectroscopy (XPS) and Low-Energy Ion Scattering (LEIS) [
25]. This technique clearly showed how important stable vanadium-enriched or pure vanadia outermost surface layers are for a selective catalyst requiring an optimum electron and oxygen (O
2−) transport in the surface layers for best performance. In contrast, mixed oxide-containing outermost layers are detrimental for the catalytic behavior. Among all, NbVO
5 and LaVO
4 solids showed superior performance; however, under optimized Nb:V [
24] and La:V ratios, respectively, the La
0.1V
0.9O
x solid has displayed the best performance [
26].
Therefore, this latter catalyst composition was investigated further with respect to optimizing product yield and selectivity. Another significant goal was the increase of the space-time-yield as an important measure for industrial applicability. Therefore, most of the catalytic tests were carried out at, more or less, complete MP conversion. In this contribution, we describe the optimization of the reaction parameters to reach the above-mentioned aims.
2. Results and Discussion
In our previous investigations, we have examined the effect of various metals (Al, Fe, Cr, Nb, La, Bi) in metal vanadate (MVs) catalysts (e.g., AlVO
4, FeVO
4, CrVO
4, NbVO
5, LaVO
4, and BiVO
4) with constant metal-to-vanadium molar ratio (
i.e., 1:1) and tested their potentiality towards the ammoxidation of MP to CP [
21,
22]. We have also previously explored the effect of reaction temperature on the catalytic performance of MP to CP for all solids mentioned above [
21]. The conversion of MP (X-MP) reached almost 100% over most of the MVs when the reaction temperature window was tuned in the range from 320 to 460 °C in every case except BiVO
4, which has shown only
ca. 75% conversion of MP at >440 °C [
21]. In contrast, the total X-MP is already reached at much lower temperature (
i.e., 340 °C) over the AlVO
4 solid [
21]. The catalyst activity increases in the following order: BiVO
4 < LaVO
4 < NbVO
5 < CrVO
4 ≈ FeVO
4 < AlVO
4. The selectivity to CP (S-CP), in general, is found to increase to a peak temperature and then decreases with a further rise in temperature. At lower reaction temperatures, mainly pyrazinamide is formed as an intermediate whereas at higher reaction temperatures, pyrazine and carbon oxides are the dominant by-products formed. Only in case of the AlVO
4 solid the S-CP decreases steadily with an increase in reaction temperature. Further details on the temperature dependency of the conversion of MP and product yields were already reported earlier [
21,
26]. On the other hand, the LaVO
4 solid under optimized La:V ratio exhibited the best performance and seemed to be a good balance with respect to conversion and selectivity [
21,
26]. Nevertheless, this catalyst requires a relatively high reaction temperature (420 °C) to obtain 100% conversion of MP. Despite such a high reaction temperature, there is no considerable loss of selectivity towards CP, which is indeed remarkable.
In general, the nature of the metal in the synthesized metal vanadate catalysts and its distribution is a crucial parameter, which appears to control both the conversion of MP and the distribution of products. From the above results, it seems that orthovanadate materials exhibit a high selectivity of the desired products when the VO4 units are separated from each other by MOx units and all the lattice oxygen ions are involved in the solid bridge (M-O-V) between V5+ and Mn+ ions. The absence of V5+-O-V5+ bonds in which the lattice oxygen can be readily removed due to the high reducibility of the two V5+ ions is the reason for the low combustion (total oxidation) activity of the metal vanadates compared to V2O5. A similar situation can be expected from the present LaVO4 and BiVO4 catalysts due to the formation of well crystalline pure metal vanadate phases and, hence, the low combustion activity (i.e., high selectivity of CP). On the other hand, the reverse trend is observed in the case of AlVO4 catalysts, where the free crystalline V2O5 phase is present in major proportion.
The LaVO
4 catalyst system was selected for further variation of the V: ratio due to its relatively superior catalytic performance in the ammoxidation of MP. Therefore, different La
aV
bO
x (
a = 0 to 1.0 and
b = 1.0 to 0) solids with varying La:V ratios were prepared and tested; several solid characterization results with respect to surface, electronic and phase properties can be found elsewhere [
21,
22,
25]. Thus, the optimized combination of La and V ratios remarkably improved the performance. The conversion of MP is observed to increase continuously with an increase in the vanadium content and reached almost 100% conversion on La
0.1V
0.9O
x catalysts using the reaction conditions given below, where the yield of CP has also been observed to be high. However, further increase in the vanadium content causes a decrease in the yield of CP. Among different La
aV
bO
x solids studied, the catalyst with a La:V molar ratio of 0.1:0.9 (
i.e., La
0.1V
0.9O
x) exhibited the best performance. The best yield of CP is found to be >85% at almost total conversion of MP (
T = 420 °C, GHSV = 11883 h
−1, MP:NH
3:air:H
2O:N
2 = 1:7:26:13:22). Additionally, the best space-time-yield (STY) of CP of over 500 g
CP/(kg
cat∙h) was obtained successfully for the first time. This is the highest value obtained so far compared to all other existing literature reports on this reaction. Further details on the effect of varying the lanthanum-to-vanadium mole ratio and the dependency of the conversion of MP and product yields can be found elsewhere [
26].
Taking into consideration this best composition and the best performance of the La0.1V0.9Ox catalyst, we have further optimized various other reaction variables such as the concentration of MP, ammonia, air, and H2O vapor in the reactant feed mixture. Besides, attempts were also made to check the effect of contact time as well as the time-on-stream behavior of the best catalyst system. By studying these reaction variables, quite interesting results were observed, which are discussed in detail in the following sections.
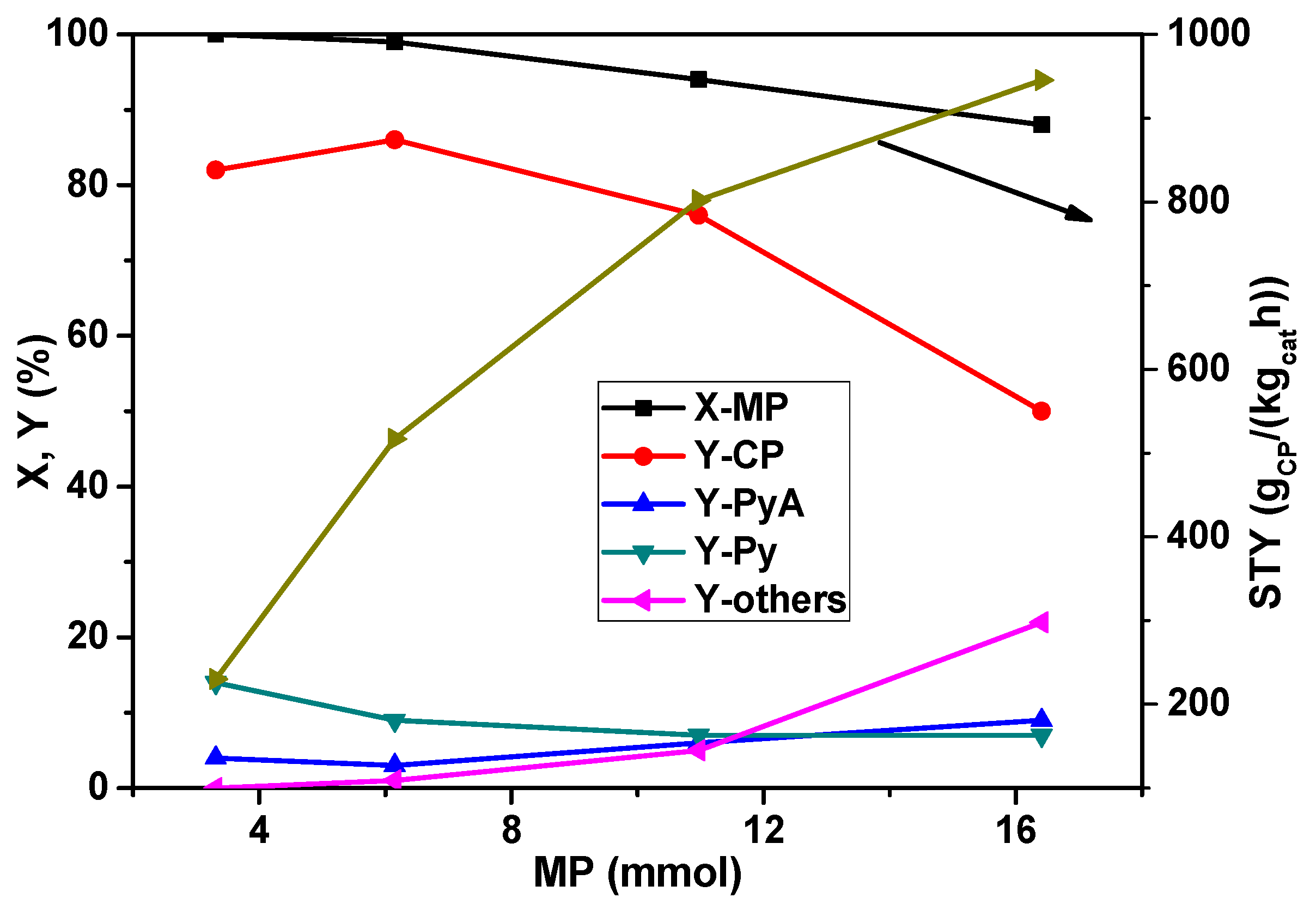
At first, the effect of MP concentration in the feed mixture was explored and the results are presented in
Figure 1. With an increase in the MP feed rate from 3.0 to 16 mmol
MP/h, the conversion is decreased from 100% to 88% while the yield of CP is increased up to 6.0 mmol
MP/h feed and then decreased with a further increase in the MP concentration. At lower MP content (3.0 mmol
MP/h), the by-product Py is received in yields up to 10%–15%. Py formation is due to demethylation as already mentioned above. At higher MP content, the Py yield dropped but the amide (PyA) and other by-product (carbon oxides mainly) concentrations are raised. The increase in carbon oxide formation is caused by an expanding hot-spot region in the catalyst bed because ammoxidation is a highly exothermic reaction. This process is pushed due to the increased MP feed rate. In addition, much more water is produced by total oxidation, leading to an increase in PyA formation, too. However, the very interesting result is that extremely high space-time-yield of CP (
i.e., >900 g
CP/(kg
cat∙h)) could be achieved for the first time at higher MP content in the feed despite a decrease in the conversion of MP. It should be noted that the total flow of the feed mixture is kept constant all the time by adjusting the diluent gas flow (
i.e., N
2 flow) so that a constant GHSV is maintained.
Figure 1.
Effect of 2-methylpyrazine (MP) feed rate on the conversion of MP and product yields in the ammoxidation reaction over La0.1V0.9Ox solid (reaction conditions: T = 420 °C; feed molar ratio: NH3:air:H2O = 7:26:13; GHSV = 11883 h−1; τ = 0.3 s; catalyst weight = 1 g; CP = 2-cyanopyrazine, PyA = pyrazinamide, Py = pyrazine, others = carbon oxides).
Figure 1.
Effect of 2-methylpyrazine (MP) feed rate on the conversion of MP and product yields in the ammoxidation reaction over La0.1V0.9Ox solid (reaction conditions: T = 420 °C; feed molar ratio: NH3:air:H2O = 7:26:13; GHSV = 11883 h−1; τ = 0.3 s; catalyst weight = 1 g; CP = 2-cyanopyrazine, PyA = pyrazinamide, Py = pyrazine, others = carbon oxides).
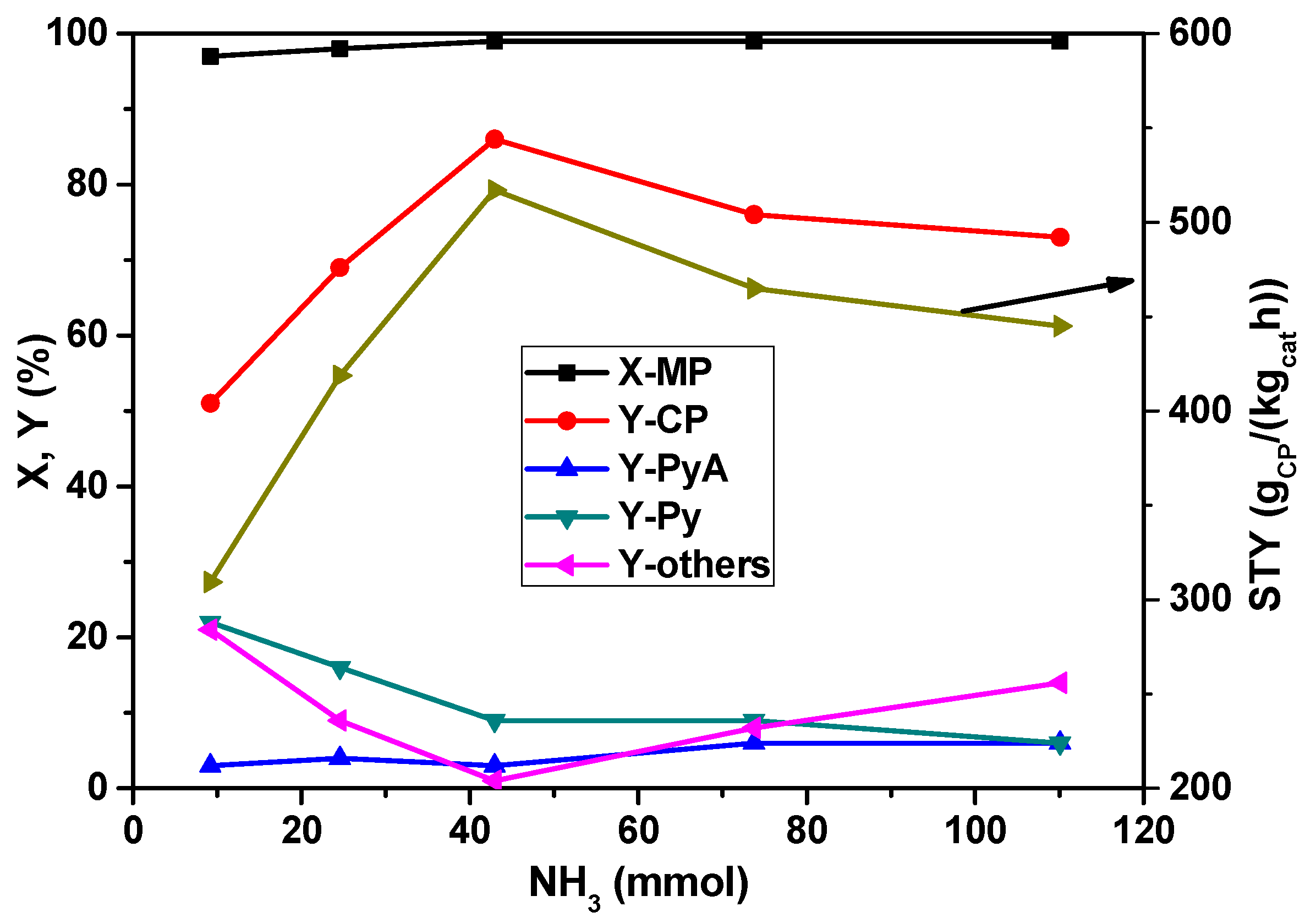
The dependency of MP conversion and product yield on the concentration of NH
3 in the feed mixture is illustrated in
Figure 2. The conversion of MP remained almost constant irrespective of the ammonia flow in the feed; however, the product selectivities have been dramatically affected. The yield of CP is increased from 50 to >80% up to a NH
3 flow rate of 42 mmol
NH3/h and then decreased with the further increase of the ammonia proportion. Obviously, high ammonia concentration leads to an occupation of reaction sites; as a consequence, MP conversion drops. At lower ammonia content, the by-products such as Py and carbon oxides are more than 20%, which are, however, decreased with the increase of the ammonia flow rate. The high yields of Py and carbon oxides are expected mainly due to the demethylation of MP easily running at low ammonia availability and simultaneous total oxidation reactions preferentially proceeding with oxygen-containing intermediates. From this study, it appears that 42 mmol
NH3/h flow of NH
3 seemed to be optimum for the improved yield of CP.
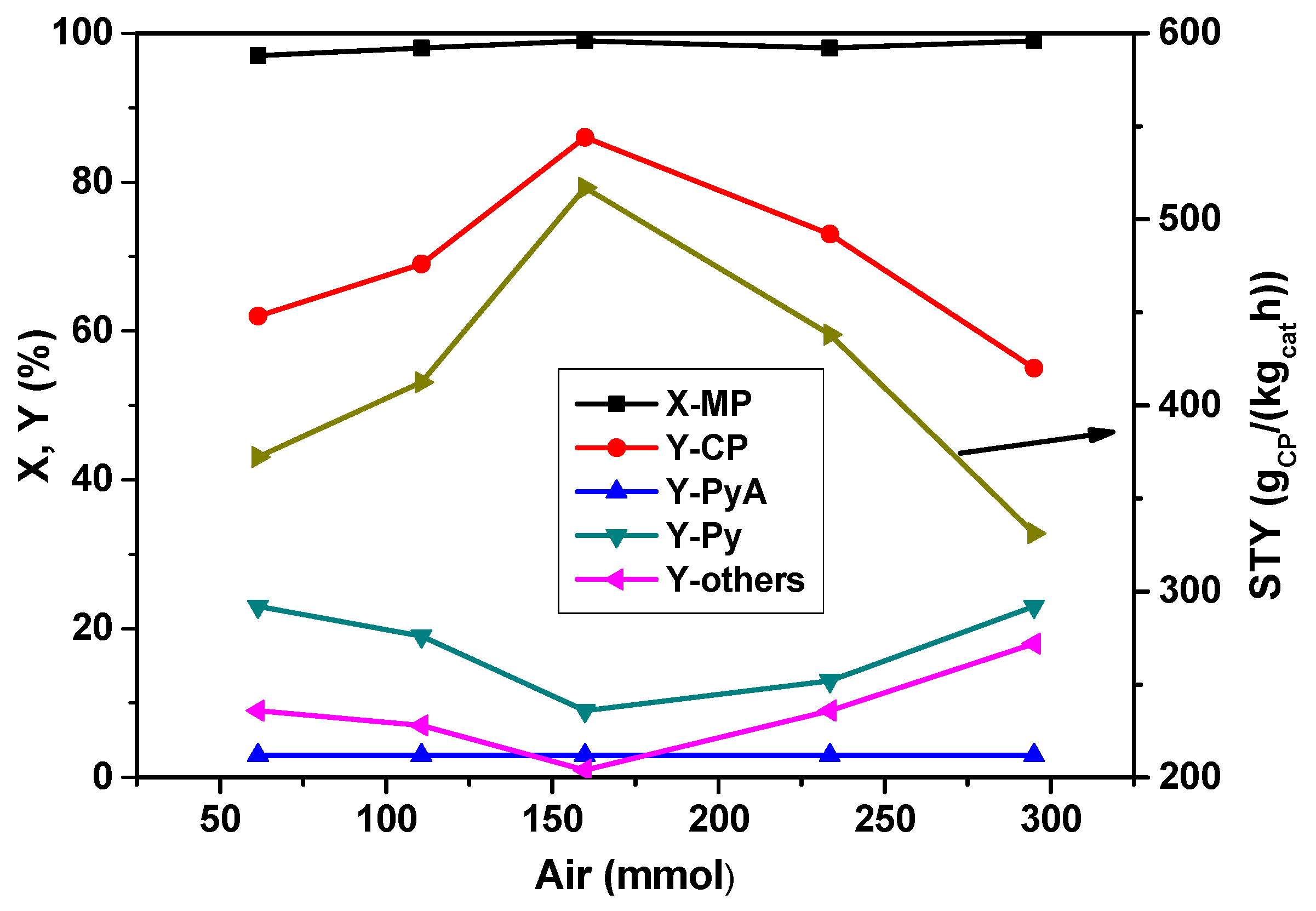
The influence of air (oxygen) on the product distribution in the ammoxidation of MP is summarized in
Figure 3. It has been noticed that the air flow has a clear effect on the catalyst performance. Even though the conversion of MP remained constant at ~100% with increasing the air content, the yields and selectivities of products, respectively, are considerably varied. The yield of CP increased from 62% to ~85% up to an air flow rate of 160 mmol
air/h and then decreased with the further increase of air content. At low air contents, the formation of Py is more likely due to possible demethylation of MP. Higher air contents lead to an increasing total oxidation. This series reveals that
ca. 160 mmol
air/h is optimum for obtaining enhanced yields of CP.
Figure 2.
Impact of ammonia flow rate on X-MP and product yields (reaction conditions: T = 420 °C; feed molar ratio: MP:air:H2O = 1:26:13; GHSV = 11883 h−1; τ = 0.3 s; catalyst weight = 1 g; MP flow: 6.2 mmol/h).
Figure 2.
Impact of ammonia flow rate on X-MP and product yields (reaction conditions: T = 420 °C; feed molar ratio: MP:air:H2O = 1:26:13; GHSV = 11883 h−1; τ = 0.3 s; catalyst weight = 1 g; MP flow: 6.2 mmol/h).
Figure 3.
Effect of air feed proportion on X-MP and product yields (reaction conditions: T = 420 °C; feed molar ratio: MP:NH3:H2O = 1:7:13; GHSV = 11883 h−1; τ = 0.3 s; catalyst weight = 1 g; MP flow: 6.2 mmol/h).
Figure 3.
Effect of air feed proportion on X-MP and product yields (reaction conditions: T = 420 °C; feed molar ratio: MP:NH3:H2O = 1:7:13; GHSV = 11883 h−1; τ = 0.3 s; catalyst weight = 1 g; MP flow: 6.2 mmol/h).
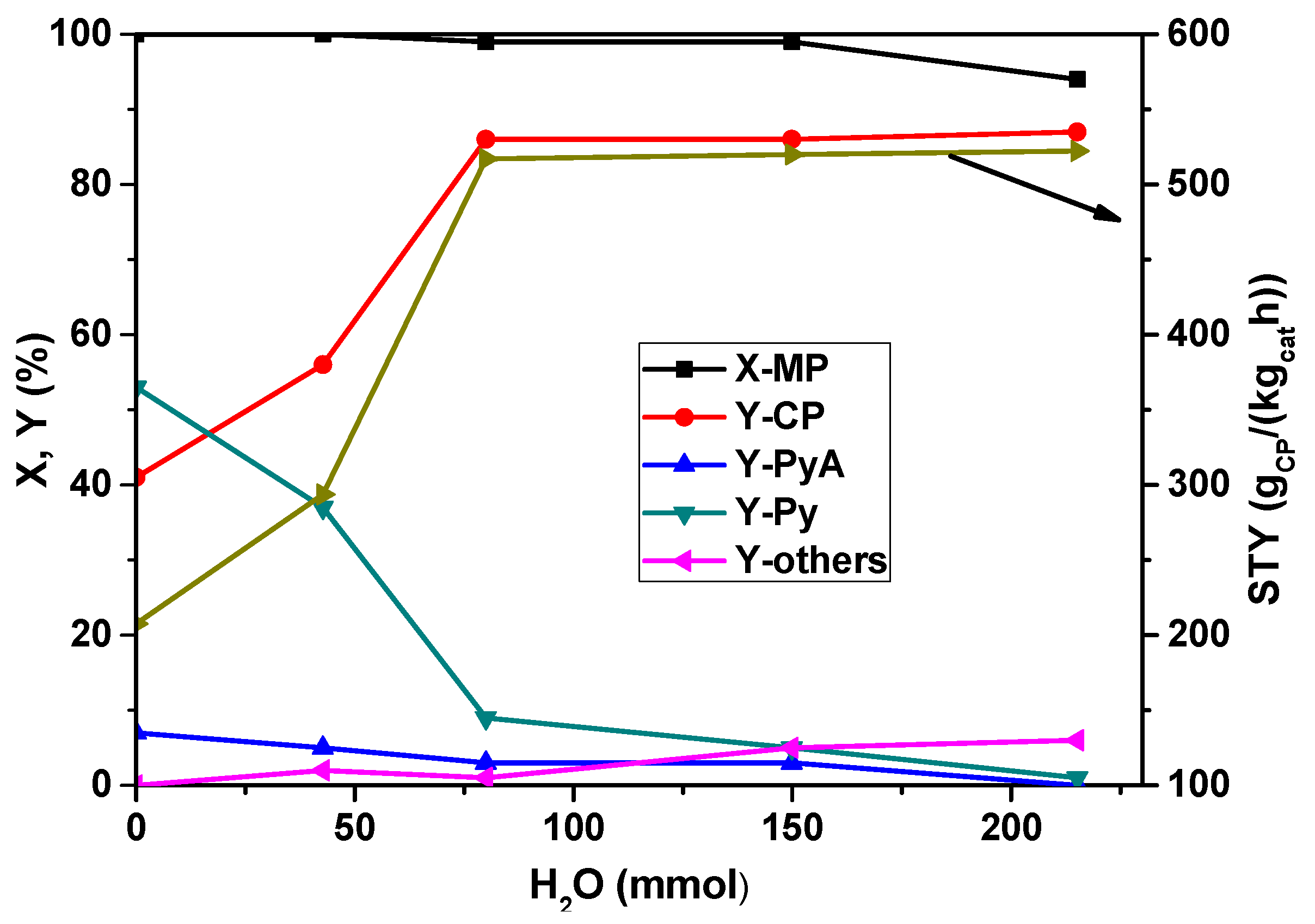
Furthermore, the influence of the water vapor feed proportion on the conversion of MP and the product distribution in the ammoxidation of MP was explored and is depicted in
Figure 4. It is obvious from the figure that in the absence of water vapor in the feed mixture, Py is the main product at complete MP conversion. Only with additionally feeding water vapor CP becomes the main product and the product distribution turns dramatically. The increased water content beyond 80 mmol/h has not shown considerable influence on the conversion of MP, but the yield of CP increased from 40 to ~85% up to 80 mmol/h water vapor and then remained almost constant beyond this value. However, the opposite trend is observed for Py formation. At lower water content (<50 mmol/h), Py formation is appreciably high (53%), which is then decreased drastically to 9% with an increase in water content to 80 mmol/h and then remains constant beyond that. It is known that the presence of water vapor is essential to facilitate the easy desorption of the products and also to control the exothermicity of the reaction. This study implies that a water vapor flow rate of ~80 mmol/h seems to be optimum for the enhanced yield of CP.
Figure 4.
Influence of water vapor content on X-MP and product yields (reaction conditions: T = 420 °C; feed molar ratio: MP:NH3:air = 1:7:26; GHSV = 11883 h−1; τ = 0.3 s; catalyst weight = 1 g; MP flow: 6.2 mmol/h).
Figure 4.
Influence of water vapor content on X-MP and product yields (reaction conditions: T = 420 °C; feed molar ratio: MP:NH3:air = 1:7:26; GHSV = 11883 h−1; τ = 0.3 s; catalyst weight = 1 g; MP flow: 6.2 mmol/h).
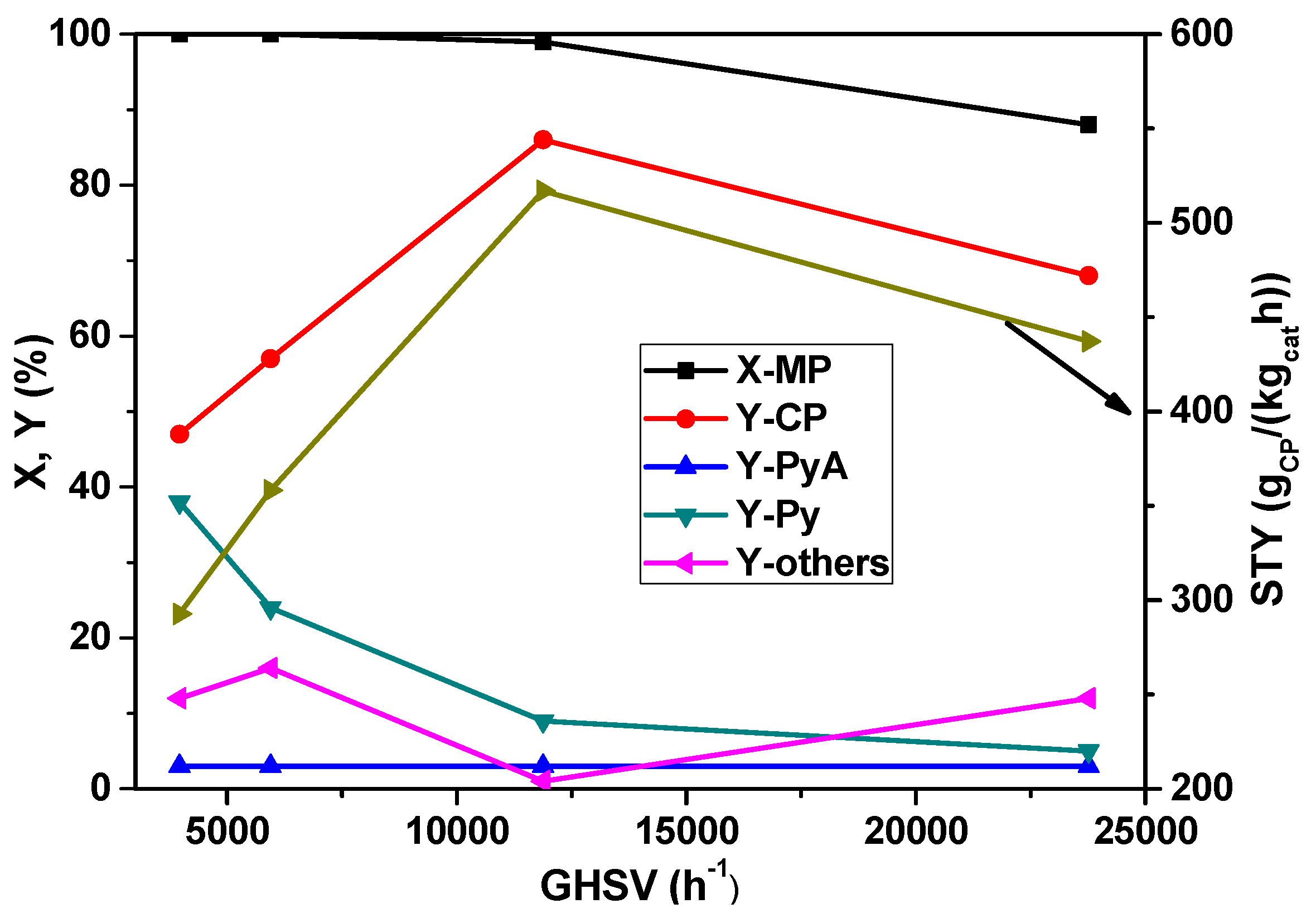
In subsequent studies, the influence of the space velocity on the MP ammoxidation was investigated by varying the amount of the catalyst in the range from 0.4 to 2.4 g and keeping the total flow rate of the feed mixture constant.
Figure 5 depicts the changes in the conversion of MP and the yields of the reaction products as a function of space velocity under steady-state conditions. With the increase in GHSV, the conversion of MP remained constant up to 11883 h
−1 and then decreased with a further increase. However, the yield of CP is increased from 47% to 85% up to 11883 h
−1 GHSV, and then decreased with a further increase. At low GHSV, a by-product such as pyrazine formation is increased, but it is, however, decreased with the increase of GHSV beyond 11883 h
−1. On the whole, a GHSV of 11883 h
−1 appears to be optimum for better performance.
Figure 5.
Effect of increasing GHSV on X-MP and product yields (reaction conditions: T = 420 °C; feed molar ratio: MP:NH3:air:H2O:N2 = 1:7:26:13:22; τ = 0.91–0.15 s; catalyst weight = 0.4–2.4 g).
Figure 5.
Effect of increasing GHSV on X-MP and product yields (reaction conditions: T = 420 °C; feed molar ratio: MP:NH3:air:H2O:N2 = 1:7:26:13:22; τ = 0.91–0.15 s; catalyst weight = 0.4–2.4 g).
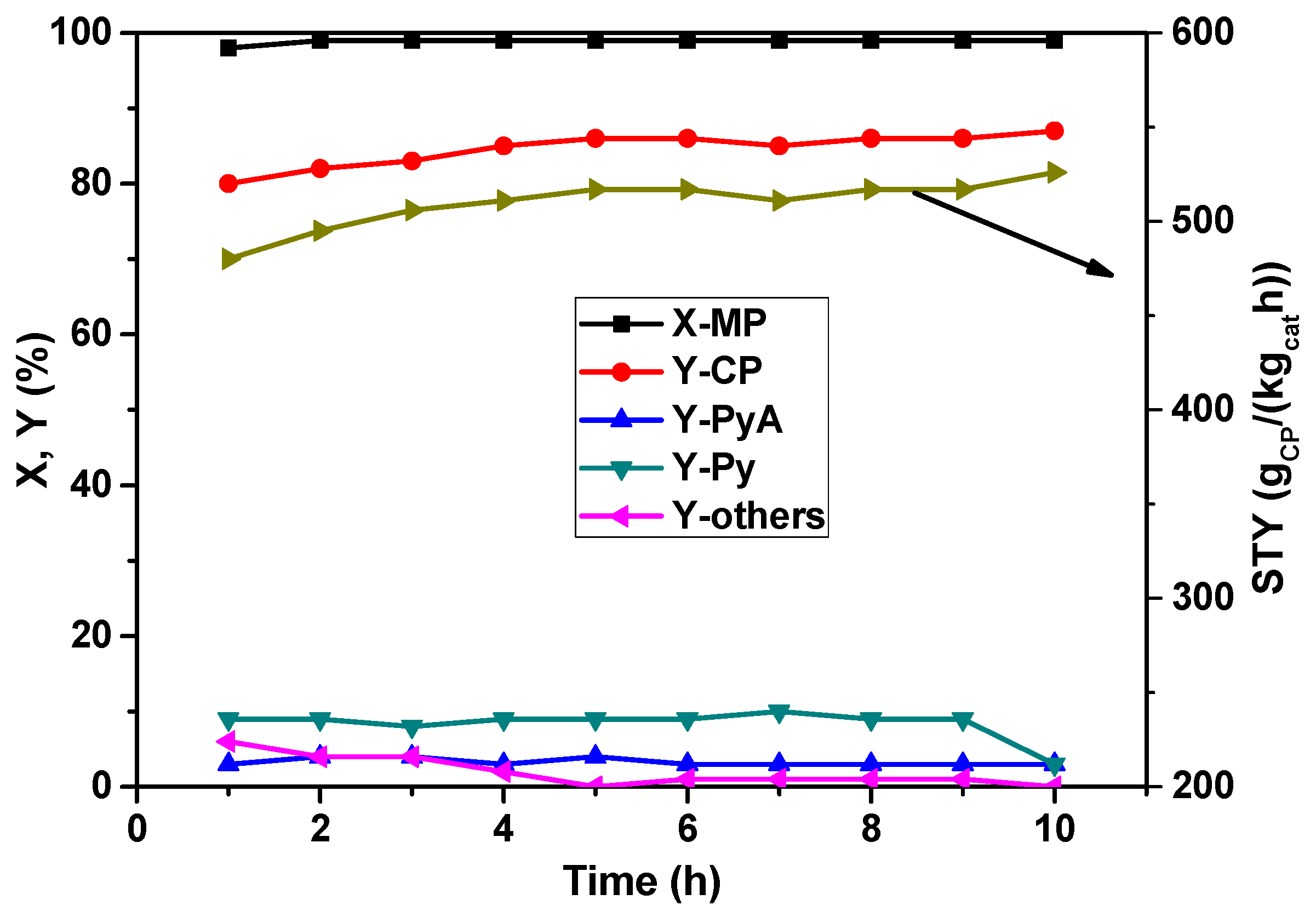
Finally, the time-on-stream behavior of the catalyst under study using the best parameters set was also investigated and is presented in
Figure 6. It is evident from the figure that the novel La
0.1V
0.9O
x catalyst has exhibited an acceptably good stability over a period of 10 h as tested in our laboratory. The conversion of MP remained more or less constant at 100% throughout the testing period, and interestingly, the yield of CP appears to increase slightly with time. From the above results we can conclude that this catalyst composition is more resistant towards deactivation by coke formation and/or agglomeration by sintering of VO
x and/or LaVO
x species compared to other VO
x-containing catalyst systems. In general, sintering leads to larger particles showing a decrease in conversion due to reduced active surface area and sometimes changes in product selectivity due to alteration in bulk properties such as increasing oxygen (O
2−) and/or electron transport. Hence, this particular catalyst composition has good potential for its operation on large-scale production of CP.
Figure 6.
Time-on-stream behavior of La0.1V0.9Ox solid in the MP ammoxidation reaction (reaction conditions: T = 420 °C; feed molar ratio: MP:NH3:air:H2O:N2 = 1:7:26:13:22; GHSV = 11883 h−1; τ = 0.3 s; MP flow: 6.2 mmol/h).
Figure 6.
Time-on-stream behavior of La0.1V0.9Ox solid in the MP ammoxidation reaction (reaction conditions: T = 420 °C; feed molar ratio: MP:NH3:air:H2O:N2 = 1:7:26:13:22; GHSV = 11883 h−1; τ = 0.3 s; MP flow: 6.2 mmol/h).