Effects of Catalyst Preparation on Hydrocarbon Product Distribution in Hydrocracking of the Fischer-Tropsch Product with Low Pt-Loaded Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
| Catalyst | BET Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (nm) | Micropore Area (m2/g) | Mesopore Area (m2/g) | |
|---|---|---|---|---|---|---|
| Entry | Remarks | |||||
| 1 | I-Pt(0.1)/SiAl8.7 | 333 | 0.83 | 10.0 | - | 368 |
| 2 | I-Pt(0.1)/SiAl(28.6) | 399 | 0.84 | 8.4 | - | 453 |
| 3 | I-Pt(0.1)/SiAl(13) | 515 | 0.67 | 5.2 | - | 523 |
| 4 | I-Pt(0.1)/SiAl5.5 | 426 | 0.44 | 4.1 | - | 339 |
| 5 | I-Pt(0.1)/S-ZrO2 | 108 | 0.19 | 7.1 | - | 123 |
| 6 | I-Pt(0.1)/FAU110 | 758 | 0.55 | 2.9 | 918 | 112 |
| 7 | I-Pt(0.1)/MOR18 | 495 | 0.27 | 2.2 | 645 | 33 |
| 8 | BEA28 | 701 | 0.67 | 3.8 | 849 | 209 |
| 9 | I-Pt(0.01)/BEA28 | 719 | 0.58 | 3.2 | 876 | 211 |
| 10 | I-Pt(0.02)/BEA28 | 709 | 0.57 | 3.2 | 864 | 211 |
| 11 | I-Pt(0.04)/BEA28 | 709 | 0.66 | 3.7 | 862 | 211 |
| 12 | I-Pt(0.06)/BEA28 | 719 | 0.63 | 3.5 | 871 | 215 |
| 13 | I-Pt(0.1)/BEA28 | 719 | 0.62 | 3.4 | 874 | 213 |
| 14 | I-Pt(0.1)/BEA40 | 640 | 0.30 | 1.9 | 836 | 59 |
| 15 | I-Pt(0.1)/BEA41.6 | 603 | 0.31 | 2.0 | 784 | 76 |
| 16 | I-Pt(0.1)/BEA510 | 608 | 0.39 | 2.6 | 781 | 96 |
| 17 | D-Pt(CTAC)/BEA28 | 723 | 0.65 | 3.6 | 880 | 211 |
| 18 | D-Pt(TPAB)/BEA28 | 727 | 0.62 | 3.4 | 884 | 209 |
| 19 | D-Pt(TEAC)/BEA28 | 731 | 0.73 | 4.0 | 887 | 213 |
| 20 | D-Pt(HTAB)/BEA28 | 740 | 0.67 | 3.6 | 894 | 214 |
| Catalyst | Acid Amount a (mmol/g) | Weak b (mmol/g) | Medium c (mmol/g) | Strong d (mmol/g) | |
|---|---|---|---|---|---|
| Entry | Remarks | ||||
| 1 | I-Pt(0.1)/SiAl8.7 | 0.43 | 0.19 | 0.15 | 0.09 |
| 2 | I-Pt(0.1)/SiAl(28.6) | 0.80 | 0.30 | 0.22 | 0.28 |
| 3 | I-Pt(0.1)/SiAl(13) | 0.70 | 0.27 | 0.23 | 0.20 |
| 4 | I-Pt(0.1)/SiAl5.5 | 0.56 | 0.25 | 0.20 | 0.11 |
| 5 | I-Pt(0.1)/S-ZrO2 | 0.63 | 0.16 | 0.18 | 0.30 |
| 6 | I-Pt(0.1)/FAU110 | 0.13 | 0.05 | 0.05 | 0.03 |
| 7 | I-Pt(0.1)/MOR18 | 1.18 | 0.68 | 0.22 | 0.28 |
| 8 | BEA28 | 1.37 | 0.65 | 0.67 | 0.04 |
| 9 | I-Pt(0.01)/BEA28 | 1.38 | 0.68 | 0.63 | 0.07 |
| 10 | I-Pt(0.02)/BEA28 | 1.35 | 0.70 | 0.61 | 0.05 |
| 11 | I-Pt(0.04)/BEA28 | 1.41 | 0.70 | 0.64 | 0.07 |
| 12 | I-Pt(0.06)/BEA28 | 1.36 | 0.66 | 0.64 | 0.06 |
| 13 | I-Pt(0.1)/BEA28 | 1.32 | 0.62 | 0.61 | 0.08 |
| 14 | I-Pt(0.1)/BEA40 | 1.08 | 0.52 | 0.50 | 0.06 |
| 15 | I-Pt(0.1)/BEA41.6 | 0.77 | 0.34 | 0.34 | 0.09 |
| 16 | I-Pt(0.1)/BEA510 | 0.14 | 0.03 | 0.03 | 0.08 |
| 17 | D-Pt(CTAC)/BEA28 | 1.46 | 0.70 | 0.70 | 0.06 |
| 18 | D-Pt(TPAB)/BEA28 | 1.38 | 0.67 | 0.65 | 0.06 |
| 19 | D-Pt(TEAC)/BEA28 | 1.38 | 0.69 | 0.64 | 0.05 |
| 20 | D-Pt(HTAB)/BEA28 | 1.44 | 0.71 | 0.67 | 0.06 |
2.2. Screening of the Hydrocracking Reaction
| Content/% on a Carbon Basis | Elemental Analysis/wt % | |||||||
|---|---|---|---|---|---|---|---|---|
| C5–C8 | C9–C15 a | C16 b | C17+ | C | H | N | S | O c |
| 0.1 | 9.7 | 61.7 | 28.5 | 84.4 | 14.8 | 0.0 | 0.0 | 0.8 |
| Catalyst | Product Yield/% on a Carbon Basis | dpulse (nm) | ||||
|---|---|---|---|---|---|---|
| Entry | Remarks | YC1–C8 | YC9–C15 | YC16+ | Loss | |
| 1 | I-Pt(0.1)/SiAl8.7 | 0.8 | 10.2 | 86.6 | 2.5 | 42.5 |
| 2 | I-Pt(0.1)/SiAl(28.6) | 0.3 | 11.5 | 89.2 | −1.0 | 56.5 |
| 3 | I-Pt(0.1)/SiAl(13) | 0.2 | 9.2 | 90.6 | 0.0 | 66.6 |
| 4 | I-Pt(0.1)/SiAl5.5 | 0.2 | 10.3 | 89.2 | 0.2 | 28.8 |
| 5 | I-Pt(0.1)/S-ZrO2 | 0.8 | 12.9 | 87.3 | −1.0 | 13.4 |
| 6 | I-Pt(0.1)/FAU110 | 1.0 | 9.8 | 89.0 | 0.2 | 26.3 |
| 7 | I-Pt(0.1)/MOR18 | 2.3 | 13.3 | 85.8 | −1.4 | 13.0 |
| 8 | BEA28 | 19.4 | 20.7 | 58.5 | 1.4 | - |
| 9 | I-Pt(0.01)/BEA28 | 24.1 | 22.4 | 54.2 | −0.7 | 1.8 |
| 10 | I-Pt(0.02)/BEA28 | 26.5 | 23.9 | 47.9 | 1.7 | 1.9 |
| 11 | I-Pt(0.04)/BEA28 | 28.0 | 24.0 | 44.3 | 3.7 | 2.3 |
| 12 | I-Pt(0.06)/BEA28 | 30.9 | 24.7 | 42.2 | 2.2 | 3.5 |
| 13 | I-Pt(0.1)/BEA28 | 37.4 | 23.1 | 37.5 | 2.0 | 4.6 |
| 14 | I-Pt(0.1)/BEA40 | 23.1 | 22.1 | 53.2 | 1.6 | 3.1 |
| 15 | I-Pt(0.1)/BEA41.6 | 15.7 | 20.6 | 65.1 | −1.4 | 4.8 |
| 16 | I-Pt(0.1)/BEA510 | 4.2 | 10.6 | 83.8 | 1.4 | 35.4 |
| 17 | D-Pt(CTAC)/BEA28 | 29.2 | 21.5 | 46.5 | 2.8 | 2.6 |
| 18 | D-Pt(TPAB)/BEA28 | 27.5 | 23.9 | 48.6 | 0.0 | 2.0 |
| 19 | D-Pt(TEAC)/BEA28 | 25.1 | 22.6 | 47.9 | 4.4 | 2.3 |
| 20 | D-Pt(HTAB)/BEA28 | 27.4 | 22.7 | 47.5 | 2.4 | 1.0 |
| 21 | None | 0.8 | 9.4 | 90.9 | −1.0 | - |

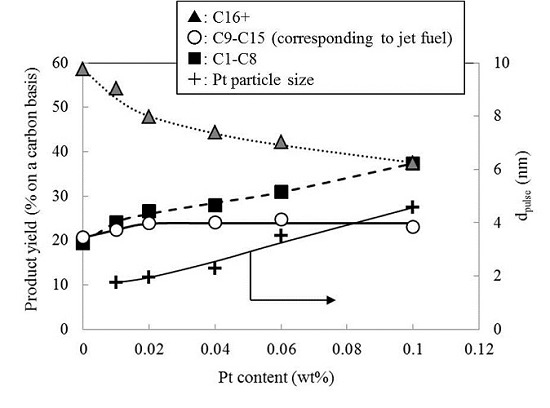
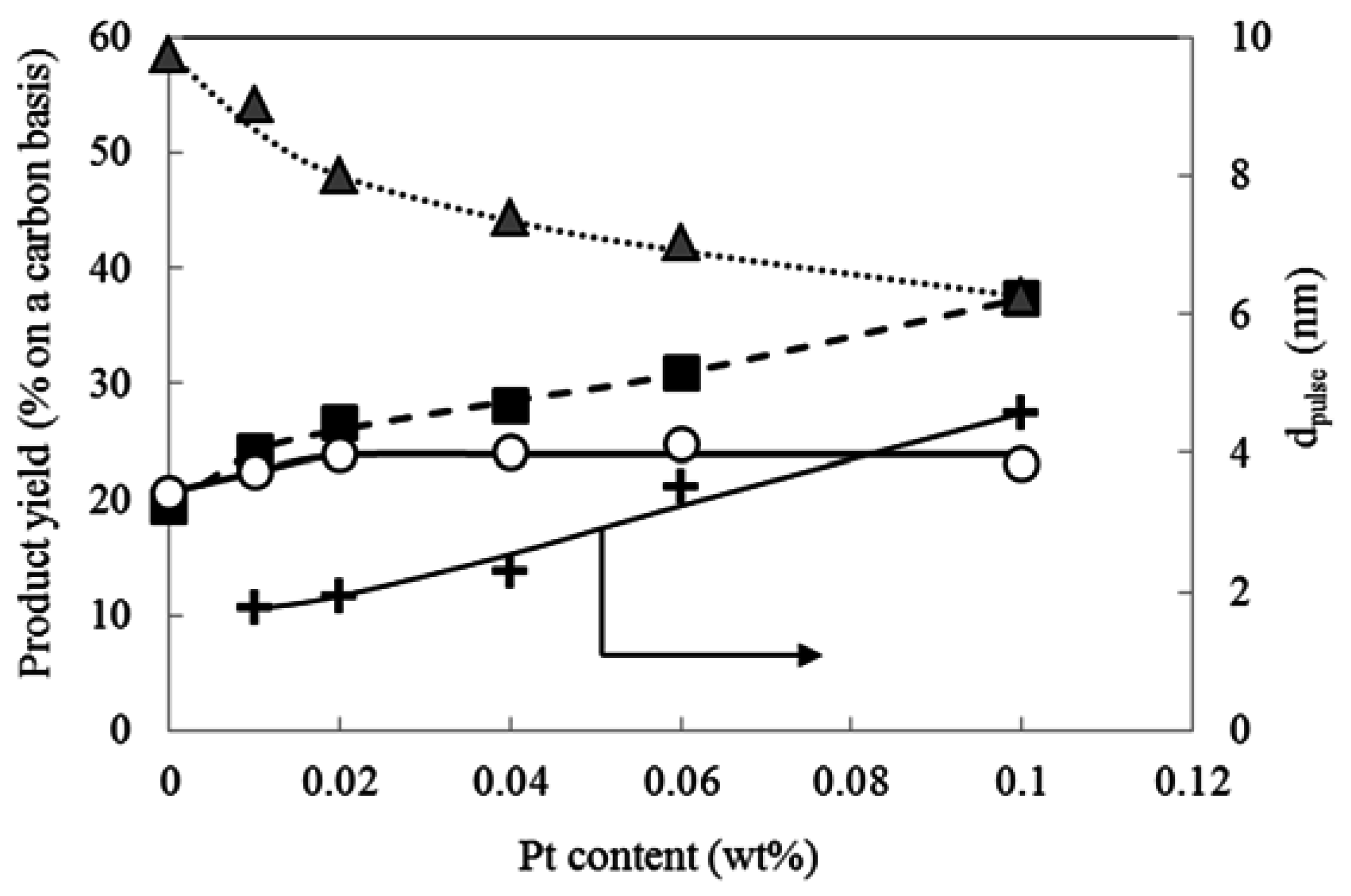
2.3. Effect of Pt Content on the Hydrocracking Reaction over Pt-Loaded BEA28 Catalysts
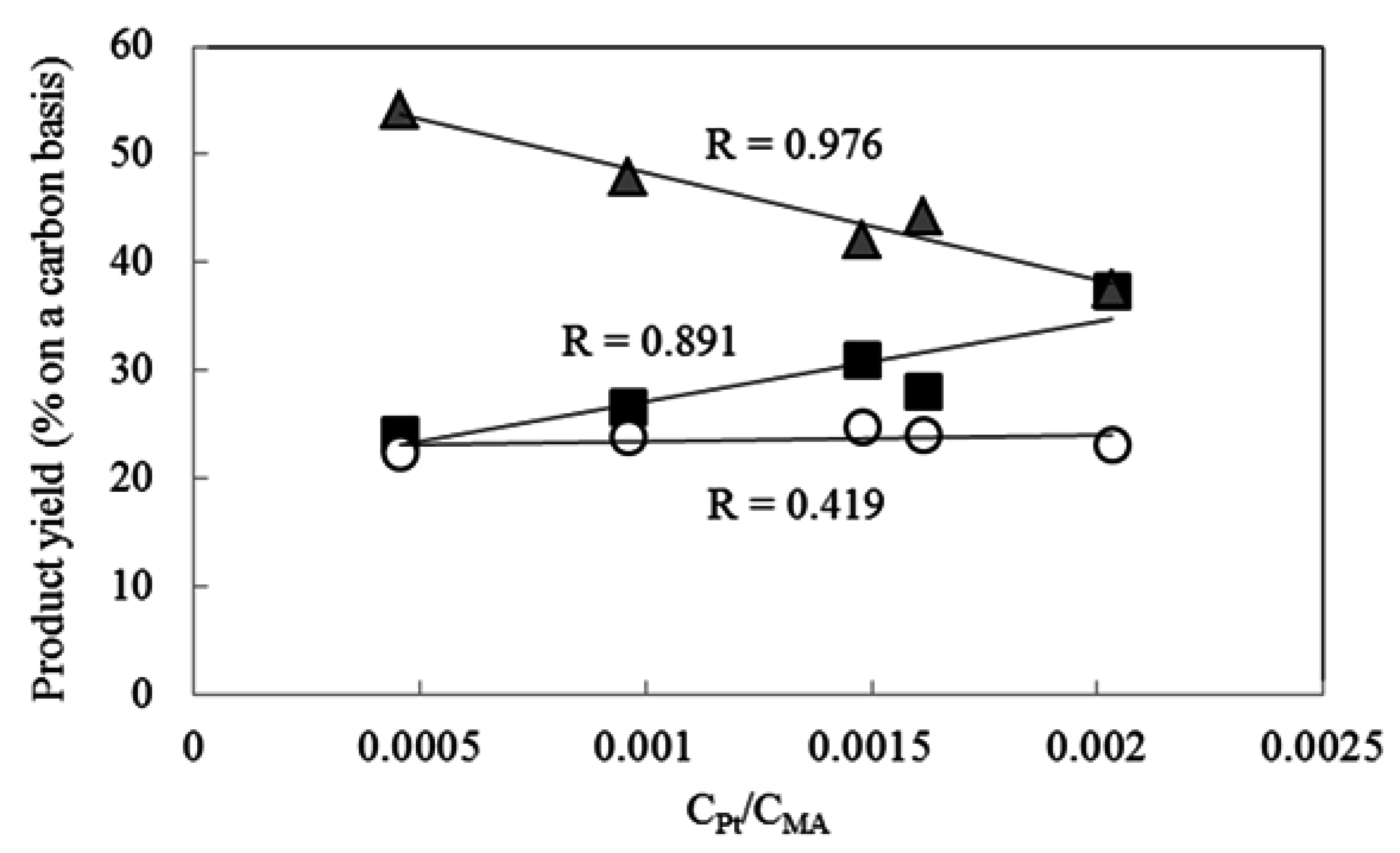
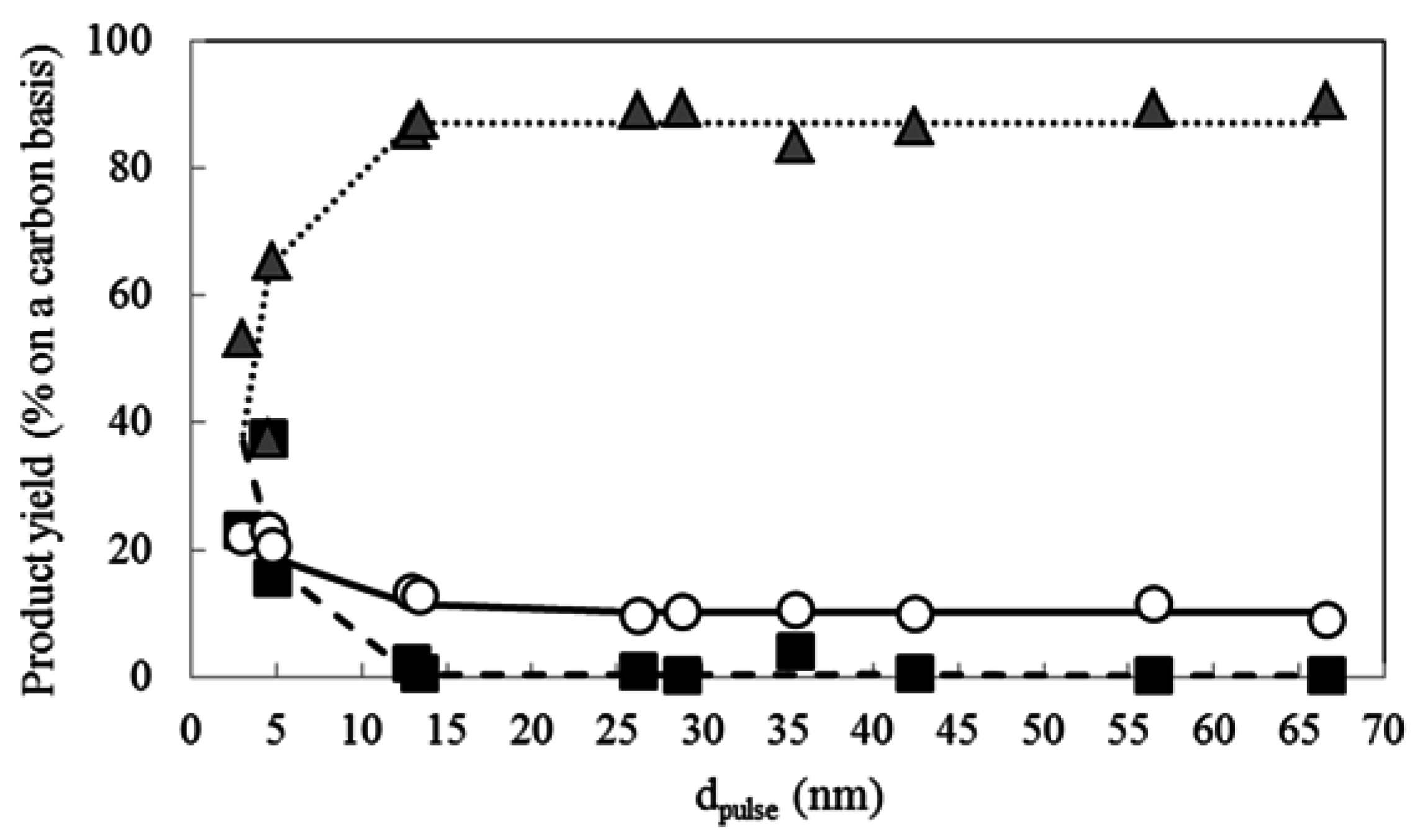
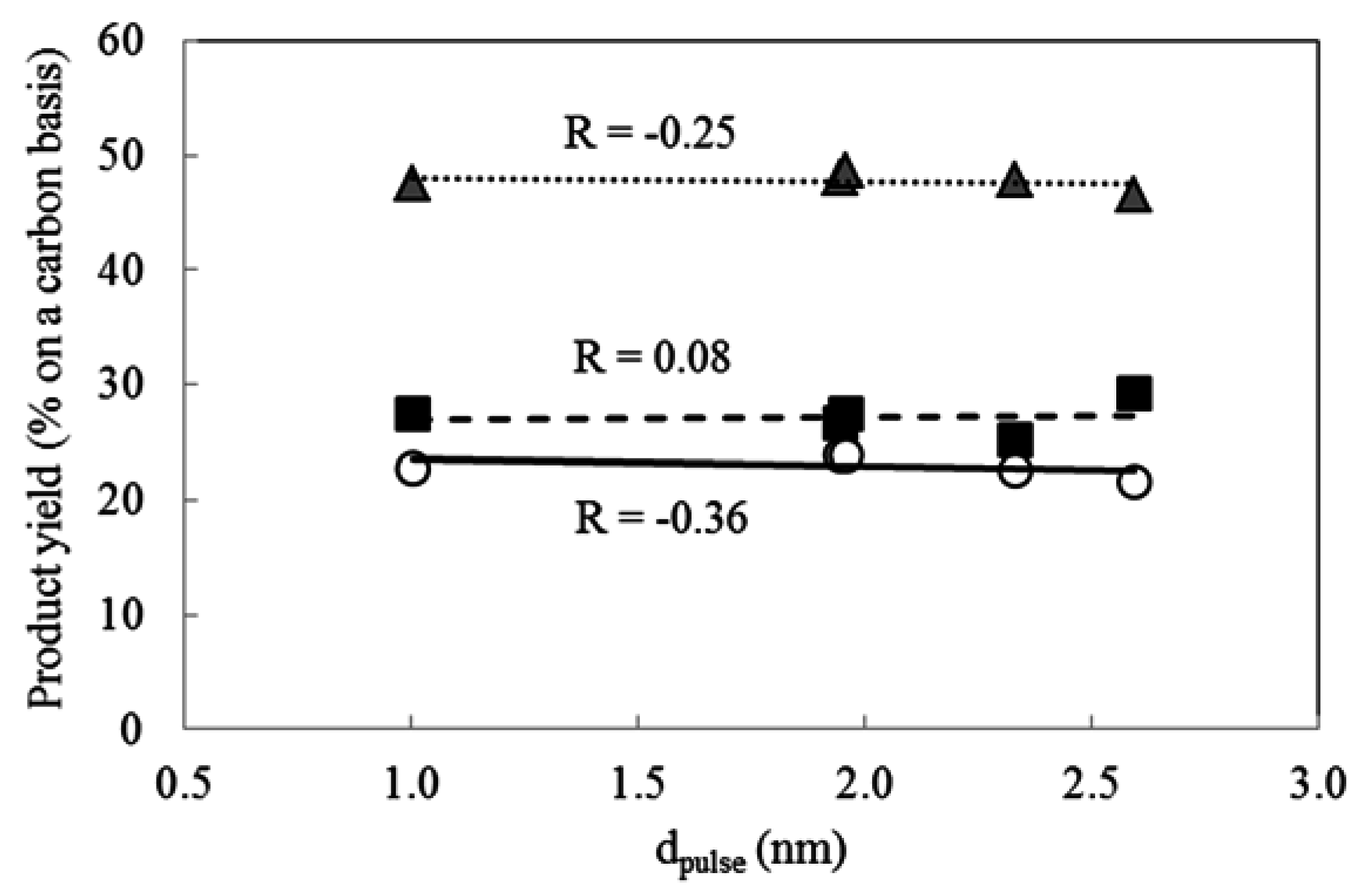
2.4. Effect of Pt Particle Size on the Hydrocracking Reaction over Pt-Loaded Catalysts with Low Pt Content
| Catalyst | Pt Complex Forming Agent | Pt Particle Type | dpulse (nm) | ||
|---|---|---|---|---|---|
| Name | Rational Formula | Name | Rational Formula | ||
| D-Pt(CATC)/BEA28 | CTAC | C16H33(CH3)3N+Cl− | (CTA)2PtCl6 | [C16H33(CH3)3N]2PtCl6 | 2.6 |
| D-Pt(TPAB)/BEA28 | HTAB | C6H13(CH3)3N+Cl− | (HTA)2PtCl6 | [C6H13(CH3)3N]2PtCl6 | 2.0 |
| D-Pt(TEAC)/BEA28 | TPAB | (C3H7)4N+Br− | (TPA)2PtCl6 | [(C3H7)4N]2PtCl6 | 2.3 |
| D-Pt(HTAB)/BEA28 | TEAC | (C2H5)4N+Cl− | (TEA)2PtCl6 | [(C2H5)4N]2PtCl6 | 1.0 |
3. Experimental Section
3.1. Catalyst Preparation
3.2. Characterization
3.3. Hydrocracking Tests
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sunde, K.; Brekke, A.; Solberg, B. Environment impacts and costs of woody Biomass-To-Liquid (BTL) production and use—A review. For. Policy Econ. 2011, 13, 591–602. [Google Scholar] [CrossRef]
- Fujimoto, S.; Yanagita, T.; Ogata, M.; Minowa, T. Evaluation of CO2 mitigation by BTL biofuels from woody biomass through simulated case studies. Int. Energy J. 2008, 9, 73–80. [Google Scholar]
- Calemma, V.; Peratello, S.; Perego, C. Hydroisomerization and hydrocracking of long chain n-alkanes on Pt/amorphous SiO2-Al2O3 catalyst. Appl. Catal. A 2000, 190, 207–218. [Google Scholar] [CrossRef]
- Kang, J.; Ma, W.; Keogh, R.A.; Shafer, W.D.; Jacobs, G.; Davis, B.H. Hydrocracking and hydroisomerization of n-hexadecane, n-octacosane and Fischer-Tropsch wax over a Pt/SiO2-Al2O3 catalyst. Catal. Lett. 2012, 142, 1295–1305. [Google Scholar] [CrossRef]
- Kim, M.Y.; Kim, Y.A.; Jeong, K.E.; Chae, H.J.; Kim, C.U.; Jeong, S.Y.; Han, J.; Park, E.D. Effect of Al content on hydrocracking of n-paraffin over Pt/SiO2-Al2O3. Catal. Commun. 2012, 26, 78–82. [Google Scholar] [CrossRef]
- Keogh, R.A.; Srinivasan, R.; Davis, B.H. The effect of Pt concentration on the activity and selectivity of SO42−-ZrO2 catalysts for the hydrocracking and hydroisomerization of n-hexadecane. Appl. Catal. A 1996, 140, 47–57. [Google Scholar] [CrossRef]
- Busto, M.; Vera, C.R.; Grau, J.M. Optimal process conditions for the isomerization-cracking of long-chain n-paraffins to high octane isomerizate gasoline over Pt/SO42−-ZrO2 catalysts. Fuel Process. Technol. 2011, 92, 1675–1684. [Google Scholar] [CrossRef]
- Grau, J.M.; Yori, J.C.; Parera, J.M. Hydroisomerization-cracking of n-octane on Pt/WO42−-ZrO2 and Pt/SO42−-ZrO2. Effect of Pt load on catalyst performance. Appl. Catal. A 2001, 213, 247–257. [Google Scholar] [CrossRef]
- Busto, M.; Lovato, M.E.; Vera, C.R.; Shimizu, K.; Grau, J.M. Silica supported tungsta-zirconia catalysts for hydroisomerization—Cracking of long alkenes. Appl. Catal. A 2009, 335, 123–131. [Google Scholar] [CrossRef]
- Busto, M.; Benitez, V.M.; Vera, C.R.; Grau, J.M.; Yori, J.C. Pt-Pd/WO3-ZrO2 catalysts for isomerization-cracking of long paraffins. Appl. Catal. A 2008, 347, 117–125. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Tierney, J.W.; Wender, I. Anion-modified zirconia: Effect of metal promotion and hydrogen reduction on hydroisomerization of n-hexadecane and Fischer-Tropsch waxes. Fuel Process. Technol. 2001, 69, 59–71. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Tierney, J.W.; Wender, I. Hydroisomerization of normal hexadecane with platinum-promoted tungstate-modified zirconia catalysts. Appl. Catal. A 2000, 193, 155–171. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, Y.; Tienery, J.W.; Wender, I. Hybrid zirconia catalysts for conversion of Fischer-Tropsch waxy products to transportation fuels. Fuel Process. Technol. 2003, 83, 67–80. [Google Scholar] [CrossRef]
- Leu, L.J.; Hou, L.Y.; Kang, B.C.; Li, C.; Wu, S.T.; Wu, J.C. Synthesis of zeolite β and catalytic isomerization of n-hexane over Pt/H-β catalysts. Appl. Catal. 1991, 69, 49–63. [Google Scholar] [CrossRef]
- Hanaoka, T.; Miyazawa, T.; Shimura, K.; Hirata, S. Jet fuel synthesis from Fischer-Tropsch product under mild hydrocracking conditions using Pt-loaded catalysts. Chem. Eng. J. 2015, 263, 178–185. [Google Scholar] [CrossRef]
- Iliopoulou, E.F.; Heracleous, E.; Delimitis, E.; Lappas, A.A. Producing high quality biofuel: Pt-based hydroisomerization catalysts evaluated using BtL-naphtha surrogates. Appl. Catal. B 2014, 145, 177–186. [Google Scholar] [CrossRef]
- Heracleous, E.; Iliopoulou, E.F.; Lappas, A.A. Microporous/mesoporous Pt/ZSM-5 catalysts for hydroisomerization of BTL-naphtha. Ind. Eng. Chem. Res. 2013, 52, 14567–14573. [Google Scholar] [CrossRef]
- Alvarez, F.; Ribeiro, F.R.; Giannetto, G.; Chevalier, F.; Perot, G.; Guisnet, M. Hydroisomerization and hydrocracking of alkanes. 5. Hydroisomerization and hydrocracking of n-hexane and n-heptane on PtHY catalysts. Effect of the distribution of metallic and acid sites. Stud. Surf. Sci. Catal. 1989, 49, 1339–1348. [Google Scholar]
- Alvarez, F.; Giannetto, G.; Guisnet, M.; Perot, G. Hydroisomerization and hydrocracking of n-alkanes. 2. n-Heptane transformation on a Pt-dealuminated Y zeolite—Comparison with a Pt-Y zeolite. Appl. Catal. 1987, 34, 353–365. [Google Scholar] [CrossRef]
- Guisnet, M.; Alvarez, F.; Giannetto, G.; Perot, G. Hydroisomerization and hydrocracking of n-heptane on PtH zeolites. Effect of the porosity and of the distribution of metallic and acid sites. Catal. Today 1987, 1, 415–433. [Google Scholar] [CrossRef]
- Alvarez, F.; Ribeiro, F.R.; Perot, G.; Thomazeau, C.; Guisnet, M. Hydroisomerization and hydrocracking of alkanes. 7. Influence of the balance between acid and hydrogenating functions on the transformation of n-decane on PtHY catalysts. J. Catal. 1996, 162, 179–189. [Google Scholar] [CrossRef]
- Hanaoka, T.; Miyazawa, T.; Shimura, K.; Hirata, S. Jet fuel synthesis in hydrocracking of Fischer-Tropsch product over Pt-loaded zeolite catalysts prepared using microemulsions. Fuel Process. Technol. 2015, 129, 139–146. [Google Scholar] [CrossRef]
- Hanaoka, T.; Miyazawa, T.; Shimura, K.; Hirata, S. Preparation for Pt-loaded zeolite catalysts using w/o microemulsion and their hydrocracking behaviors on Fischer-Tropsch product. Catalysts 2015, 5, 88–105. [Google Scholar] [CrossRef]
- Lippens, B.C.; Boer, J.H. Studies on pore systems in catalysts. V. The t method. J. Catal. 1965, 4, 319–323. [Google Scholar] [CrossRef]
- Batalha, N.; Pinard, L.; Pouilloux, Y.; Guisnet, M. Bifunctional hydrogenating/acid catalysis: Quantification of the intimacy criterion. Catal. Lett. 2013, 143, 587–591. [Google Scholar] [CrossRef]
- Thybaut, J.W.; Narasimhan, C.S.L.; Denayer, J.F.; Baron, G.V.; Jacobs, P.A.; Martens, J.A.; Marin, G.B. Acid-metal balance of a hydrocracking catalyst: Ideal versus nonideal behavior. Ind. Eng. Chem. Res. 2005, 44, 5159–5169. [Google Scholar] [CrossRef]
- Hanaoka, T.; Miyazawa, T.; Shimura, K.; Hirata, S. Effect of Pt particle density on the hydrocracking of Fischer-Tropsch products over Pt-loaded zeolite catalysts prepared using water-in-oil microemulsions. Chem. Eng. J. 2015, 274, 256–264. [Google Scholar] [CrossRef]
- Ikeda, M.; Takeshima, S.; Tago, T.; Kishida, M.; Wakabayashi, K. Preparation of size-controlled Pt catalysts supported alumina. Catal. Lett. 1999, 58, 195–197. [Google Scholar] [CrossRef]
- Hardeveld, R.; Hartog, F. The statistics of surface atoms and surface sites on metal crystals. Surface Sci. 1969, 15, 189–230. [Google Scholar] [CrossRef]
- Dauscher, A.; Garin, F.; Maire, G. Correlations between the surface structure of platinum single crystals and hydrocarbon skeletal rearrangement mechanisms: Approach to the nature of the active sites. J. Catal. 1987, 105, 233–244. [Google Scholar] [CrossRef]
- Stace, T. How small is a solid? Nature 1988, 331, 116–117. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Hanaoka, T.; Miyazawa, T.; Nurunnabi, M.; Hirata, S.; Sakanishi, K. Liquid fuel production from woody biomass via oxygen-enriched air/CO2 gasification on a bench scale. J. Jpn. Inst. Energy 2011, 90, 1072–1080. [Google Scholar] [CrossRef]
- Miyazawa, T.; Hanaoka, T.; Shimura, K.; Hirata, S. Mn and Zr modified Co/SiO2 catalysts development in slurry-phase Fischer-Tropsch synthesis. Appl. Catal. A 2013, 467, 47–54. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanaoka, T.; Miyazawa, T.; Shimura, K.; Hirata, S. Effects of Catalyst Preparation on Hydrocarbon Product Distribution in Hydrocracking of the Fischer-Tropsch Product with Low Pt-Loaded Catalysts. Catalysts 2015, 5, 1983-2000. https://doi.org/10.3390/catal5041983
Hanaoka T, Miyazawa T, Shimura K, Hirata S. Effects of Catalyst Preparation on Hydrocarbon Product Distribution in Hydrocracking of the Fischer-Tropsch Product with Low Pt-Loaded Catalysts. Catalysts. 2015; 5(4):1983-2000. https://doi.org/10.3390/catal5041983
Chicago/Turabian StyleHanaoka, Toshiaki, Tomohisa Miyazawa, Katsuya Shimura, and Satoshi Hirata. 2015. "Effects of Catalyst Preparation on Hydrocarbon Product Distribution in Hydrocracking of the Fischer-Tropsch Product with Low Pt-Loaded Catalysts" Catalysts 5, no. 4: 1983-2000. https://doi.org/10.3390/catal5041983