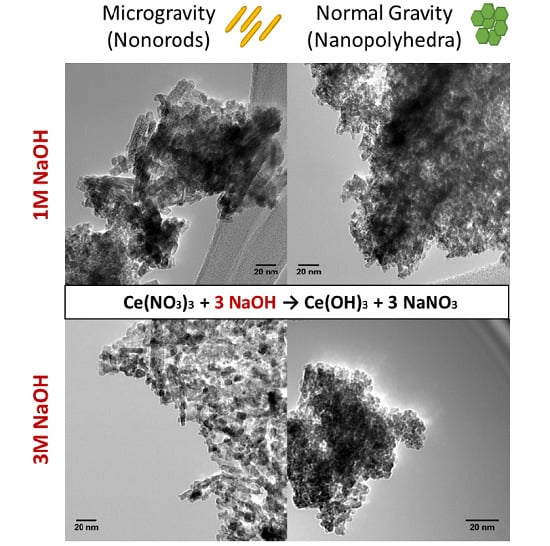
Effect of Microgravity on Synthesis of Nano Ceria
Abstract
:1. Introduction
2. Results and Discussion
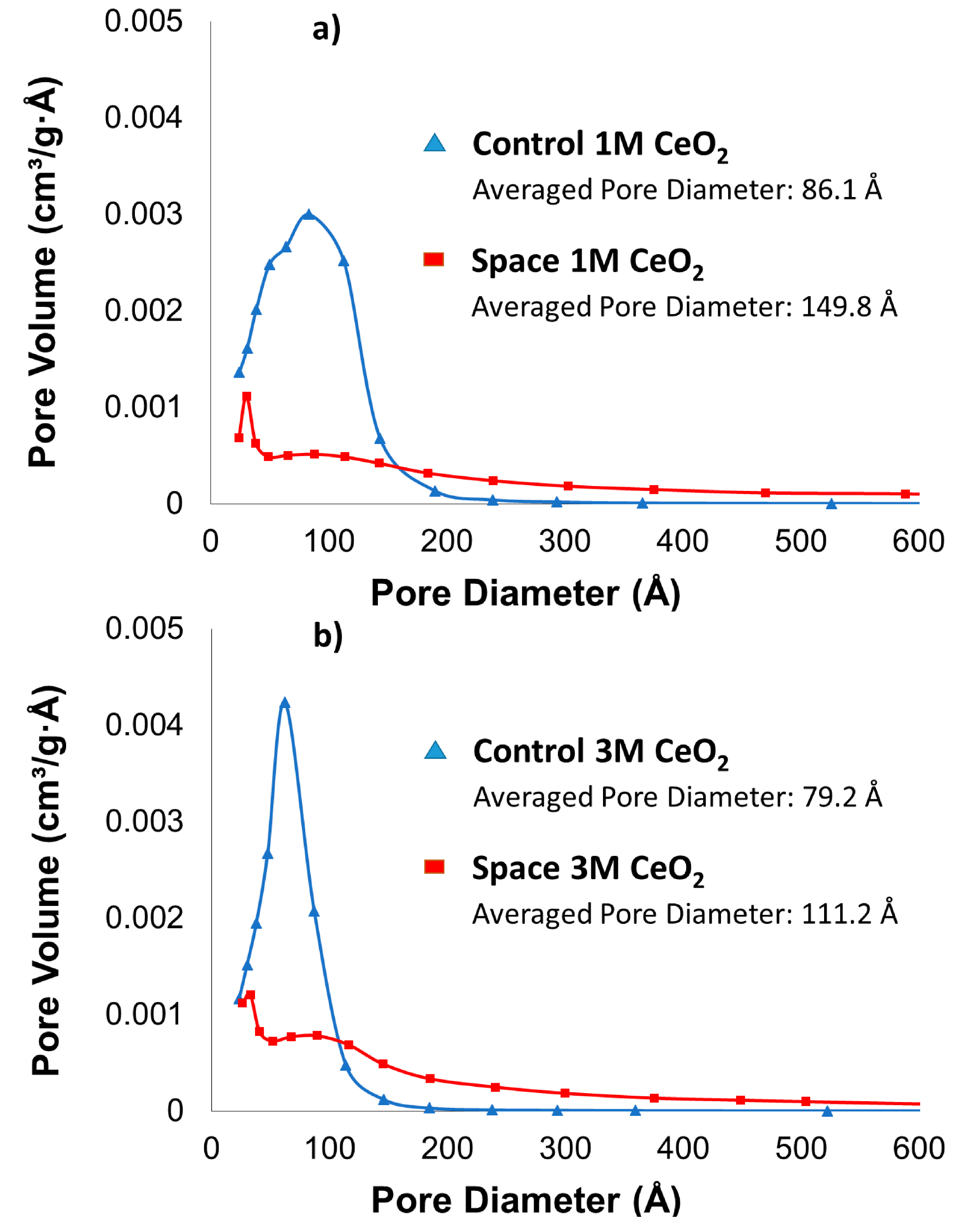
2.1. BET Surface Area, Pore Size and Pore Volume
| Samples | BET Surface Area (m2/g) | Pore Volume (cm3/g) |
|---|---|---|
| Control 1 M | 138 | 0.3 |
| Control 3 M | 118 | 0.23 |
| Space 1 M | 47 | 0.18 |
| Space 3 M | 69 | 0.19 |
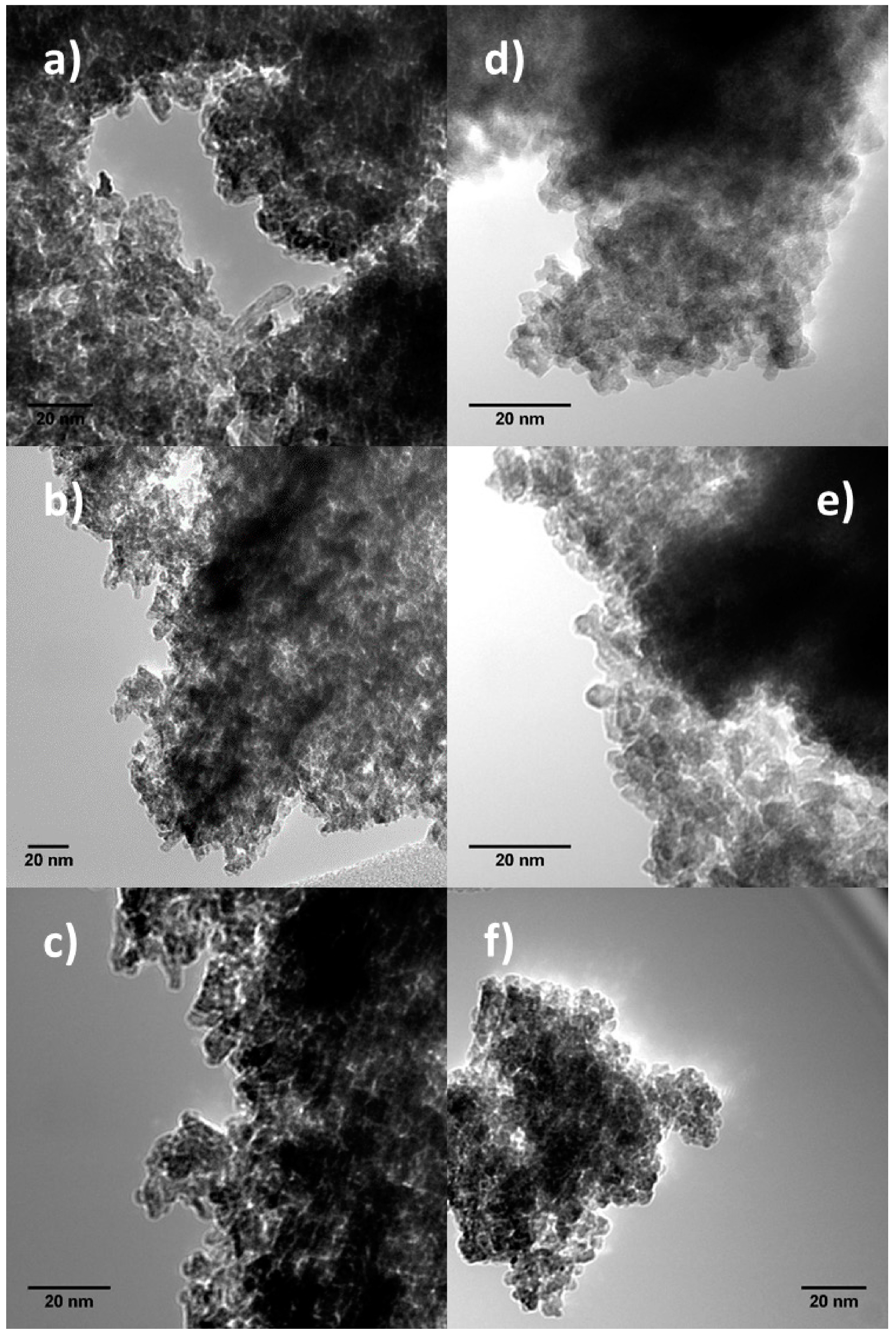
2.2. Transmission Electron Microscopy (TEM)
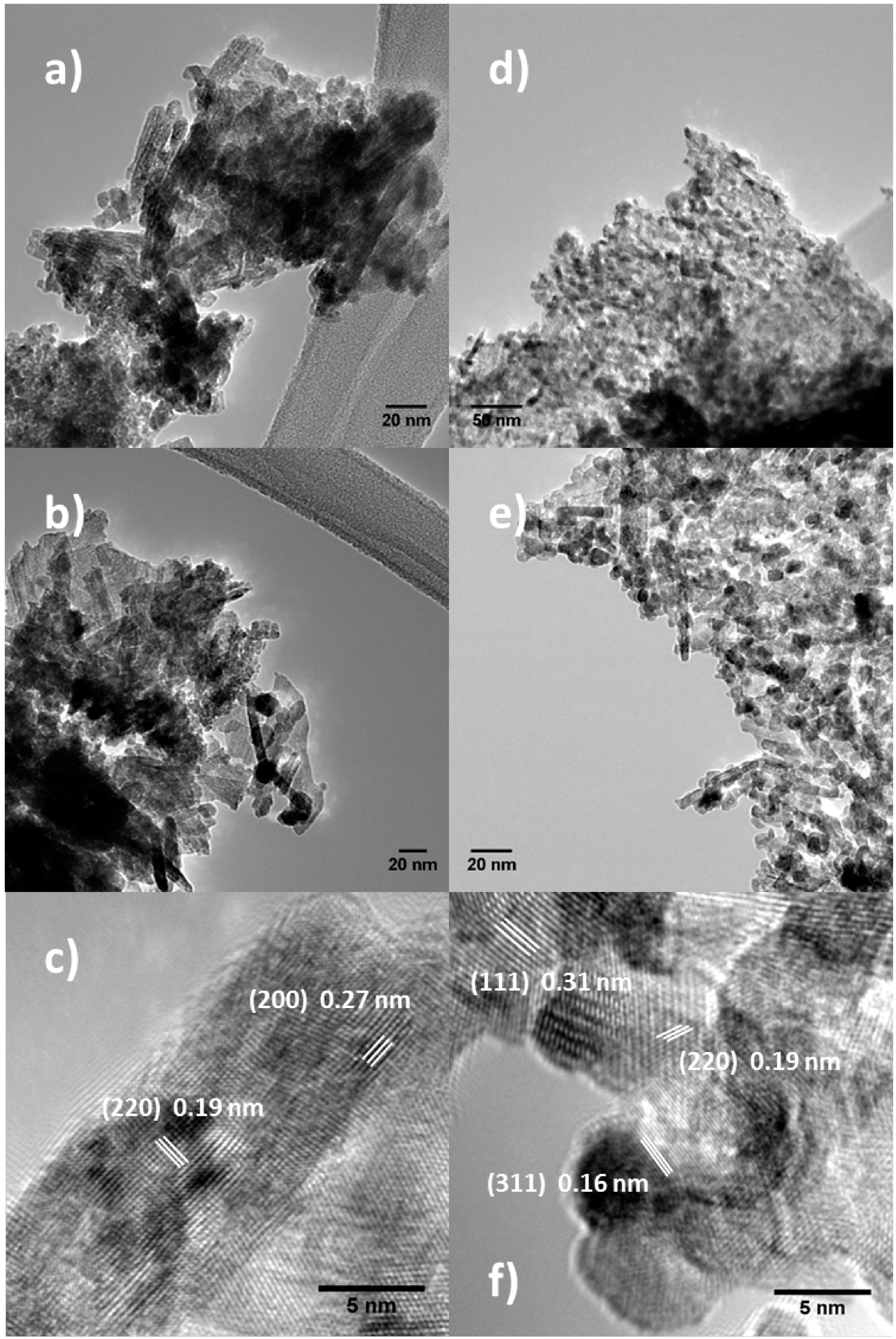
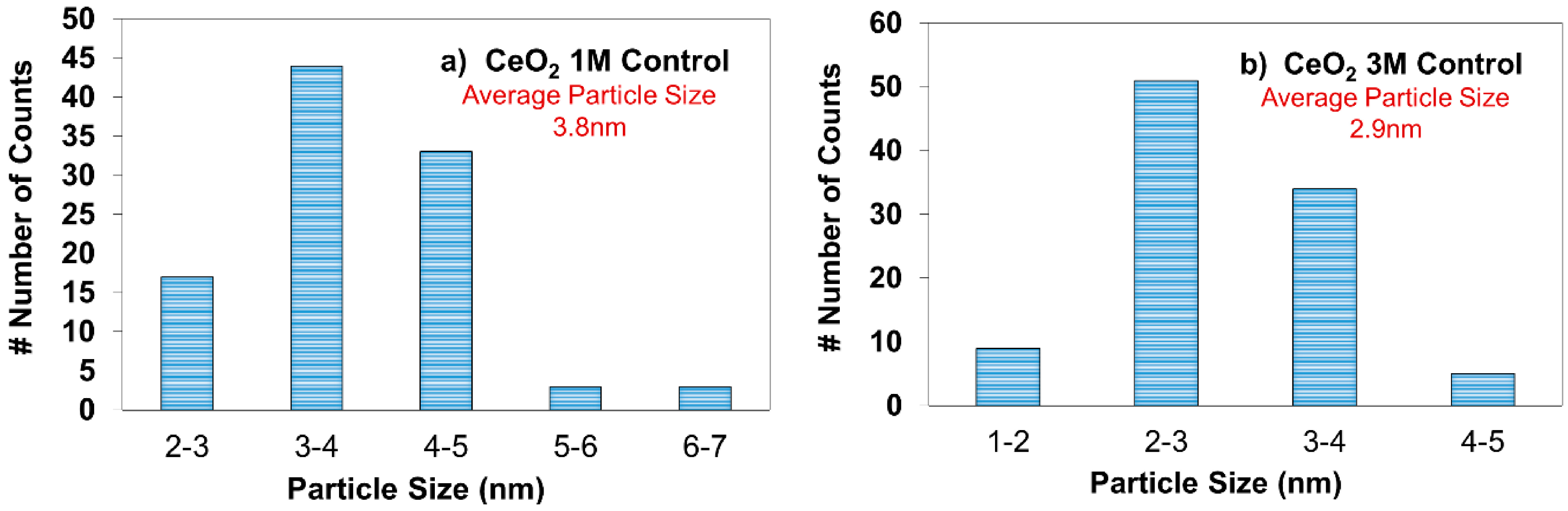
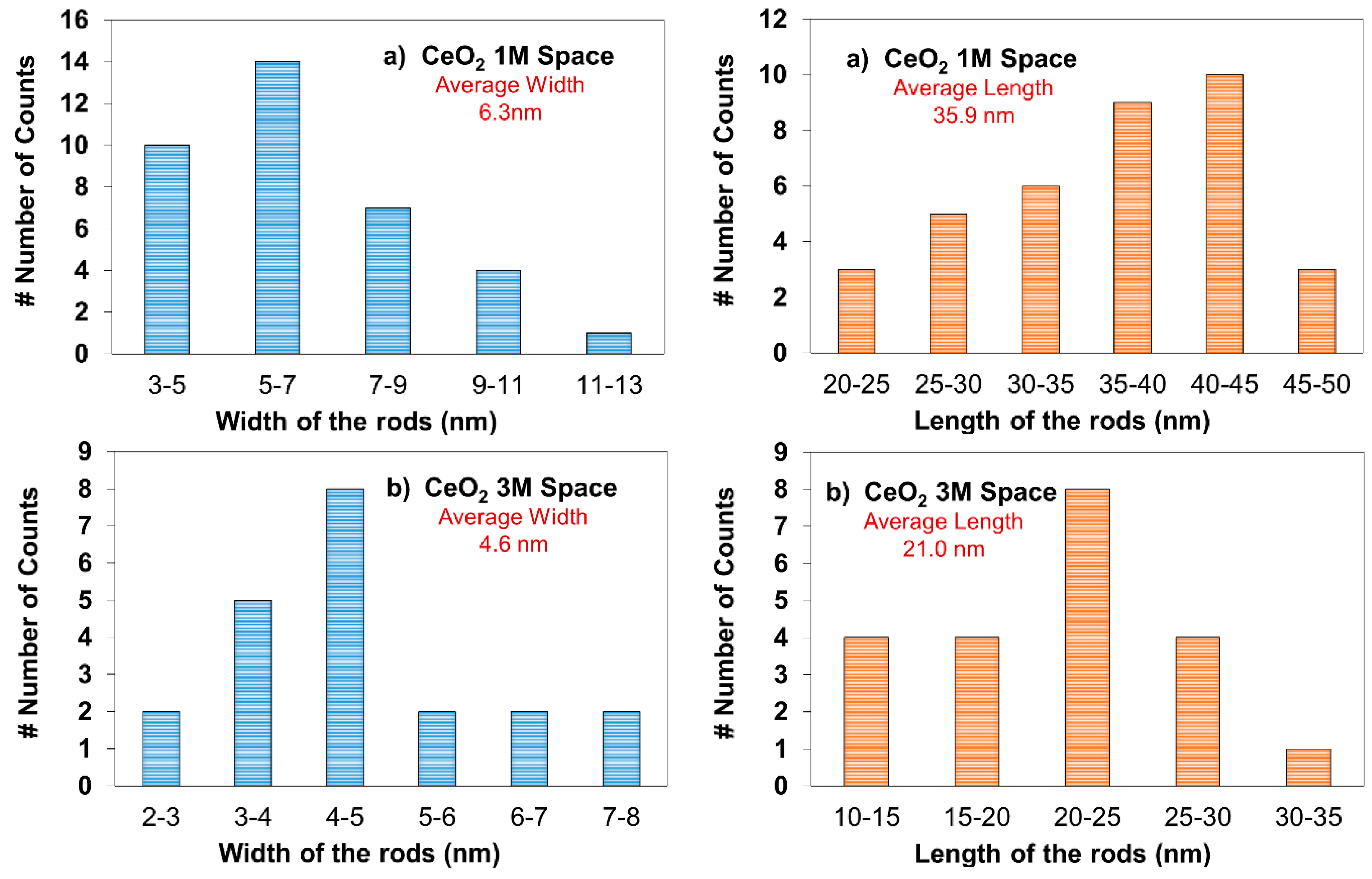
2.3. X-ray Diffraction (XRD) Analysis
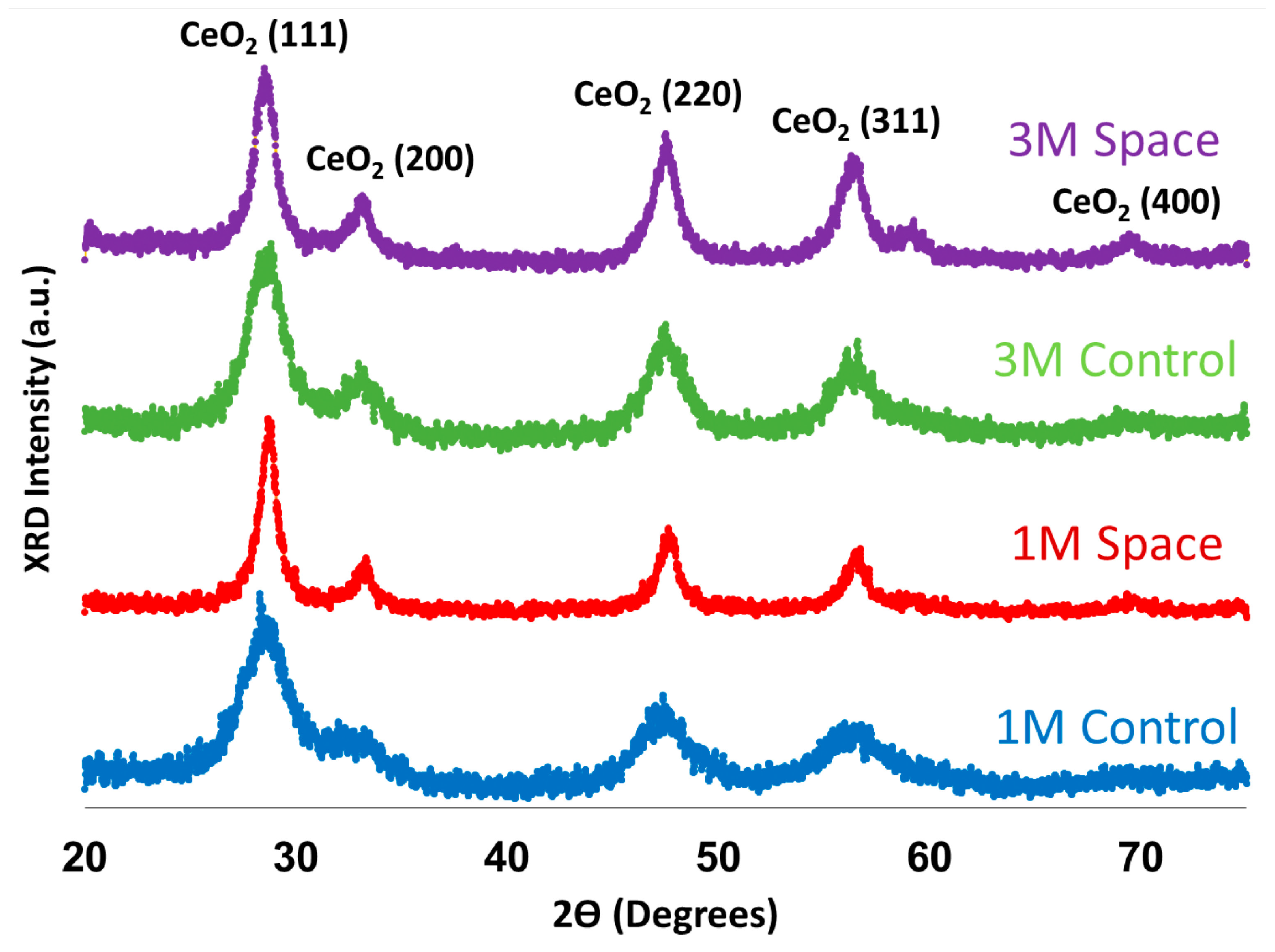
| Mean Crystal Size (nm) | |
|---|---|
| 1 M Control | 3.2 |
| 3 M Control | 4.0 |
| 1 M Space | 6.8 |
| 3 M Space | 5.3 |
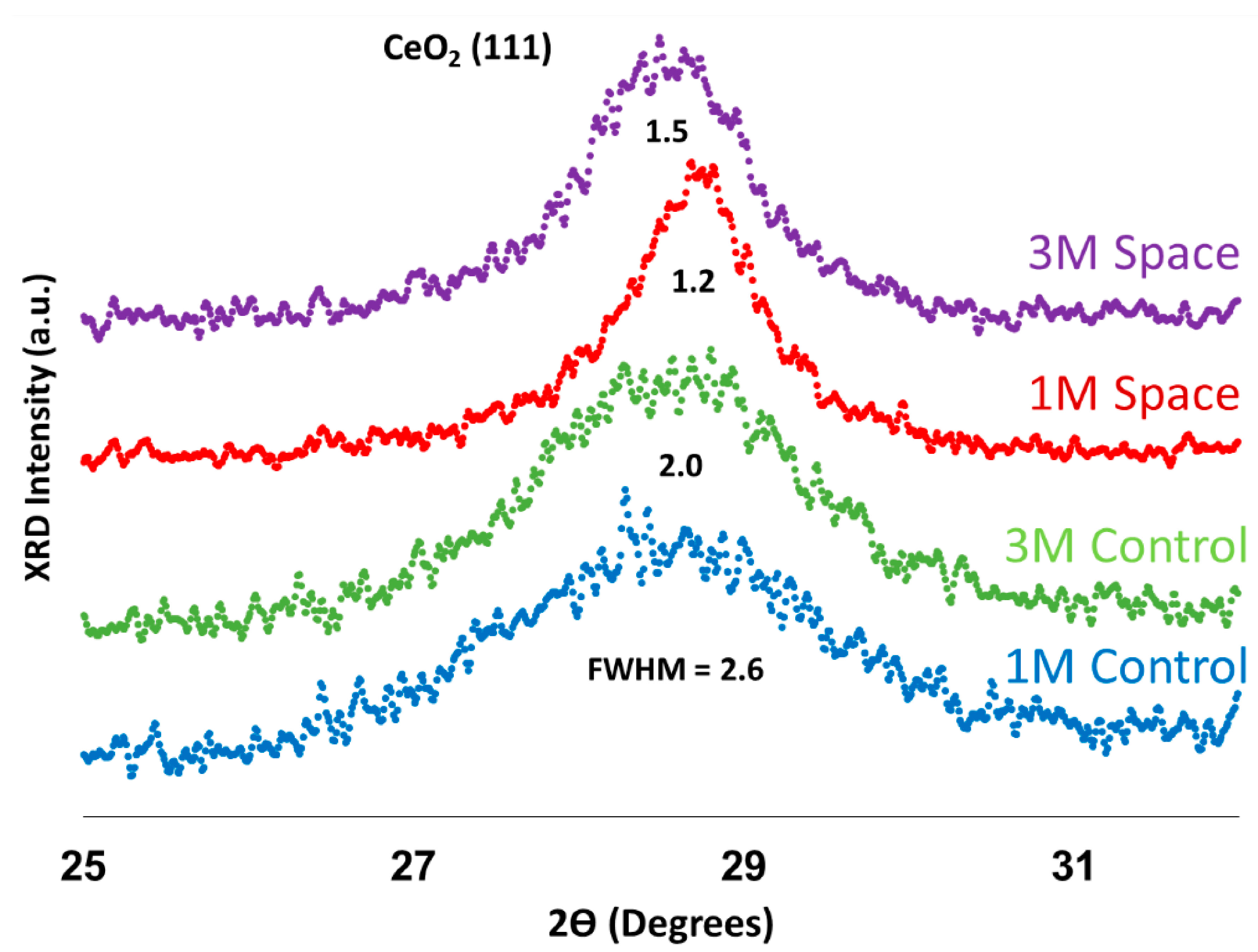
3. Experimental Methods
3.1. Catalyst Preparation
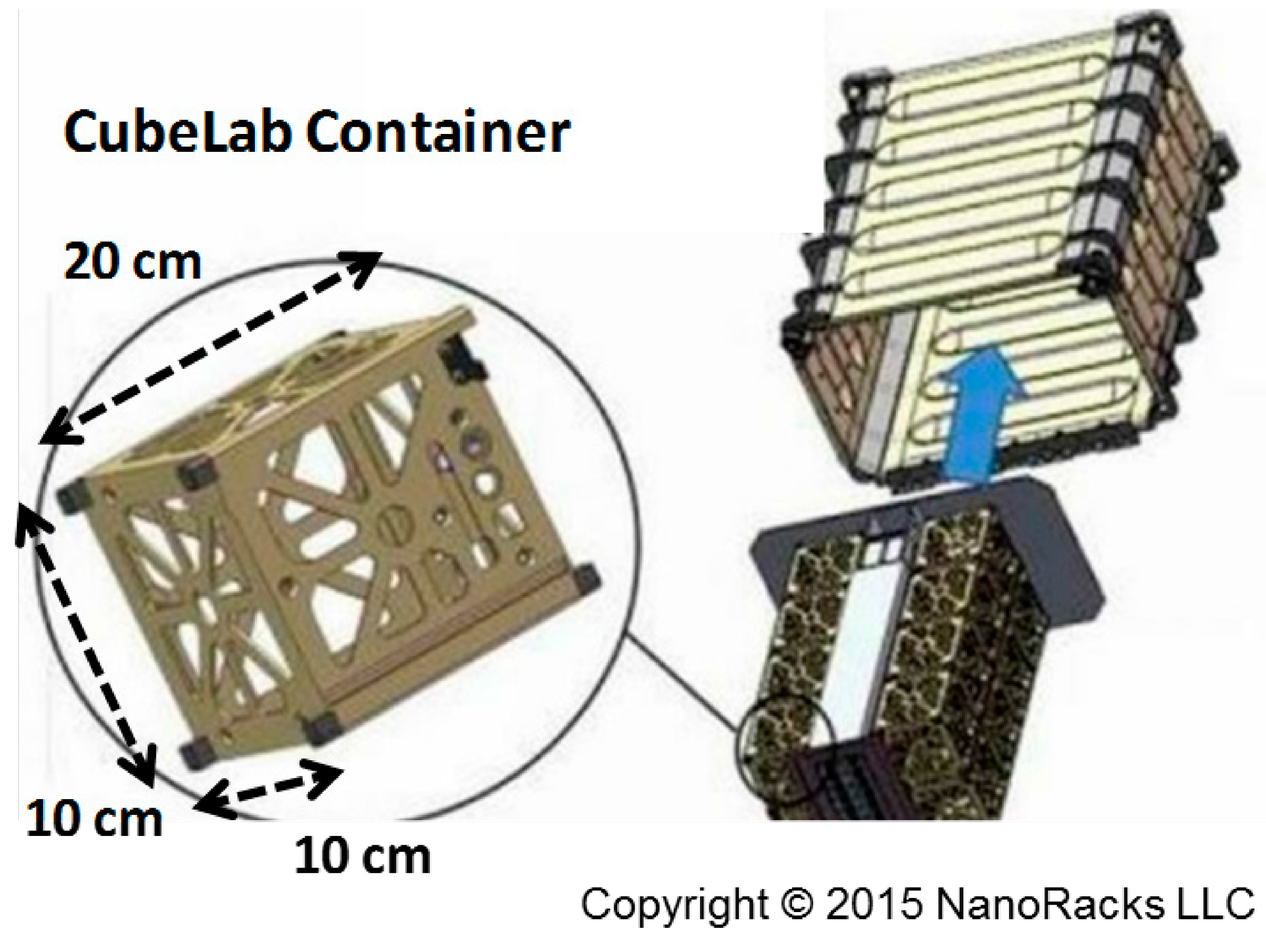
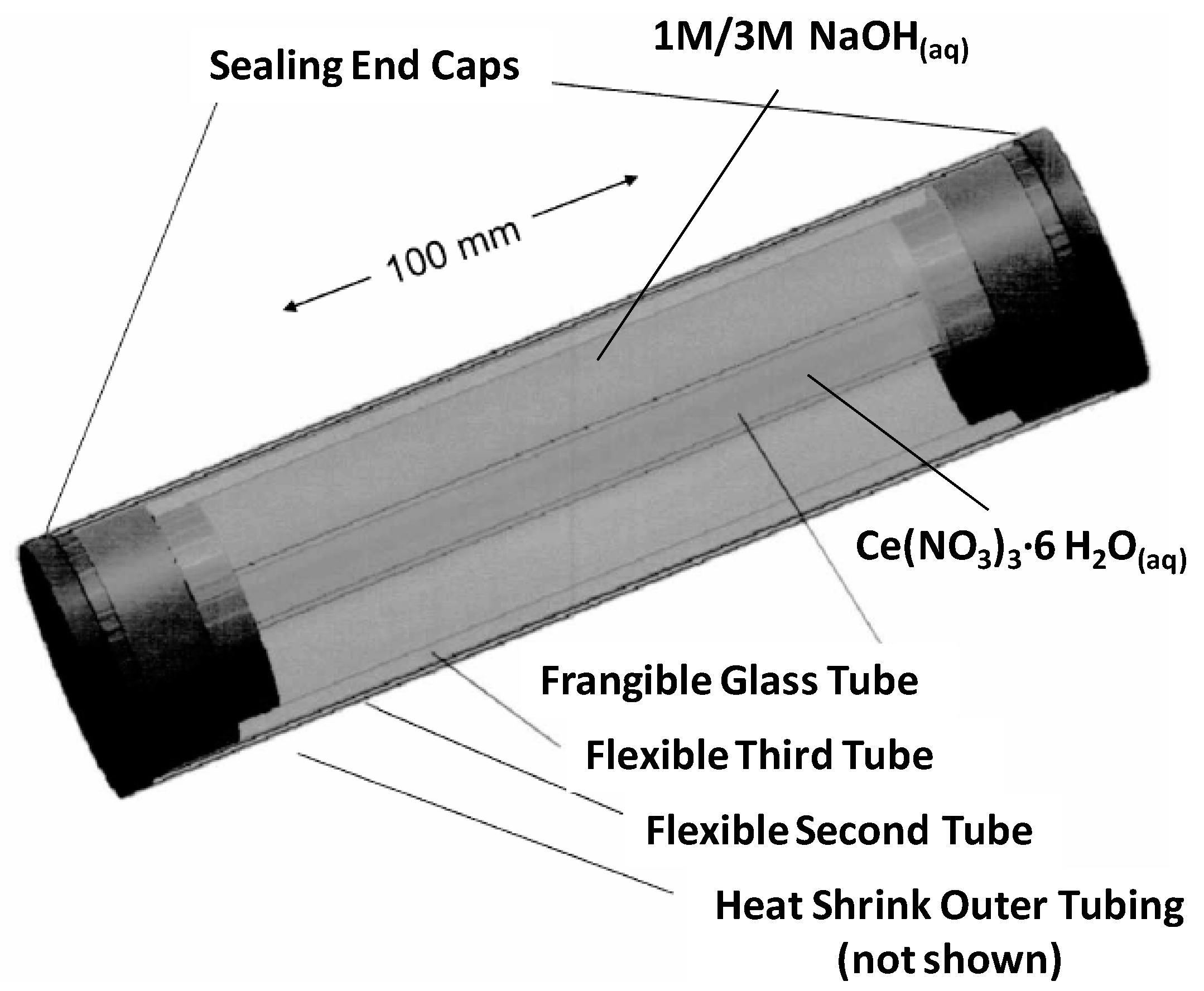
3.2. Surface Analysis
3.3. Transmission Electron Microscopy (TEM)
3.4. X-ray Diffraction (XRD)
4. Conclusions
Acknowledgment
Author Contributions
Conflicts of Interest
References
- Sun, C.; Li, H.; Chen, L. Nanostructured ceria-based materials: Synthesis, properties, and applications. Energy Environ. Sci. 2012, 5, 8475–8505. [Google Scholar] [CrossRef]
- Trovarelli, A. Catalytic Properties of Ceria and CeO2-Containing Materials. Catal. Rev. Sci. Eng. 1996, 38, 439–520. [Google Scholar] [CrossRef]
- Soykal, I.I.; Sohn, H.; Ozkan, U.S. Effect of Support Particle Size in Steam Reforming of Ethanol over Co/CeO2 Catalysts. ACS Catal. 2012, 2, 2335–2348. [Google Scholar] [CrossRef]
- Soykal, I.I.; Sohn, H.; Singh, D.; Miller, J.T.; Ozkan, U.S. Reduction Characteristics of Ceria under Ethanol Steam Reforming Conditions: Effect of the Particle Size. ACS Catal. 2014, 4, 585–592. [Google Scholar] [CrossRef]
- Kaspar, J.; Fornasiero, P.; Graziani, M. Use of CeO2-based oxides in the three-way catalysis. Catal. Today 1999, 50, 285–298. [Google Scholar] [CrossRef]
- Diwell, A.; Rajaram, R.; Shaw, H.; Truex, T. The Role of Ceria in Three-Way Catalysts. Stud. Surf. Sci. Catal. 1991, 71, 139–152. [Google Scholar]
- Whittington, B.; Jiang, C.; Trimm, D. Vehicle exhaust catalysis: I. The relative importance of catalytic oxidation, steam reforming and water-gas shift reactions. Catal. Today 1995, 26, 41–45. [Google Scholar] [CrossRef]
- Murray, E.P.; Tsai, T.; Barnett, S. A direct-methane fuel cell with a ceria-based anode. Nature 1999, 400, 649–651. [Google Scholar] [CrossRef] [PubMed]
- Steele, B.C.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Carrettin, S.; Concepcion, P.; Corma, A.; Nieto, J.M.L.; Puntes, V.F. Nanocrystalline CeO2 Increases the Activity of Au for CO Oxidation by Two Orders of Magnitude. Angew. Chem. 2004, 43, 2538–2540. [Google Scholar] [CrossRef] [PubMed]
- Diagne, C.; Idriss, H.; Pearson, K.; Gómez-García, M.A.; Kiennemann, A.R. Efficient hydrogen production by ethanol reforming over Rh catalysts. Effect of addition of Zr on CeO2 for the oxidation of CO to CO2. C.R. Chim. 2004, 7, 617–622. [Google Scholar] [CrossRef]
- Biswas, P.; Kunzru, D. Steam reforming of ethanol on Ni–CeO2–ZrO2 catalysts: Effect of doping with copper, cobalt and calcium. Catal. Lett. 2007, 118, 36–49. [Google Scholar] [CrossRef]
- Cai, W.; Wang, F.; van Veen, A.C.; Provendier, H.; Mirodatos, C.; Shen, W. Autothermal reforming of ethanol for hydrogen production over an Rh/CeO2 catalyst. Catal. Today 2008, 138, 152–156. [Google Scholar] [CrossRef]
- Cai, W.; Zhang, B.; Li, Y.; Xu, Y.; Shen, W. Hydrogen production by oxidative steam reforming of ethanol over an Ir/CeO2 catalyst. Catal. Commun. 2007, 8, 1588–1594. [Google Scholar] [CrossRef]
- Frusteri, F.; Freni, S.; Chiodo, V.; Donato, S.; Bonura, G.; Cavallaro, S. Steam and auto-thermal reforming of bio-ethanol over MgO and CeO2/Ni supported catalysts. Int. J. Hydrogen Energy 2006, 31, 2193–2199. [Google Scholar] [CrossRef]
- Laosiripojana, N.; Assabumrungrat, S. Catalytic steam reforming of ethanol over high surface area CeO2: The role of CeO2 as an internal pre-reforming catalyst. Appl. Catal. 2006, 66, 29–39. [Google Scholar] [CrossRef]
- Song, H.; Ozkan, U.S. Changing the Oxygen Mobility in Co/Ceria Catalysts by Ca Incorporation: Implications for Ethanol Steam Reforming. J. Phys. Chem. A 2010, 114, 3796–3801. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, G.; Chenu, E.; Patterson, P.M.; Williams, L.; Sparks, D.; Thomas, G.; Davis, B.H. Water-gas shift: Comparative screening of metal promoters for metal/ceria systems and role of the metal. Appl. Catal. A 2004, 258, 203–214. [Google Scholar] [CrossRef]
- Yi, N.; Si, R.; Saltsburg, H.; Flytzani-Stephanopoulos, M. Active gold species on cerium oxide nanoshapes for methanol steam reforming and the water gas shift reactions. Energy Environ. Sci. 2010, 3, 831–837. [Google Scholar] [CrossRef]
- Anupriya, K.; Vivek, E.; Subramanian, B. Facile synthesis of ceria nanoparticles by precipitation route for UV blockers. J. Alloys Compd. 2014, 590, 406–410. [Google Scholar] [CrossRef]
- Shih, S.J.; Tzeng, W.L.; Kuo, W.L. Fabrication of ceria particles using glycine nitrate spray pyrolysis. Surf. Coat. Technol. 2014, 259, 302–309. [Google Scholar] [CrossRef]
- Christensen, J.; Deiana, D.; Grunwaldt, J.D.; Jensen, A. Ceria Prepared by Flame Spray Pyrolysis as an Efficient Catalyst for Oxidation of Diesel Soot. Catal. Lett. 2014, 144, 1661–1666. [Google Scholar] [CrossRef]
- Hirano, M.; Inagaki, M. Preparation of monodispersed cerium(IV) oxide particles by thermalhydrolysis: Influence of the presence of urea and Gd doping on their morphology and growth. J. Mater. Chem. 2000, 10, 473–477. [Google Scholar] [CrossRef]
- Bumajdad, A.; Zaki, M.I.; Eastoe, J.; Pasupulety, L. Microemulsion-Based Synthesis of CeO2 Powders with High Surface Area and High-Temperature Stabilities. Langmuir 2004, 20, 11223–11233. [Google Scholar] [CrossRef] [PubMed]
- Charojrochkul, S.; Nualpaeng, W.; Laosiripojana, N.; Assabumrungrat, S. Materials Challenges and Testing for Supply of Energy and Resources; Springer: Berlin, Germany, 2012; pp. 189–202. [Google Scholar]
- Derakhshandeh, P.G.; Soleimannejad, J.; Janczak, J. Sonochemical synthesis of a new nano-sized cerium(III) coordination polymer and its conversion to nanoceria. Ultrason. Sonochem. 2015, 26, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.Y.; He, J.X.; Lu, N.P.; Zheng, Y.Y.; Dong, W.J.; Tang, W.H.; Chen, B.Y.; Li, C.R. Morphology and size control of cerium carbonate hydroxide and ceria micro/nanostructures by hydrothermal technology. Mater. Chem. Phys. 2010, 121, 314–319. [Google Scholar] [CrossRef]
- Zawadzki, M. Preparation and characterization of ceria nanoparticles by microwave-assisted solvothermal process. J. Alloys Compd. 2008, 454, 347–351. [Google Scholar] [CrossRef]
- Zhang, D.; Niu, F.; Li, H.; Shi, L.; Fang, J. Uniform ceria nanospheres: Solvothermal synthesis, formation mechanism, size-control and catalytic activity. Powder Technol. 2011, 207, 35–41. [Google Scholar] [CrossRef]
- Abbasi, Z.; Haghighi, M.; Fatehifar, E.; Rahemi, N. Comparative synthesis and physicochemical characterization of CeO2 nanopowder via redox reaction, precipitation and sol-gel methods used for total oxidation of toluene. Asia Pac. J. Chem. Eng. 2012, 7, 868–876. [Google Scholar] [CrossRef]
- Zhou, X.D.; Huebner, W. Size-induced lattice relaxation in CeO2 nanoparticles. Appl. Phys. Lett. 2001, 79, 3512–3514. [Google Scholar] [CrossRef]
- Deshpande, S.; Patil, S.; Kuchibhatla, S.V.N.T.; Seal, S. Size dependency variation in lattice parameter and valency states in nanocrystalline cerium oxide. Appl. Phys. Lett. 2005, 87, 133113. [Google Scholar] [CrossRef]
- Zhang, F.; Chan, S.W.; Spanier, J.E.; Apak, E.; Jin, Q.; Robinson, R.D.; Herman, I.P. Cerium oxide nanoparticles: Size-selective formation and structure analysis. Appl. Phys. Lett. 2002, 80, 127–129. [Google Scholar] [CrossRef]
- Si, R.; Flytzani-Stephanopoulos, M. Shape and Crystal-Plane Effects of Nanoscale Ceria on the Activity of Au–CeO2 Catalysts for the Water-Gas Shift Reaction. Angew. Chem. Int. Ed. 2008, 47, 2884–2887. [Google Scholar] [CrossRef] [PubMed]
- Yi, N.; Si, R.; Saltsburg, H.; Flytzani-Stephanopoulos, M. Steam reforming of methanol over ceria and gold-ceria nanoshapes. Appl. Catal. B 2010, 95, 87–92. [Google Scholar] [CrossRef]
- Lee, Y.; He, G.; Akey, A.J.; Si, R.; Flytzani-Stephanopoulos, M.; Herman, I.P. Raman Analysis of Mode Softening in Nanoparticle CeO2−δ and Au–CeO2−δ during CO Oxidation. J. Am. Chem. Soc. 2011, 133, 12952–12955. [Google Scholar] [CrossRef] [PubMed]
- Boucher, M.B.; Goergen, S.; Yi, N.; Flytzani-Stephanopoulos, M. “Shape effects” in metal oxide supported nanoscale gold catalysts. Phys. Chem. Chem. Phys. 2011, 13, 2517–2527. [Google Scholar] [CrossRef] [PubMed]
- Coker, E.N.; Jansen, J.C.; DiRenzo, F.; Fajula, F.; Martens, J.A.; Jacobs, P.A.; Microporous, A.S., Jr. Zeolite ZSM-5 synthesized in space: Catalysts with reduced external surface activity. Microporous Mesoporous Mater. 2001, 46, 223–236. [Google Scholar] [CrossRef]
- Coker, E.N.; Jansen, J.C.; Martens, J.A.; Jacobs, P.A.; DiRenzo, F.; Fajula, F.; Microporou, A.S., Jr. The synthesis of zeolites under micro-gravity conditions: A review. Microporous Mesoporous Mater. 1998, 23, 119–136. [Google Scholar] [CrossRef]
- Sano, T.; Mizukami, F.; Kawamura, M.; Takaya, H.; Mouri, T.; Inaoka, W.; Toida, Y.; Watanabe, M.; Toyoda, K. Crystallization of ZSM-5 zeolite under microgravity. Zeolites 1992, 12, 801–805. [Google Scholar] [CrossRef]
- Warzywoda, J.; Baç, N.; Rossetti, G.A., Jr.; van der Puil, N.; Jansen, J.C.; Bekkum, H.V.; Sacco, A., Jr. Synthesis of high-silica ZSM-5 in microgravity. Microporous Mesoporous Mater. 2000, 38, 423–432. [Google Scholar] [CrossRef]
- Tsuchida, A.; Taguchi, K.; Takyo, E.; Yoshimi, H.; Kiriyama, S.; Okubo, T.; Ishikawa, M. Microgravity experiments on colloidal crystallization of silica spheres in the presence of sodium chloride. Colloid Polym. Sci. 2000, 278, 872–877. [Google Scholar] [CrossRef]
- Smith, D.D.; Sibille, L.; Cronise, R.J.; Hunt, A.J.; Oldenburg, S.J.; Wolfe, D.; Halas, N.J. Effect of Microgravity on the Growth of Silica Nanostructures. Langmuir 2000, 16, 10055–10060. [Google Scholar] [CrossRef]
- Ahari, H.; Bedard, R.L.; Bowes, C.L.; Coombs, N.; Dag, O.; Jiang, T.; Ozin, G.A.; Petrov, S.; Sokolov, I.; Verma, A.; et al. Effect of microgravity on the crystallization of a self-assembling layered material. Nature 1997, 388, 857–860. [Google Scholar]
- Fujii, H.; Nogi, K. Formation and disappearance of pores in aluminum alloy molten pool under microgravity. Sci. Technol. Adv. Mater. 2004, 5, 219–223. [Google Scholar] [CrossRef]
- Soykal, I.I.; Bayram, B.; Sohn, H.; Gawade, P.; Miller, J.T.; Ozkan, U.S. Ethanol steam reforming over Co/CeO2 catalysts: Investigation of the effect of ceria morphology. Appl. Catal. A 2012, 449, 47–58. [Google Scholar] [CrossRef]
- Ho, C.M.; Yu, J.C.; Kwong, T.; Mak, A.C.; Lai, S.Y. Morphology-Controllable Synthesis of Mesoporous CeO2 Nano- and Microstructures. Chem. Mater. 2005, 17, 4514–4522. [Google Scholar] [CrossRef]
- Huang, P.X.; Wu, F.; Zhu, B.L.; Gao, X.P.; Zhu, H.Y.; Yan, T.Y.; Huang, W.P.; Wu, S.H.; Song, D.Y. CeO2 Nanorods and Gold Nanocrystals Supported on CeO2 Nanorods as Catalyst. J. Phys. Chem. B 2005, 109, 19169–19174. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yao, Z.; Deng, Y.; Gao, F.; Liu, B.; Dong, L. Morphology and Crystal-Plane Effects of Nanoscale Ceria on the Activity of CuO/CeO2 for NO Reduction by CO. ChemCatChem 2011, 3, 978–989. [Google Scholar] [CrossRef]
- Tok, A.I.Y.; Boey, F.Y.C.; Dong, Z.; Sun, X.L. Hydrothermal synthesis of CeO2 nano-particles. J. Mater. Process. Technol. 2007, 190, 217–222. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, K.; Liu, X.; Tian, Q.; Lu, D.; Yang, S. Single-crystalline ceria nanocubes: Size-controlled synthesis, characterization and redox property. Nanotechnology 2007, 18, 185606. [Google Scholar] [CrossRef]
- Mai, H.X.; Sun, L.D.; Zhang, Y.W.; Si, R.; Feng, W.; Zhang, H.P.; Liu, H.C.; Yan, C.H. Shape-Selective Synthesis and Oxygen Storage Behavior of Ceria Nanopolyhedra, Nanorods, and Nanocubes. J. Phys. Chem. B 2005, 109, 24380–24385. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.C.; Shi, E.W.; Zheng, Y.Q.; Li, W.J. Effect of pH of Medium on Hydrothermal Synthesis of Nanocrystalline Cerium(IV) Oxide Powders. J. Am. Ceram. Soc. 2002, 85, 2462–2468. [Google Scholar] [CrossRef]
- Li, J.G.; Ikegami, T.; Wang, Y.; Mori, T. Reactive Ceria Nanopowders via Carbonate Precipitation. J. Am. Ceram. Soc. 2002, 85, 2376–2378. [Google Scholar] [CrossRef]
- Levine, S.; Snyder, M. Microgravity Cube Lab Experiment Design: Setting New Precedents for Micro-Gravity Testing. In Proceedings of the AIAA SPACE 2012 Conference & Exposition, Pasadena, CA, USA, 11–13 September 2012.
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soykal, I.I.; Sohn, H.; Bayram, B.; Gawade, P.; Snyder, M.P.; Levine, S.E.; Oz, H.; Ozkan, U.S. Effect of Microgravity on Synthesis of Nano Ceria. Catalysts 2015, 5, 1306-1320. https://doi.org/10.3390/catal5031306
Soykal II, Sohn H, Bayram B, Gawade P, Snyder MP, Levine SE, Oz H, Ozkan US. Effect of Microgravity on Synthesis of Nano Ceria. Catalysts. 2015; 5(3):1306-1320. https://doi.org/10.3390/catal5031306
Chicago/Turabian StyleSoykal, Ilgaz I., Hyuntae Sohn, Burcu Bayram, Preshit Gawade, Michael P. Snyder, Stephen E. Levine, Hayrani Oz, and Umit S. Ozkan. 2015. "Effect of Microgravity on Synthesis of Nano Ceria" Catalysts 5, no. 3: 1306-1320. https://doi.org/10.3390/catal5031306