Microwave Synthesis of High Activity FeSe2/C Catalyst toward Oxygen Reduction Reaction
Abstract
:1. Introduction
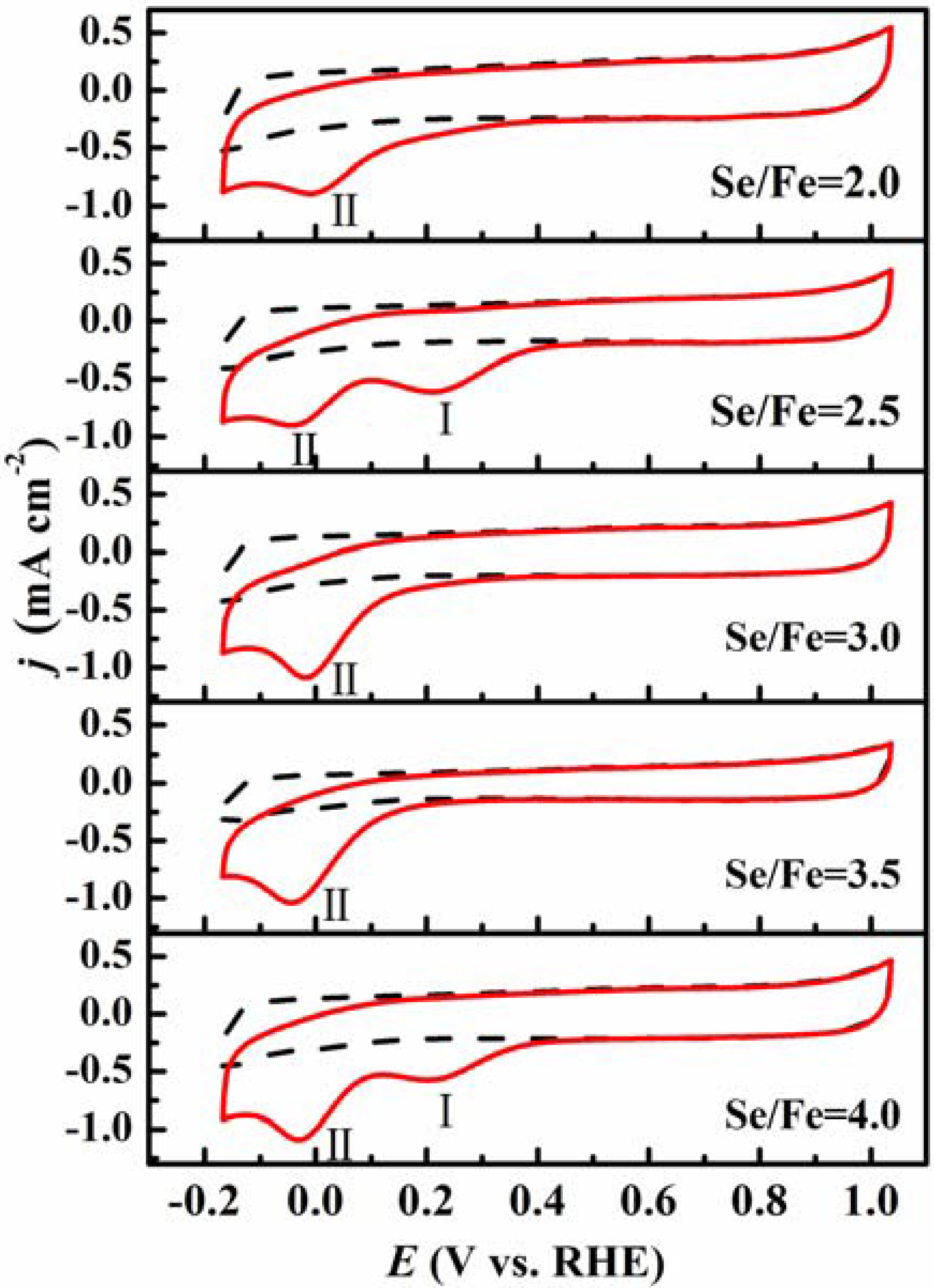
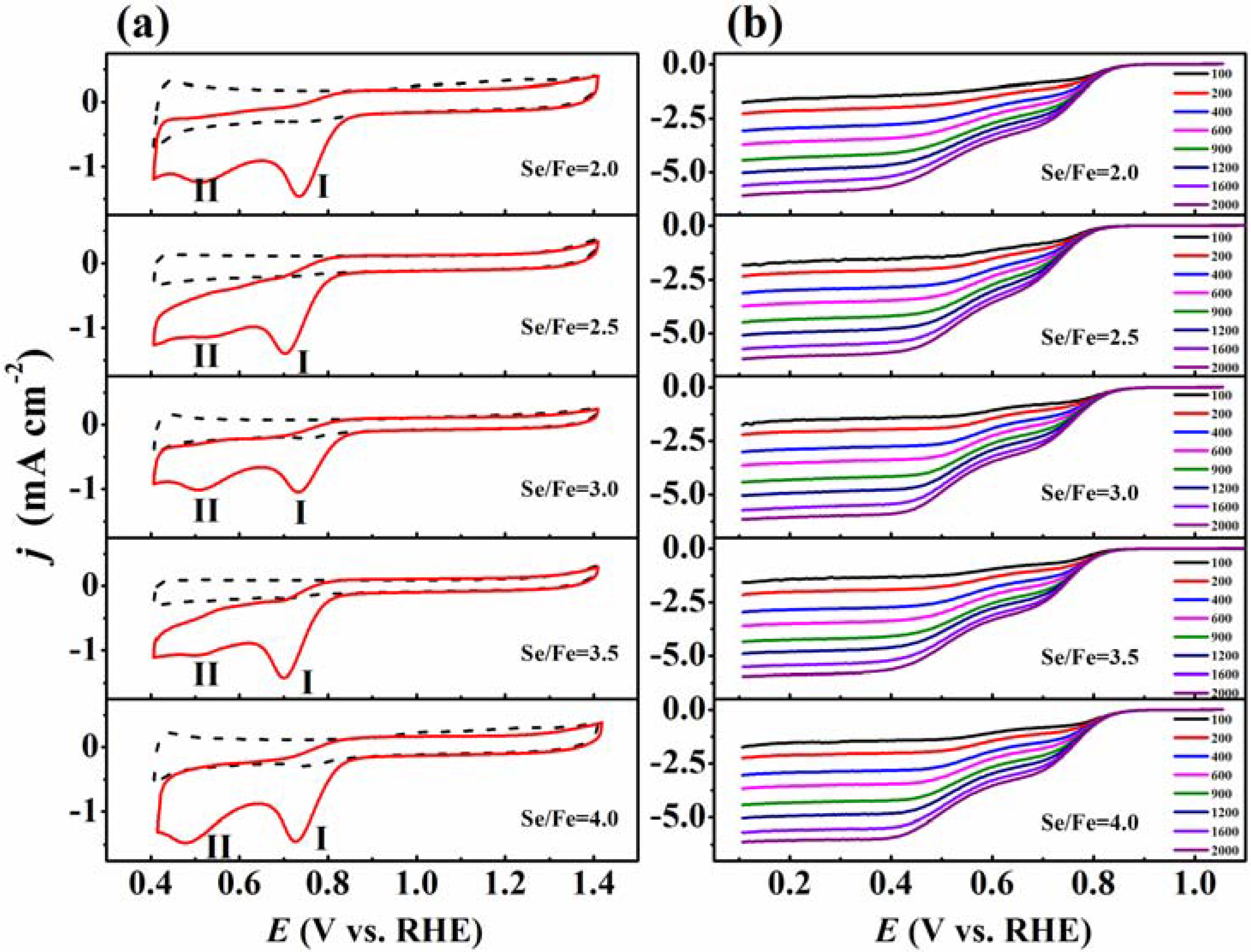
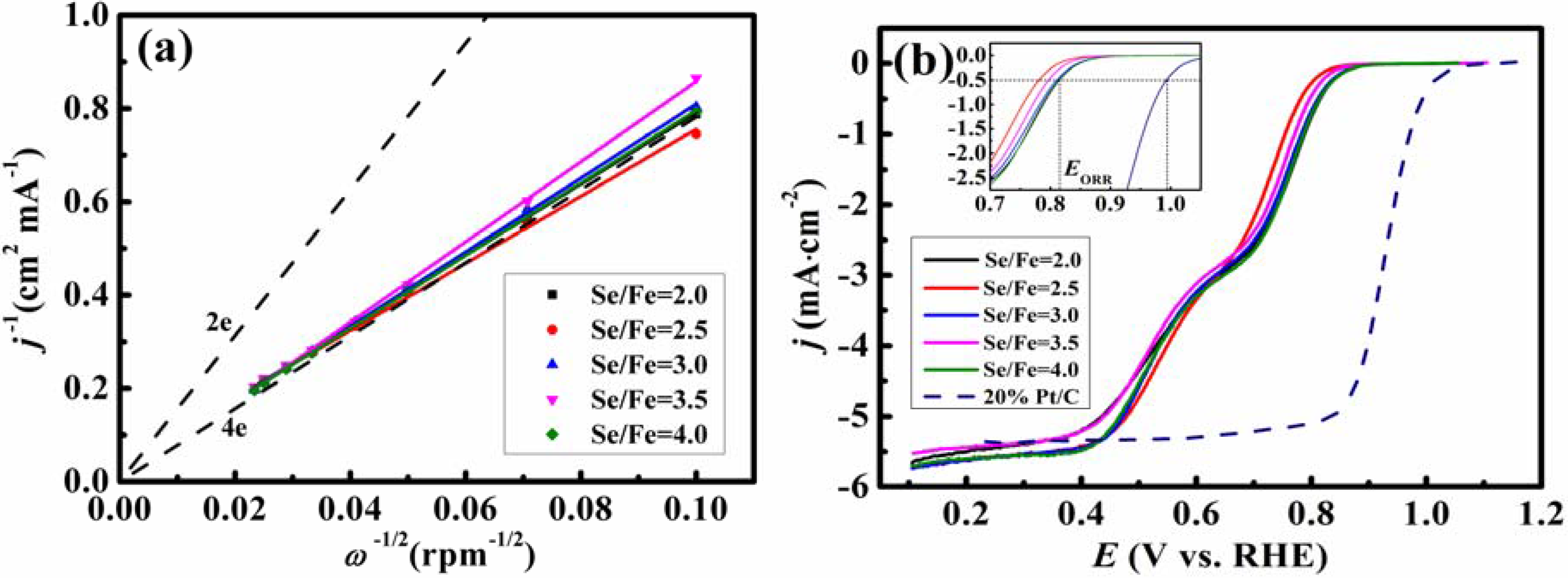
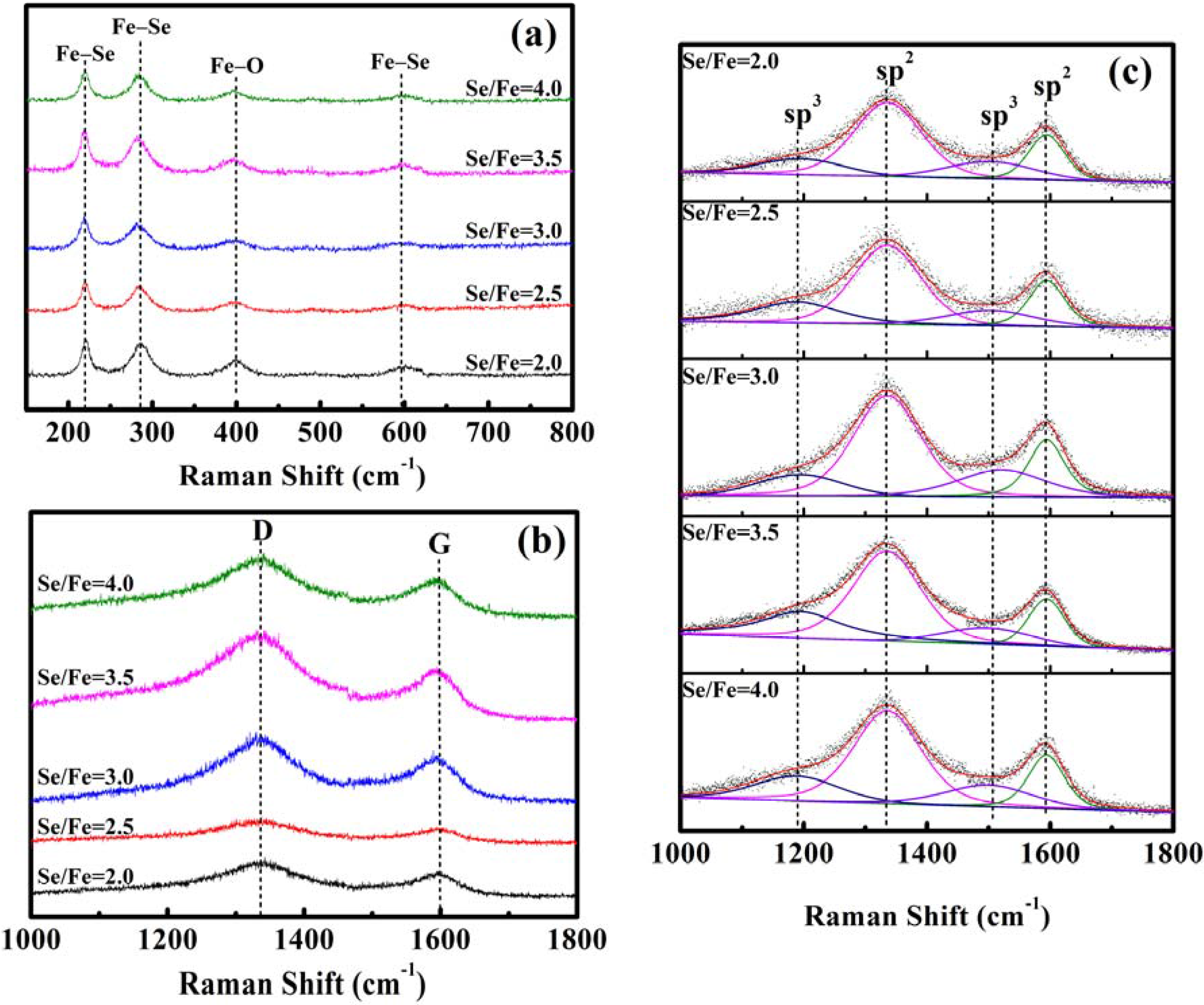
2. Results and Discussion

| Parameter | Se/Fe ratio | |||||
|---|---|---|---|---|---|---|
| Nominal | 2.0 | 2.5 | 3.0 | 3.5 | 4.0 | |
| Evaluated by EDS | 2.1 | 2.5 | 3.3 | 3.6 | 4.2 | |
| Crystallite Size (nm) | 36.2 | 35.6 | 32.9 | 33.1 | 35.4 | |
| EP (V, vs. RHE) | (I) | 0.733 | 0.704 | 0.733 | 0.699 | 0.727 |
| (II) | 0.511 | 0.514 | 0.509 | 0.499 | 0.478 | |
| n at 0.3 V (vs. RHE) | 3.7 | 3.9 | 3.5 | 3.3 | 3.6 | |
| EORR (V, vs. RHE) | 0.814 | 0.781 | 0.809 | 0.795 | 0.814 | |
| ID/IG | 1.64 | 1.71 | 1.76 | 1.90 | 1.74 | |
| Asp3/Asp2 | 0.37 | 0.48 | 0.44 | 0.51 | 0.46 | |


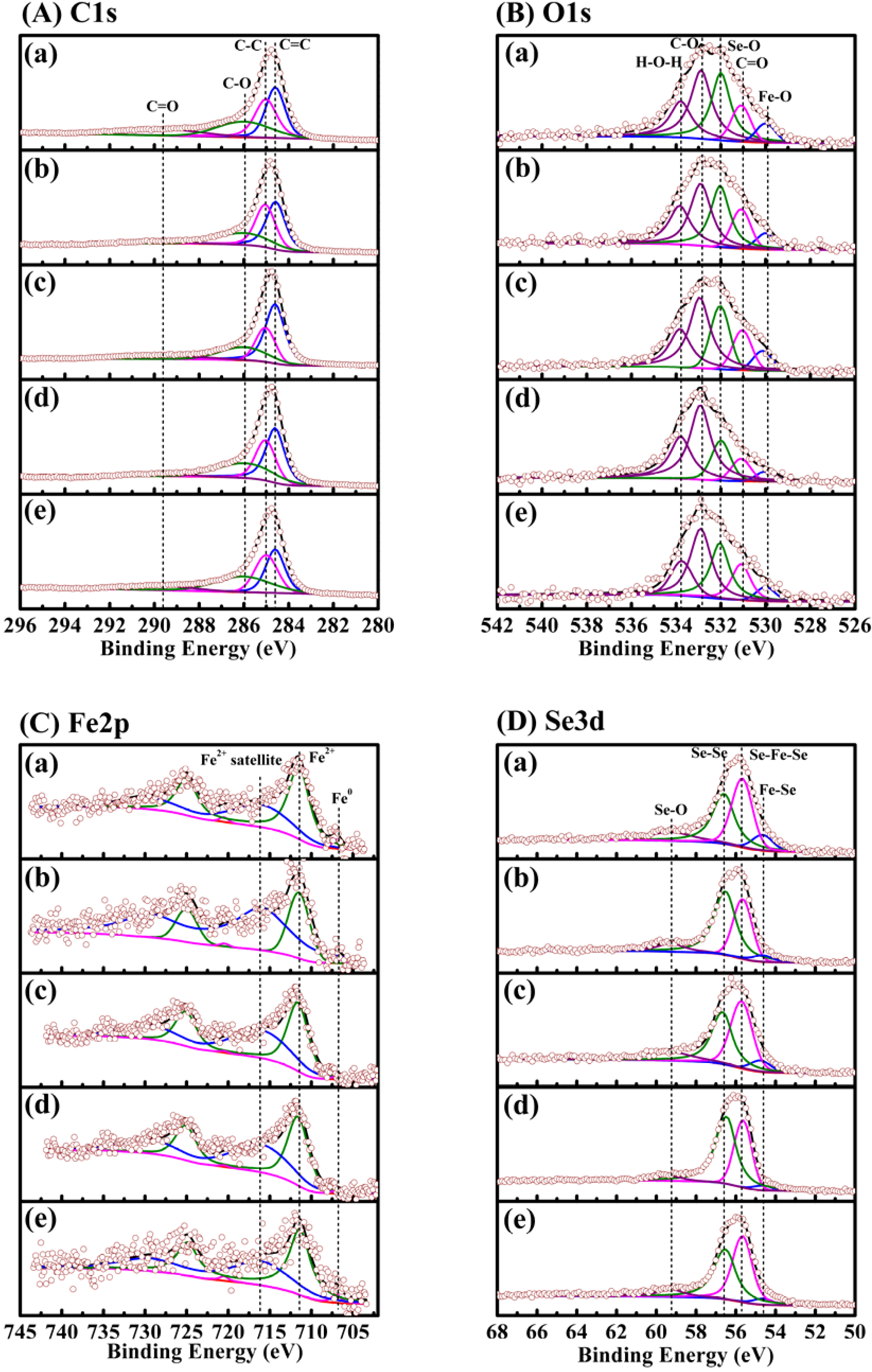
| Parameter | Fe/Se ratio | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nominal | 2.0 | 2.5 | 3.0 | 3.5 | 4.0 | ||||||
| Calculated | 2.0 | 2.5 | 3.0 | 3.5 | 4.0 | ||||||
| Species | EB (eV) | R.A. (%) | EB (eV) | R.A. (%). | EB (eV) | R.A. (%). | EB (eV) | R.A. (%) | EB (eV) | R.A. (%) | |
| C1s | C=C | 284.6 | 31.8 | 284.6 | 39.8 | 284.6 | 47.1 | 284.6 | 41.2 | 284.6 | 31.3 |
| C–C | 285.0 | 29.5 | 285.0 | 34.3 | 285.0 | 22.3 | 285.0 | 28.6 | 285.0 | 28.7 | |
| C–O | 286.0 | 24.3 | 286.0 | 22.4 | 286.0 | 21.3 | 286.0 | 26.5 | 286.0 | 31.0 | |
| C=O | 289.6 | 14.4 | 289.6 | 3.5 | 289.6 | 9.3 | 289.6 | 3.7 | 289.6 | 9.0 | |
| O1s | Fe–O | 530.0 | 5.9 | 530.0 | 6.4 | 530.1 | 6.5 | 530.0 | 3.7 | 530.0 | 5.1 |
| C=O | 531.1 | 11.2 | 531.1 | 13.0 | 531.1 | 13.2 | 531.1 | 8.3 | 531.1 | 15.8 | |
| Se–O | 532.0 | 30.0 | 532.0 | 28.2 | 532.0 | 20.9 | 532.0 | 16.5 | 532.0 | 27.9 | |
| C–O | 532.9 | 33.8 | 532.9 | 30.6 | 532.9 | 37.8 | 532.9 | 44.8 | 532.9 | 35.5 | |
| H–O–H | 533.8 | 19.0 | 533.8 | 21.7 | 533.8 | 21.5 | 533.8 | 26.6 | 533.8 | 15.7 | |
| Fe2p | Fe° | 707.0 | 3.7 | 706.9 | 3.3 | 707.0 | 1.5 | 707.0 | 1.4 | 707.0 | 5.3 |
| Fe2+ | 711.5 | 96.3 | 711.5 | 96.7 | 711.6 | 98.5 | 711.6 | 98.6 | 711.4 | 94.7 | |
| Satellite | 716.0 | / | 715.9 | / | 716.0 | / | 716.0 | / | 716.0 | / | |
| Se3d | Fe–Se | 54.5 | 8.8 | 54.5 | 5.1 | 54.6 | 6.4 | 54.5 | 2.7 | 54.5 | 4.5 |
| Se–Fe–Se | 55.5 | 38.4 | 55.5 | 32.0 | 55.6 | 40.7 | 55.5 | 35.5 | 55.5 | 43.7 | |
| Se–Se | 56.4 | 38.4 | 56.4 | 53.5 | 56.5 | 38.5 | 56.4 | 57.0 | 56.4 | 43.4 | |
| Se–O | 59.1 | 14.3 | 59.1 | 9.3 | 59.1 | 14.4 | 59.2 | 4.8 | 59.1 | 8.4 | |
3. Experimental Section
3.1. Materials
3.2. Catalyst Synthesis
3.3. Electrochemical Characterization
3.4. Physicochemical Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McLean, G.; Niet, T.; Prince-Richard, S.; Djilali, N. An assessment of alkaline fuel cell technology. Int. J. Hydrogen Energy 2002, 27, 507–526. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, H.; Zhou, J.; Li, Y.; Wang, J.; Regier, T.; Dai, H. Covalent hybrid of spinel manganese–cobalt oxide and graphene as advanced oxygen reduction electrocatalysts. J. Am. Chem. Soc. 2012, 134, 3517–3523. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, M.; Proietti, E.; Jaouen, F.; Dodelet, J.P. Iron-based catalysts with improved oxygen reduction activity in polymer electrolyte fuel cells. Science 2009, 324, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Thapa, R.; Kim, H.; Xu, X.; Kim, M.G.; Li, Q.; Park, N.; Liu, M.; Cho, J. Promotion of oxygen reduction by a bio-inspired tethered iron phthalocyanine carbon nanotube-based catalyst. Nat. Commun. 2013, 4, 2076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, D.; Jeong, B.; Mun, B.S.; Jeon, H.; Shin, H.J.; Baik, J.; Lee, J. On the Origin of Electrocatalytic Oxygen Reduction Reaction on Electrospun Nitrogen–Carbon Species. J. Phys. Chem. C 2013, 117, 11619–11624. [Google Scholar] [CrossRef]
- Ding, L.; Xin, Q.; Zhou, X.; Qiao, J.; Li, H.; Wang, H. Electrochemical behavior of nanostructured nickel phthalocyanine (NiPc/C) for oxygen reduction reaction in alkaline media. J. Appl. Electrochem. 2013, 43, 43–51. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Z.; Bao, J.; Lan, Y.; Tu, W.; Han, M.; Dai, Z. Controllable Synthesis of Tetragonal and Cubic Phase Cu2Se Nanowires Assembled by Small Nanocubes and Their Electrocatalytic Performance for Oxygen Reduction Reaction. J. Phys. Chem. C 2013, 117, 15164–15173. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, S.; Zhao, Y.; Zhu, L.; Chen, S.; Wang, X.; Wu, Q.; Ma, J.; Ma, Y.; Hu, Z. Boron-Doped Carbon Nanotubes as Metal-Free Electrocatalysts for the Oxygen Reduction Reaction. Angew. Chem. 2011, 123, 7270–7273. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Hibino, T.; Kobayashi, K.; Heo, P. Oxygen reduction reaction over nitrogen-doped graphene oxide cathodes in acid and alkaline fuel cells at intermediate temperatures. Electrochim. Acta 2013, 112, 82–89. [Google Scholar] [CrossRef]
- Wu, J.; Yang, Z.; Li, X.; Sun, Q.; Jin, C.; Strasser, P.; Yang, R. Phosphorus-doped porous carbons as efficient electrocatalysts for oxygen reduction. J. Mater. Chem. A 2013, 1, 9889–9896. [Google Scholar] [CrossRef]
- Zhu, J.; He, G.; Liang, L.; Wan, Q.; Shen, P.K. Direct anchoring of platinum nanoparticles on nitrogen and phosphorus-dual-doped carbon nanotube arrays for oxygen reduction reaction. Electrochim. Acta 2015, 158, 374–382. [Google Scholar] [CrossRef]
- Liang, J.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. Sulfur and Nitrogen Dual-Doped Mesoporous Graphene Electrocatalyst for Oxygen Reduction with Synergistically Enhanced Performance. Angew. Chem. Int. Ed. 2012, 51, 11496–11500. [Google Scholar] [CrossRef] [PubMed]
- Susac, D.; Zhu, L.; Teo, M.; Sode, A.; Wong, K.C.; Wong, P.C.; Parsons, R.R.; Bizzotto, D.; Mitchell, K.A.R.; Campbell, S.A. Characterization of FeS2-based thin films as model catalysts for the oxygen reduction reaction. J. Phys. Chem. C 2007, 111, 18715–18723. [Google Scholar] [CrossRef]
- Jin, Z.; Nie, H.; Yang, Z.; Zhang, J.; Liu, Z.; Xu, X.; Huang, S. Metal-free selenium doped carbon nanotube/graphene networks as a synergistically improved cathode catalyst for oxygen reduction reaction. Nanoscale 2012, 4, 6455–6460. [Google Scholar] [CrossRef] [PubMed]
- Nekooi, P.; Akbari, M.; Amini, M.K. CoSe nanoparticles prepared by the microwave-assisted polyol method as an alcohol and formic acid tolerant oxygen reduction catalyst. Int. J. Hydrogen Energy 2010, 35, 6392–6398. [Google Scholar] [CrossRef]
- Li, H.; Gao, D.; Cheng, X. Simple microwave preparation of high activity Se-rich CoSe2/C for oxygen reduction reaction. Electrochim. Acta 2014, 138, 232–239. [Google Scholar] [CrossRef]
- Feng, Y.; Alonso-Vante, N. Carbon-supported cubic CoSe2 catalysts for oxygen reduction reaction in alkaline medium. Electrochim. Acta 2012, 72, 129–133. [Google Scholar] [CrossRef]
- Oyler, K.D.; Ke, X.; Sines, I.T.; Schiffer, P.; Schaak, R.E. Chemical Synthesis of Two-Dimensional Iron Chalcogenide Nanosheets: FeSe, FeTe, Fe (Se, Te), and FeTe2. Chem. Mater. 2009, 21, 3655–3661. [Google Scholar] [CrossRef]
- Han, D.S.; Batchelor, B.; Abdel-Wahab, A. Sorption of selenium (IV) and selenium (VI) onto synthetic pyrite (FeS2): Spectroscopic and microscopic analyses. J. Colloid Interface Sci. 2012, 368, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Burrard-Lucas, M.; Free, D.G.; Sedlmaier, S.J.; Wright, J.D.; Cassidy, S.J.; Hara, Y.; Corkett, A.J.; Lancaster, T.; Baker, P.J.; Blundell, S.J. Enhancement of the superconducting transition temperature of FeSe by intercalation of a molecular spacer layer. Nat. Mater. 2013, 12, 15–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Iyyamperumal, E.; Roy, A.; Xue, Y.; Yu, D.; Dai, L. Vertically Aligned BCN Nanotubes as Efficient Metal-Free Electrocatalysts for the Oxygen Reduction Reaction: A Synergetic Effect by Co-Doping with Boron and Nitrogen. Angew. Chem. Int. Ed. 2011, 50, 11756–11760. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.; de Lima, J.; Grandi, T.; Machado, K.; Pizani, P. Structural studies of iron selenides prepared by mechanical alloying. Solid State Commun. 2002, 123, 179–184. [Google Scholar] [CrossRef]
- Frost, R.L.; Xi, Y.; López, A.; Scholz, R.; de Carvalho Lana, C.; e Souza, B.F. Vibrational spectroscopic characterization of the phosphate mineral barbosalite Fe2+Fe3+2(PO4)2(OH)2 – Implications for the molecular structure. J. Mol. Struct. 2013, 1051, 292–298. [Google Scholar] [CrossRef]
- Hibino, T.; Kobayashi, K.; Nagao, M.; Kawasaki, S. High-temperature supercapacitor with a proton-conducting metal pyrophosphate electrolyte. Sci. Rep. 2015, 5, 7903. [Google Scholar] [CrossRef] [PubMed]
- Warren, B.E. X-ray Diffraction; Dover Publications: Mineola, NY, USA, 1969; Volume II, pp. 251–257. [Google Scholar]
- Kobayashi, K.; Nagao, M.; Yamamoto, Y.; Heo, P.; Hibino, T. Rechargeable PEM fuel-cell batteries using porous carbon modified with carbonyl groups as anode materials. J. Electrochem. Soc. 2015, 162, F868–F877. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Q.; Cheng, X.; Li, H. Microwave Synthesis of High Activity FeSe2/C Catalyst toward Oxygen Reduction Reaction. Catalysts 2015, 5, 1079-1091. https://doi.org/10.3390/catal5031079
Zheng Q, Cheng X, Li H. Microwave Synthesis of High Activity FeSe2/C Catalyst toward Oxygen Reduction Reaction. Catalysts. 2015; 5(3):1079-1091. https://doi.org/10.3390/catal5031079
Chicago/Turabian StyleZheng, Qiaoling, Xuan Cheng, and Hengyi Li. 2015. "Microwave Synthesis of High Activity FeSe2/C Catalyst toward Oxygen Reduction Reaction" Catalysts 5, no. 3: 1079-1091. https://doi.org/10.3390/catal5031079





