Polyaniline-Derived Ordered Mesoporous Carbon as an Efficient Electrocatalyst for Oxygen Reduction Reaction
Abstract
:1. Introduction
2. Results and Discussion
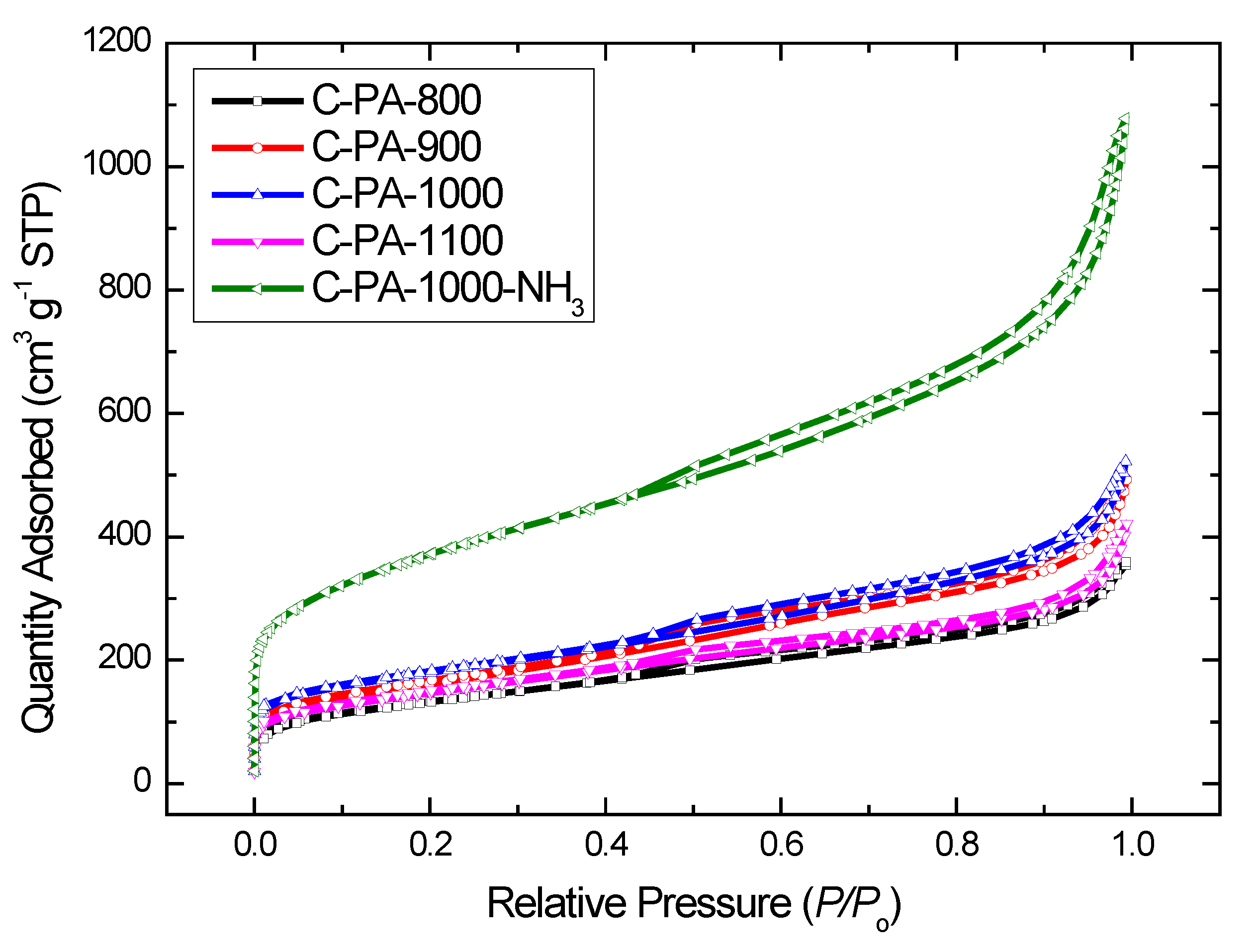
| Samples | ABET/m2·g−1 | AMP/m2·g−1 | DBJH/nm | V/cm3·g−1 |
|---|---|---|---|---|
| C-PA-800 | 470 | 67 | 5.4 | 0.58 |
| C-PA-900 | 569 | 41 | 5.9 | 0.80 |
| C-PA-1000 | 629 | 132 | 6.1 | 0.81 |
| C-PA-1100 | 517 | 61 | 5.9 | 0.67 |
| C-PA-1000-NH3 | 1312 | 229 | 5.9 | 1.73 |
| Samples | C | N | O | N:C |
|---|---|---|---|---|
| C-PA-800 | 86.63 | 5.07 | 5.33 | 0.057 |
| C-PA-900 | 92.03 | 3.13 | 4.40 | 0.034 |
| C-PA-1000 | 93.45 | 2.20 | 3.91 | 0.024 |
| C-PA-1100 | 94.88 | 1.25 | 3.57 | 0.013 |
| C-PA-1000-NH3 | 94.32 | 3.15 | 2.33 | 0.033 |

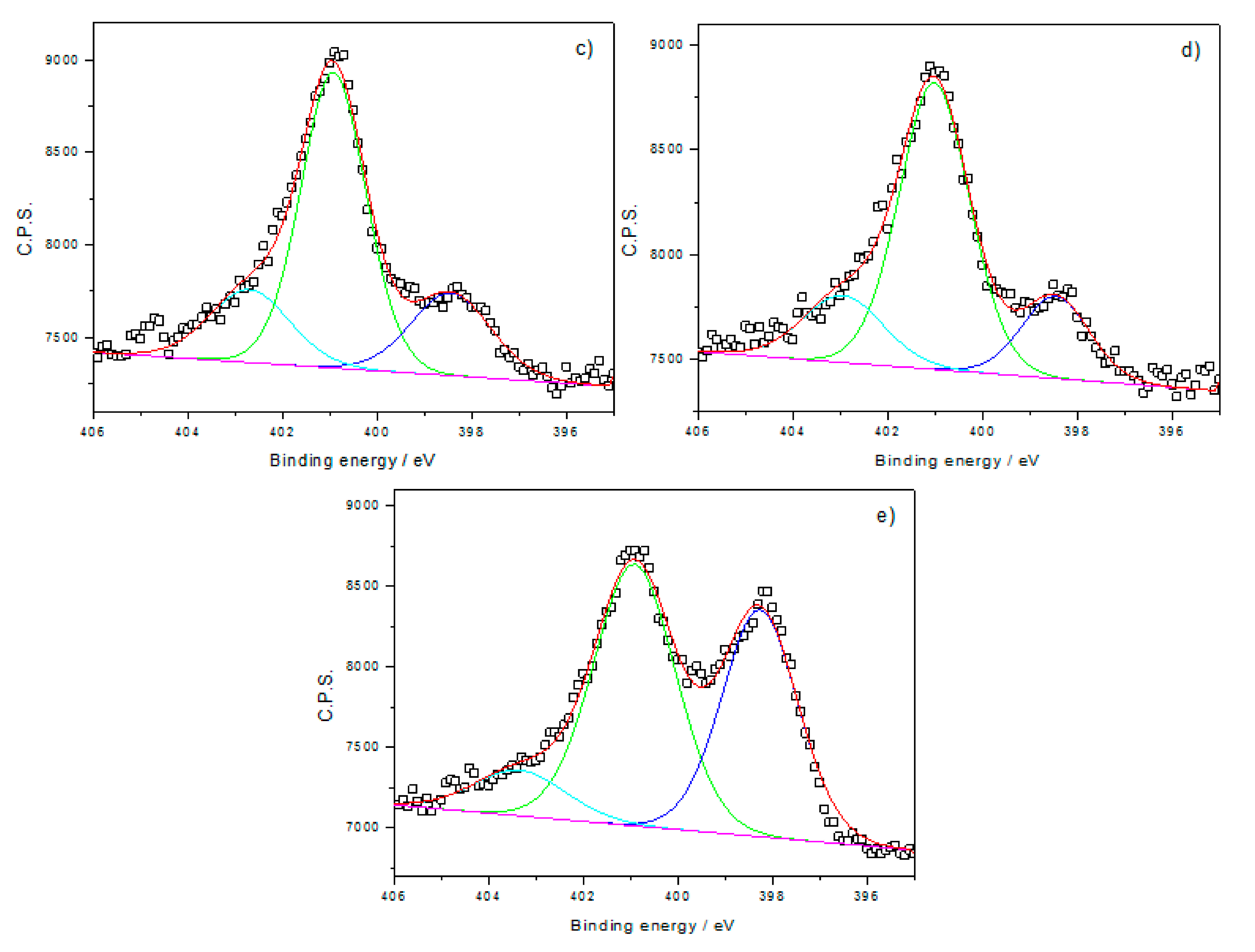
| Samples | Pyridinic-N | Graphitic-N | O-N |
|---|---|---|---|
| C-PA-800 | 31.88 | 59.10 | 9.02 |
| C-PA-900 | 26.42 | 59.55 | 14.04 |
| C-PA-1000 | 20.90 | 60.62 | 18.48 |
| C-PA-1100 | 17.90 | 64.72 | 17.38 |
| C-PA-1000-NH3 | 40.31 | 49.88 | 9.81 |
| Samples | C–C=C | C–N | C–O/C=N | C=O | COOH |
|---|---|---|---|---|---|
| C-PA-800 | 65.58 | 13.19 | 14.39 | 5.52 | 1.33 |
| C-PA-900 | 68.29 | 15.60 | 8.81 | 5.46 | 1.84 |
| C-PA-1000 | 68.84 | 18.51 | 7.53 | 3.85 | 1.28 |
| C-PA-1100 | 73.60 | 15.11 | 6.10 | 3.71 | 1.48 |
| C-PA-1000-NH3 | 67.98 | 20.49 | 7.84 | 1.65 | 2.04 |
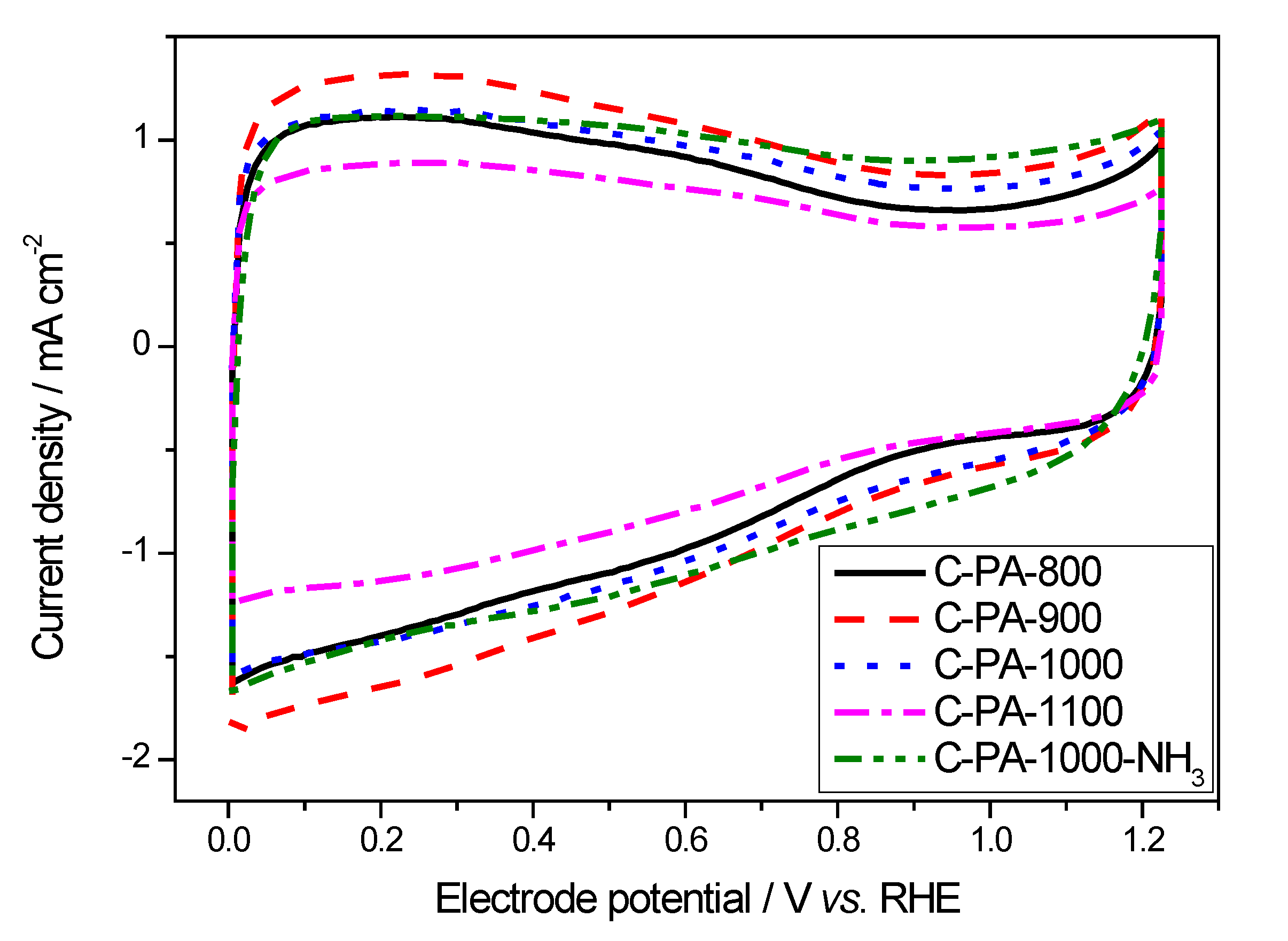
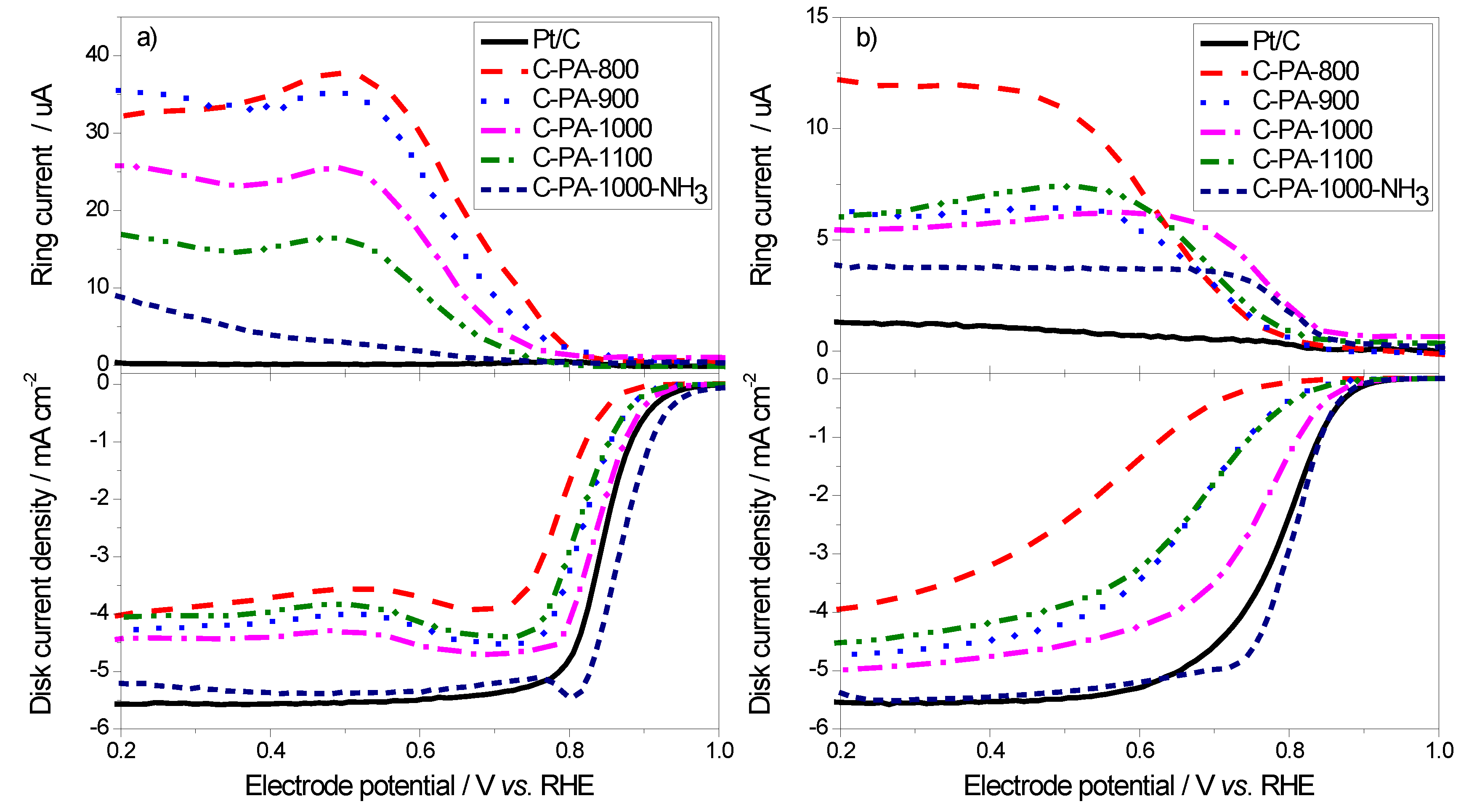
3. Experimental Section
3.1. Materials Preparation
3.2. Physical Characterizations
3.3. Electrochemical Characterization
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Job, N.; Lambert, S.; Zubiaur, A.; Cao, C.; Pirard, J.P. Design of Pt/carbon xerogel catalysts for pem fuel cells. Catalysts 2015, 5, 40–57. [Google Scholar] [CrossRef]
- Jia, Q.; Liang, W.; Bates, M.K.; Mani, P.; Lee, W.; Mukerjee, S. Activity descriptor identification for oxygen reduction on platinum-based bimetallic nanoparticles: In situ observation of the linear composition-strain-activity relationship. ACS Nano 2015, 9, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.N.; Lv, L.B.; Li, X.H.; Xu, M.; Chen, J.S. Strongly veined carbon nanoleaves as a highly efficient metal-free electrocatalyst. Angew. Chem. Int. Ed. 2014, 53, 6905–6909. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Zelenay, P. Nanostructured nonprecious metal catalysts for oxygen reduction reaction. Acc. Chem. Res. 2013, 46, 1878–1889. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhuang, X.D.; Su, Y.Z.; Zhang, F.; Feng, X.L. Polyaniline nanosheet derived B/N co-doped carbon nanosheets as efficient metal-free catalysts for oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 7742–7746. [Google Scholar] [CrossRef]
- Su, D.S.; Perathoner, S.; Centi, G. Nanocarbons for the development of advanced catalysts. Chem. Rev. 2013, 113, 5782–5816. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Dai, H.J. Strongly coupled inorganic-nano-carbon hybrid materials for energy storage. Chem. Soc. Rev. 2013, 42, 3088–3113. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Feng, X.; Wang, X.; Müllen, K. Graphene-based carbon nitride nanosheets as efficient metal-free electrocatalysts for oxygen reduction reactions. Angew. Chem. Int. Ed. 2011, 50, 5339–5343. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Jiang, C.; Wang, J.; Lu, L. High-rate oxygen electroreduction over graphitic-n species exposed on 3D hierarchically porous nitrogen-doped carbons. Angew. Chem. Int. Ed. 2014, 53, 9503–9507. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, B.; Liu, X.; Wang, D.W.; Su, D.S. Unravelling the structure of electrocatalytically active Fe–N complexes in carbon for the oxygen reduction reaction. Angew. Chem. Int. Ed. 2014, 53, 10673–10677. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Song, P.; Zhang, Y.; Liu, C.; Xu, W.; Xing, W. A class of high performance metal-free oxygen reduction electrocatalysts based on cheap carbon blacks. Sci. Rep. 2013, 3, 2505. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Voiry, D.; Chhowalla, M.; Asefa, T. Efficient metal-free electrocatalysts for oxygen reduction: Polyaniline-derived N- and O-doped mesoporous carbons. J. Am. Chem. Soc. 2013, 135, 7823–7826. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, N.; Tylus, U.; Jia, Q.; Mukerjee, S. Activity descriptor identification for oxygen reduction on nonprecious electrocatalysts: Linking surface science to coordination chemistry. J. Am. Chem. Soc. 2013, 135, 15443–15449. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.W.; Wei, W.; Wu, Z.S.; Feng, X.; Mullen, K. Mesoporous metal-nitrogen-doped carbon electrocatalysts for highly efficient oxygen reduction reaction. J. Am. Chem. Soc. 2013, 135, 16002–16005. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, X.G.; Ganesan, P.; Popov, B.N. Development of non-precious metal oxygen-reduction catalysts for pem fuel cells based on N-doped ordered porous carbon. Appl. Catal. B 2009, 93, 156–165. [Google Scholar] [CrossRef]
- Cheon, J.Y.; Kim, T.; Choi, Y.; Jeong, H.Y.; Joo, S.H. Ordered mesoporous porphyrinic carbons with very high electrocatalytic activity for the oxygen reduction reaction. Sci. Rep. 2013, 3, 2715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, H.; Zhang, C.; Liu, F.; Hou, Y. Hybrid of iron nitride and nitrogen-doped graphene aerogel as synergistic catalyst for oxygen reduction reaction. Adv. Funct. Mater. 2014, 24, 2930–2937. [Google Scholar] [CrossRef]
- Xi, P.B.; Liang, Z.X.; Liao, S.J. Stability of hemin/C electrocatalyst for oxygen reduction reaction. Int. J. Hydrogen Energy 2012, 37, 4606–4611. [Google Scholar] [CrossRef]
- Liang, Z.X.; Song, H.Y.; Liao, S.J. Hemin: A highly effective electrocatalyst mediating the oxygen reduction reaction. J. Phys. Chem. C 2011, 115, 2604–2610. [Google Scholar] [CrossRef]
- Fellinger, T.P.; Hasche, F.; Strasser, P.; Antonietti, M. Mesoporous nitrogen-doped carbon for the electrocatalytic synthesis of hydrogen peroxide. J. Am. Chem. Soc. 2012, 134, 4072–4075. [Google Scholar] [CrossRef] [PubMed]
- Sa, Y.J.; Park, C.; Jeong, H.Y.; Park, S.H.; Lee, Z.; Kim, K.T.; Park, G.G.; Joo, S.H. Carbon nanotubes/heteroatom-doped carbon core-sheath nanostructures as highly active, metal-free oxygen reduction electrocatalysts for alkaline fuel cells. Angew. Chem. Int. Ed. 2014, 53, 4102–4106. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; More, K.L.; Johnston, C.M.; Zelenay, P. High-performance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt. Science 2011, 332, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Wan, K.; Long, G.F.; Liu, M.Y.; Du, L.; Liang, Z.X.; Tsiakaras, P. Nitrogen-doped ordered mesoporous carbon: Synthesis and active sites for electrocatalysis of oxygen reduction reaction. Appl. Catal. B 2015, 165, 566–571. [Google Scholar] [CrossRef]
- Long, G.F.; Wan, K.; Liu, M.Y.; Li, X.H.; Liang, Z.X.; Piao, J.H. Effect of pyrolysis conditions on nitrogen-doped ordered mesoporous carbon electrocatalysts. Chin. J. Catal. 2015. [Google Scholar] [CrossRef]
- Wan, K.; Yu, Z.P.; Li, X.H.; Liu, M.Y.; Yang, G.; Piao, J.H.; Liang, Z.X. PH effect on electrochemistry of nitrogen-doped carbon catalyst for oxygen reduction reaction. ACS Catal. 2015, 5, 4325–4332. [Google Scholar] [CrossRef]
- Meng, H.; Larouche, N.; Lefèvre, M.; Jaouen, F.; Stansfield, B.; Dodelet, J.-P. Iron porphyrin-based cathode catalysts for polymer electrolyte membrane fuel cells: Effect of NH3 and Ar mixtures as pyrolysis gases on catalytic activity and stability. Electrochim. Acta 2010, 55, 6450–6461. [Google Scholar] [CrossRef]
- Zhao, Y.; Watanabe, K.; Hashimoto, K. Self-supporting oxygen reduction electrocatalysts made from a nitrogen-rich network polymer. J. Am. Chem. Soc. 2012, 134, 19528–19531. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.W.; Zhuang, X.; Bruller, S.; Feng, X.; Mullen, K. Hierarchically porous carbons with optimized nitrogen doping as highly active electrocatalysts for oxygen reduction. Nat. Commun. 2014, 5, 4973. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Bi, J.Y.; Zhao, Y.; Yang, L.J.; Zhang, C.; Ma, Y.W.; Wu, Q.; Wang, X.Z.; Hu, Z. Nitrogen-doped carbon nanocages as efficient metal-free electrocatalysts for oxygen reduction reaction. Adv. Mater. 2012, 24, 5593–5597. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jensen, J.O.; Zhang, W.; Cleemann, L.N.; Xing, W.; Bjerrum, N.J.; Li, Q. Hollow spheres of iron carbide nanoparticles encased in graphitic layers as oxygen reduction catalysts. Angew. Chem. Int. Ed. 2014, 53, 3675–3679. [Google Scholar] [CrossRef] [PubMed]
- Geng, D.; Chen, Y.; Chen, Y.; Li, Y.; Li, R.; Sun, X.; Ye, S.; Knights, S. High oxygen-reduction activity and durability of nitrogen-doped graphene. Energ. Environ. Sci. 2011, 4, 760–764. [Google Scholar] [CrossRef]
- Favaro, M.; Perini, L.; Agnoli, S.; Durante, C.; Granozzi, G.; Gennaro, A. Electrochemical behavior of n and ar implanted highly oriented pyrolytic graphite substrates and activity toward oxygen reduction reaction. Electrochim. Acta 2013, 88, 477–487. [Google Scholar] [CrossRef]
- Zhao, D.Y.; Feng, J.L.; Huo, Q.S.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, K.; Yu, Z.-P.; Liang, Z.-X. Polyaniline-Derived Ordered Mesoporous Carbon as an Efficient Electrocatalyst for Oxygen Reduction Reaction. Catalysts 2015, 5, 1034-1045. https://doi.org/10.3390/catal5031034
Wan K, Yu Z-P, Liang Z-X. Polyaniline-Derived Ordered Mesoporous Carbon as an Efficient Electrocatalyst for Oxygen Reduction Reaction. Catalysts. 2015; 5(3):1034-1045. https://doi.org/10.3390/catal5031034
Chicago/Turabian StyleWan, Kai, Zhi-Peng Yu, and Zhen-Xing Liang. 2015. "Polyaniline-Derived Ordered Mesoporous Carbon as an Efficient Electrocatalyst for Oxygen Reduction Reaction" Catalysts 5, no. 3: 1034-1045. https://doi.org/10.3390/catal5031034
APA StyleWan, K., Yu, Z.-P., & Liang, Z.-X. (2015). Polyaniline-Derived Ordered Mesoporous Carbon as an Efficient Electrocatalyst for Oxygen Reduction Reaction. Catalysts, 5(3), 1034-1045. https://doi.org/10.3390/catal5031034





