Downhole Upgrading of Orinoco Basin Extra-Heavy Crude Oil Using Hydrogen Donors under Steam Injection Conditions. Effect of the Presence of Iron Nanocatalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Upgrading Reaction
| Run | Solid (Reaction time) | °API b (±0.5°) | %HDS c (±1%) | % Asphaltenes Reduction d |
|---|---|---|---|---|
| - | Original Hamaca C. e | 9.1 | - | - |
| 1 | Control Exp.(0 h) f | 10.2 | 5% | 9% |
| 2 | Mineral Formation g | 14.7 | 12% | 13% |
| 3 | Fe-20/SiO2 h | 17.5 | 28% | 31% |
| 4 | Fe-60/SiO2 h | 14.5 | 22% | 19% |
| 5 | Fe-90/SiO2 h | 13.2 | 8.5% | 17.9% |
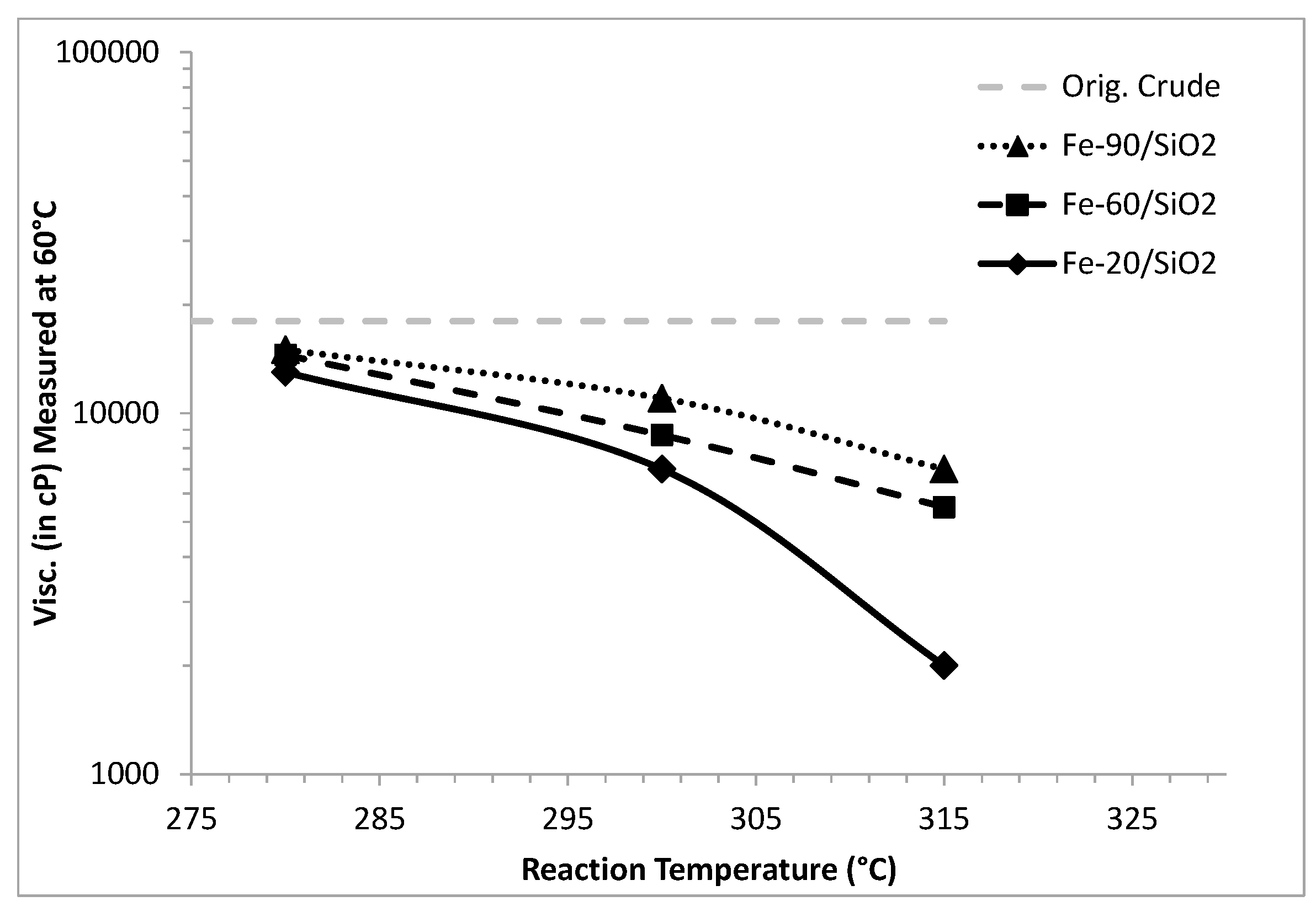
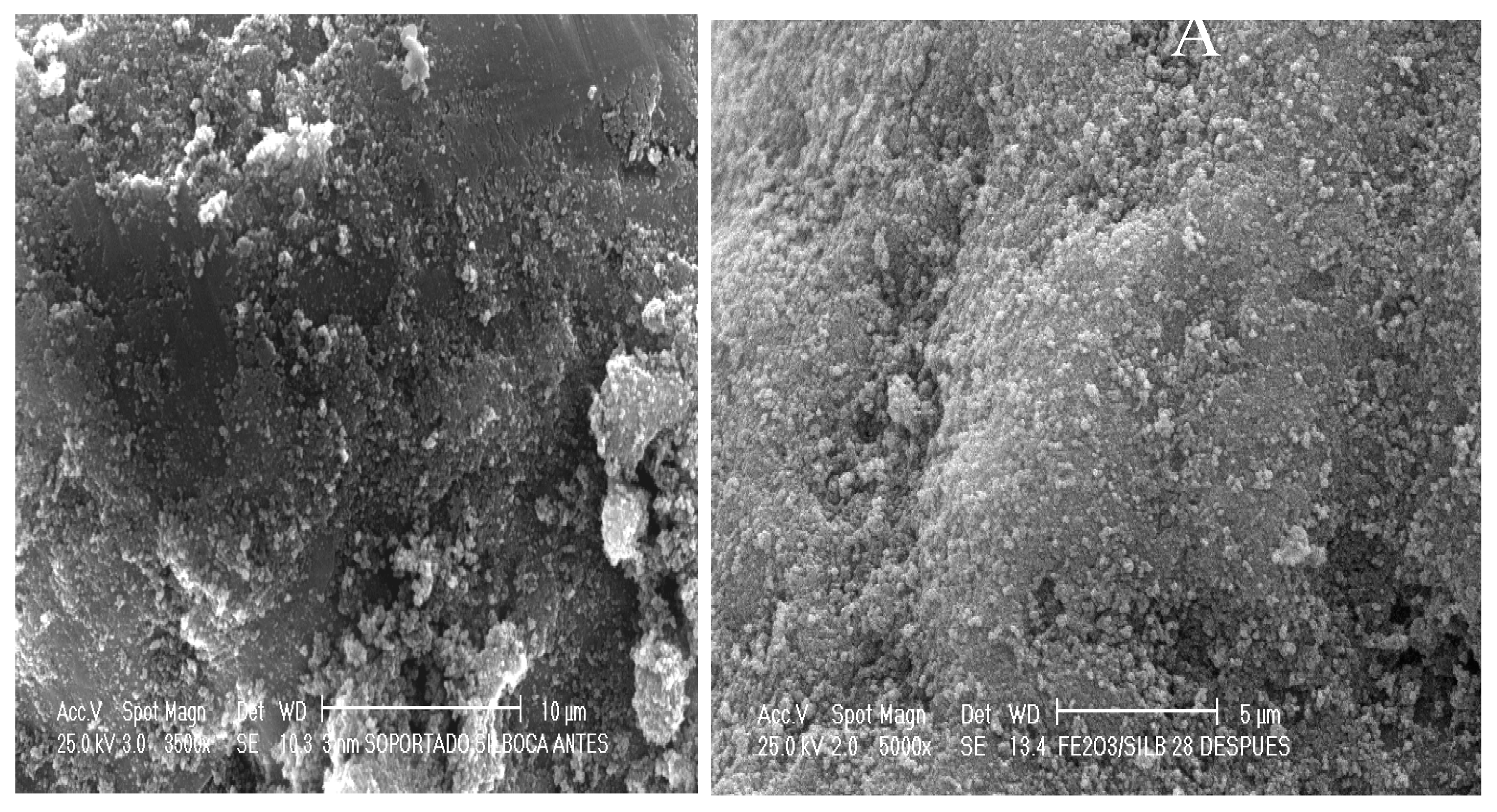
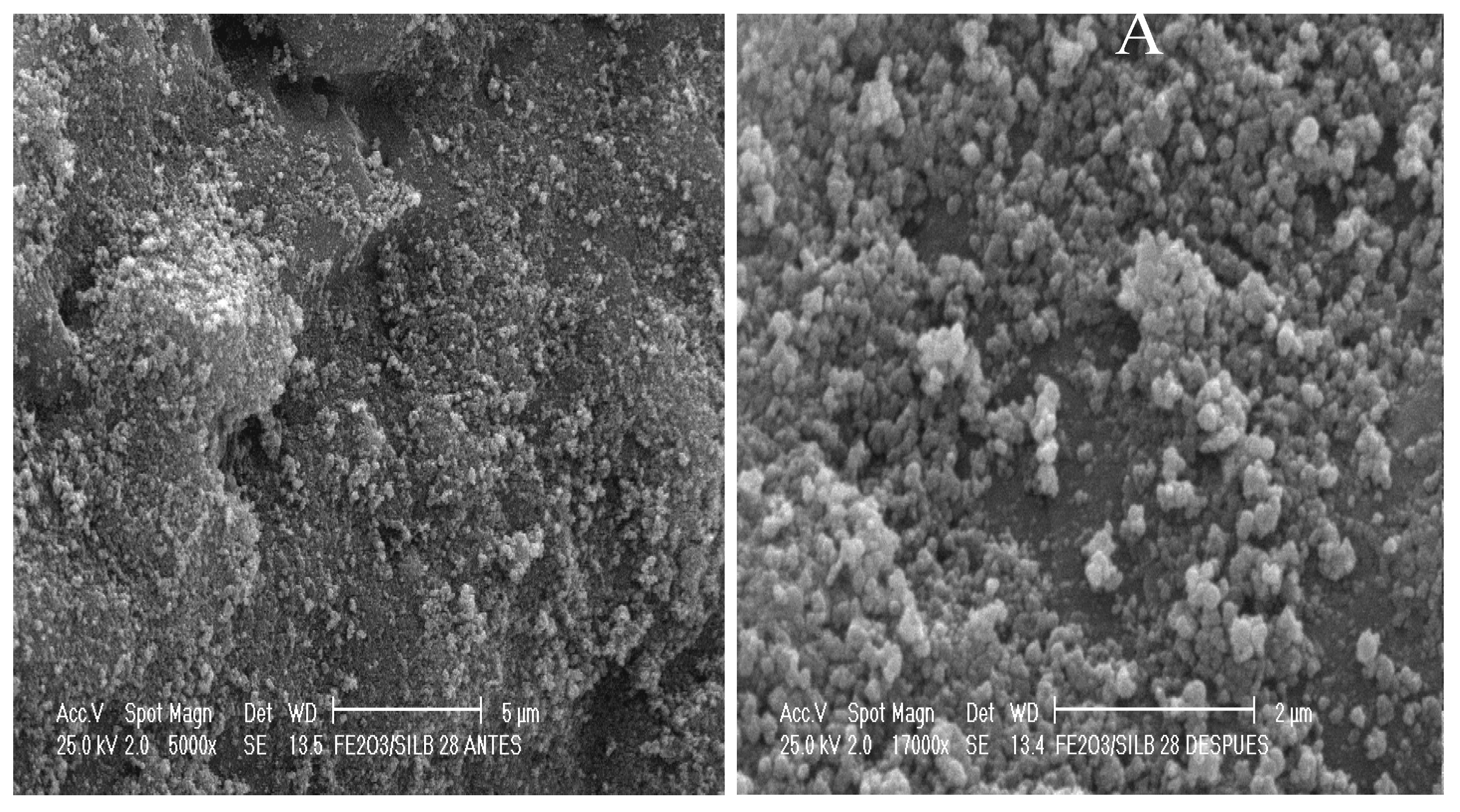
2.2. Catalyst Characterization
| Experiment a | Element Band b | Binding Energy (eV) c | % Atomic d | Fe-Dispersion e |
|---|---|---|---|---|
| Before Upgrading Reaction | Fe 2p2/3 | 710.6 | 16.27 | 4.54 |
| O 1s | 530 | 25.07 | ||
| C 1s | 284.6 | 55.09 | ||
| Si 2p | 103.1 | 3.58 | ||
| After Upgrading Reaction | Fe 2p2/3 | 711.3 | 6.86 | 1.75 |
| O 1s | 530.5 | 18.27 | ||
| C 1s | 284.6 | 69.18 | ||
| Si 2p | 103 | 5.68 |
2.3. Mechanistic Considerations


| Run | Solid (Reaction time) | Harom b | Haliph c | Hα d | Hβ e | Hγ f |
|---|---|---|---|---|---|---|
| - | Original Hamaca C. e | 5.1 | 94.1 | 14.7 | 56.2 | 24.0 |
| 1 | Control Exp.(0 h) f | 5.6 | 94.4 | 14.8 | 55.2 | 24.4 |
| 2 | Mineral Formation g | 6.9 | 93.1 | 14.9 | 53.8 | 24.4 |
| 3 | Fe-20/SiO2 h | 7.6 | 92.4 | 15.4 | 52.1 | 24.9 |
| 4 | Fe-60/SiO2 h | 7.4 | 92.6 | 15.1 | 52.9 | 24.6 |
| 5 | Fe-90/SiO2 h | 6.8 | 93.2 | 14.9 | 54.0 | 24.3 |

3. Experimental Section
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Weisman, J.G.; Kessler, R.V. Downhole heavy crude oil hydroprocessing. Appl. Catal. 1996, 140, 1–16, and references therein. [Google Scholar] [CrossRef]
- Weissman, J.G.; Kessler, R.V.; Sawicki, R.A.; Belgrave, J.D.M.; Laureshen, C.J.; Metha, S.A.; Moore, R.G.; Ursenbach, M.G. Down-Hole Catalytic Upgrading of Heavy Crude Oil. Energy Fuels 1996, 10, 883–889, and references therein. [Google Scholar] [CrossRef]
- Vallejos, C.; Vasquez, T.; Ovalles, C. Process for the downhole upgrading of extra heavy crude oil. U.S. Patent 5,891,829, April 1999. [Google Scholar]
- Ovalles, C.; Vallejos, C.; Vásquez, T.; Martinis, J.; Peréz-Peréz, A.; Cotte, E.; Castellanos, L.; Rodríguez, H. Extra-Heavy Crude Oil Downhole Upgrading Process using Hydrogen Donors under Steam Injection Conditions. In Proceedings of SPE International Thermal Operations and Heavy Oil Symposium, Margarita, Venezuela, 12–14 March 2001.
- Ovalles, C.; Vallejos, C.; Vasquez, T.; Rojas, I.; Ehrman, U.; Benitez, J.L.; Martinez, R. Downhole Upgrading of Extra-Heavy Crude Oil Using Hydrogen Donor and Methane under Steam Injection Conditions. J. Pet. Sci. Tech. 2003, 21, 255–274. [Google Scholar] [CrossRef]
- Ovalles, C.; Rengel-Unda, P.; Bruzual, J.; Salazar, A. Upgrading of Extra-Heavy Crude Using Hydrogen Donor under Steam Injection Conditions. Characterization by Pyrolysis GC-MS of the Asphaltenes and Effects of a Radical Initiator. Am. Chem. Soc. Div. Fuel. Chem. 2003, 48, 59–60. [Google Scholar]
- Hyne, J.B.; Clark, P.D.; Clarke, R.A.; Koo, J.; Greidanus, J.W.; Tyrer, J.D.; Verona, D. Aquathermolysis of Heavy Oils. Rev. Tech. INTEVEP 1982, 2, 87–94. [Google Scholar]
- Viloria, A.; Parisi, S.; Martinez, E.; Gineika, A.; Sanchez, V. Efectos de la inyección de vapor sobre la calidad de un crudo extrapesado de la F.P.O. Rev. Tech. INTEVEP 1985, 5, 69–72. [Google Scholar]
- Siskin, M.; Brons, G.; Katritzky, A.R.; Balasubramanian, M. Aqueous Organic Chemistry. 1. Aquathermolysis: Comparison with Thermolysis in the Reactivity of Aliphatic Compounds. Energy Fuels 1990, 4, 475–482, and references therein. [Google Scholar] [CrossRef]
- Rivas, O.R.; Campos, R.E.; Borges, L.G. Experimental Evaluation of Transition Metal Solutions as Additives in Steam Recovery Processes. In Presented at 63th Annual Symposium of SPE, Houston, TX, USA, 2–5 October 1988.
- Reynolds, J.G.; Thorsness, C.B. Mild Upgrading of Midway Sunset Crude Oil from The San Joaquin Valley of California by Aqueous Pyrolysis-Catalysis and Modeling. In Presented at 213th ACS National Meeting, San Francisco, CA, USA, 13–17 April 1997.
- Nares, H.R.; Schacht-Hernandez, P.; Ramirez-Garnica, M.A.; Cabrera-Reyes, M.C.; Noe-Valencia, L. Heavy-Crude-Oil Upgrading with transition Metals. In Presented at the SPE Latin America and Caribbean Petroleum Engineering Conference, Buenos Aires, Argentina, 15–18 April 2007.
- Maity, S.K.; Ancheyta, J.; Marroquin, G. Catalytic Aquathermolysis Used for Viscosity Reduction of Heavy Oils: A Review. Energy Fuels 2010, 24, 2809. [Google Scholar] [CrossRef]
- Fumoto, E.; Sato, S.; Takanohashi, T. Oxidative Cracking of Oil Sand Bitumen with Iron Oxide Catalyst in a Steam Atmosphere. In Proceedings of 11th International Conference of Petroleum Phase behavior and Fouling, Petrophase, Jersey, NJ, USA, 13–17 June 2010.
- Wang, Y.; Chen, Y.; He, J.; Li, P.; Yang, C. Viscosity Reduction of Heavy Oil through Catalytic Aquathermolysis at relatively Low Temperature. In Presented at 11th International Conference of Petroleum Phase behavior and Fouling, Petrophase, Jersey, NJ, USA, 13–17 June 2010.
- Wen, S.; Zhao, Y.; Liu, Y. A Study on Catalytic Aquathermolysis of Heavfy Crude Oil during steam Stimulation. In Presented at 2007 SPE International Symposium Oilfield Chemistry, Houston, TX, USA, 28 February–2 March 2007.
- Chen, Y.; Wang, Y.; Wu, C.; Xia, F. Laboratory Experiments and Field Tests of an Amphiphilic Metallic Chelate for Catalytic Aquathermolysis of Heavy Oil. Energy Fuels 2008, 22, 1502–1508. [Google Scholar] [CrossRef]
- Chao, K.; Chen, Y.; Liu, H.; Zhang, X.; Li, J. Laboratory Experiments and Field Test of a Difunctional Catalyst for Catalytic Aquathermolysis of Heavy Oil. Energy Fuels 2012, 26, 1152–1159. [Google Scholar] [CrossRef]
- Chao, K.; Chen, Y.; Liu, H.; Zhang, X.; Li, J. Influences on the Aquathermolysis of Heavy Oil Catalyzed by Two Different Catalytic Ions: Cu2+ and Fe3+. Energy Fuels 2013, 27, 2555–2562. [Google Scholar] [CrossRef]
- Sakanishi, K.; Taniguchi, H.; Hasuo, H.-u.; Mochida, I. Iron-Based Catalysts Supported on Carbon Nanoparticles of Hollow Structure for Coal Liquefaction. Eng. Chem. Res. 1997, 36, 306–309, and references therein. [Google Scholar] [CrossRef]
- Wagner, C.D.; Riggs, W.M.; Davis, L.E.; Moulder, J.F.; Muilenberg, G.E. Handbook of X-Ray Photoelectron Spectroscopy; Perkin-Elmer Corporation Physical Electronics Division: Eden Prairie, MN, USA, 1979. [Google Scholar]
- Ovalles, C.; Filgueiras, E.; Morales, A.; Rojas, I.; de Jesus, J.C.; Berrios, I. Use of Dispersed Molybdenum Catalyst and Mechanistic Studies for Upgrading Extra-Heavy Crude Oil Using Methane as Source of Hydrogen. Energy Fuels 1998, 12, 379–385. [Google Scholar] [CrossRef]
- Egiebor, N.O.; Gray, M.R. Evidence for methane activation during coal pyrolysis and liquefaction. Fuel 1990, 69, 1276–1282. [Google Scholar] [CrossRef]
- Ovalles, C.; Hamana, A.; Rojas, I.; Bolivar, R. Upgrading of Extra-Heavy Crude Oil by Direct Use of Methane in the Presence of Water. Deuterium Labeled Experiments and Mechanistic Consideration. Fuel 1995, 74, 1162–1168. [Google Scholar] [CrossRef]
- Leon, V.; Carrazza, J. X- ray photoelectron spectroscopy (XPS) sensitivity factors. A general and simple approach. Rev. Tech. INTEVEP 1989, 9, 81–88. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ovalles, C.; Rivero, V.; Salazar, A. Downhole Upgrading of Orinoco Basin Extra-Heavy Crude Oil Using Hydrogen Donors under Steam Injection Conditions. Effect of the Presence of Iron Nanocatalysts. Catalysts 2015, 5, 286-297. https://doi.org/10.3390/catal5010286
Ovalles C, Rivero V, Salazar A. Downhole Upgrading of Orinoco Basin Extra-Heavy Crude Oil Using Hydrogen Donors under Steam Injection Conditions. Effect of the Presence of Iron Nanocatalysts. Catalysts. 2015; 5(1):286-297. https://doi.org/10.3390/catal5010286
Chicago/Turabian StyleOvalles, Cesar, Victor Rivero, and Arelys Salazar. 2015. "Downhole Upgrading of Orinoco Basin Extra-Heavy Crude Oil Using Hydrogen Donors under Steam Injection Conditions. Effect of the Presence of Iron Nanocatalysts" Catalysts 5, no. 1: 286-297. https://doi.org/10.3390/catal5010286





