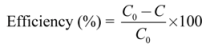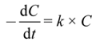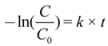2.1. Performing Microvolume-TOC Analysis in Lab Scale Photocatalytic Applications
In
Figure 1, the scheme of a standard TOC is plotted. A detailed picture of the gas injection kit is shown in
Figure 2. This new injection technique allows injecting directly on the combustion oven. In this way, large death volumes and a large amount of tubing, that needs to be cleaned with sample solution before injection, is avoided. This leads to a significant reduction of the volume needed for analysis. Applying a dilution procedure, in order to increase the sampling volume and to use the standard tubing, is not always possible. Certainly in case of degradation processes due to the often low concentrations of the organic pollutant and degradation products. Furthermore, because tubing cleaning before injection can be bypassed by direct injection on the combustion tube, the analysis time and sampling frequency can be increased. Moreover, it seriously reduces the amount of Milli-Q water needed and the possibility for errors due to dilution.
The benefit of performing microvolume TOC on small volume liquids in lab scale applications is exemplified by studying the photocatalytic degradation processes of 25 mL 4 × 10
−5 M R6G by 8 mg of the P25 commercial photocatalyst (Evonik
®) as photocatalyst. By applying multiple small volume TOC analyses at short intervals, details on the photomineralization processes, next to the information on photobleaching (UV-Vis), can be obtained to assure complete photomineralization in time. This leads to a more thorough evaluation of the photocatalyst in a very fast way. After adsorption (40 min equilibration time, not shown in the
Figure 3), the R6G solution was illuminated with UV-light (365 nm) for 120 min during which UV-Vis spectroscopy and μV-TOC-analysis were performed every 20 min. Results of both measurements are plotted in
Figure 3.
Figure 1.
Technical scheme of standard TOC equipment (black square). The part of the TOC equipment applied in μV-TOC analysis is marked in grey. The high precision syringe with Chaney Adapter is marked in red: (1) TC injection and (2) IC injection. The part of the standard TOC equipment that is bypassed in μV-TOC-analysis is marked in white. The high precision syringe (Hamilton) equipped with a Chaney Adapter allows for very precise and reproducible injected volumes for each injection, which is indispensable for accurate measurements. The gas injection kit was developed by Shimadzu and applied for the first time for injection of liquids.
Figure 1.
Technical scheme of standard TOC equipment (black square). The part of the TOC equipment applied in μV-TOC analysis is marked in grey. The high precision syringe with Chaney Adapter is marked in red: (1) TC injection and (2) IC injection. The part of the standard TOC equipment that is bypassed in μV-TOC-analysis is marked in white. The high precision syringe (Hamilton) equipped with a Chaney Adapter allows for very precise and reproducible injected volumes for each injection, which is indispensable for accurate measurements. The gas injection kit was developed by Shimadzu and applied for the first time for injection of liquids.
Figure 2.
Close up picture on top of the TOC equipment showing the microvolume injection setup: (1) Shimadzu gas injection kit; (2) Chaney Adapter; (3) Hamilton high precision syringe equipped and (4) cooling fan.
Figure 2.
Close up picture on top of the TOC equipment showing the microvolume injection setup: (1) Shimadzu gas injection kit; (2) Chaney Adapter; (3) Hamilton high precision syringe equipped and (4) cooling fan.
Figure 3.
Results of the photocatalytic test with 8 mg of P25 in 25 mL 4 × 10−5 M R6G at room temperature and after 40 min of adsorption-desorption equilibrium in dark:
Figure 3.
Results of the photocatalytic test with 8 mg of P25 in 25 mL 4 × 10−5 M R6G at room temperature and after 40 min of adsorption-desorption equilibrium in dark:
By using the high precision syringe, the standard deviation of each measuring point (TOC) is determined to be maximum 2% (see
Table 1). By using the developed micro injection technique, small volumes can be analyzed and many samplings can be performed with only minor influence on the catalyst/dye ratio. This implies that although TOC analysis is a destructive analysis method, it remains applicable in the evaluation of a photocatalyst as sampling volumes are only a few microliters in contrast to 5 mL samples required in regular TOC method. The dye concentration, measured with UV-Vis analysis, is recalculated to the corresponding amount of carbon (ppm) in order to be able to make a good comparison between both analysis methods.
Table 1.
Results of automatic and manual injection.
Table 1.
Results of automatic and manual injection.
| Automatic Injection/ppm TC | Manual Injection/ppm TC |
|---|
| 2 ppm TC | 5 ppm TC | 10 ppm TC | 2 ppm TC | 5 ppm TC | 10 ppm TC |
|---|
| 2.22 | 5.08 | 9.98 | 2.39 | 5.53 | 10.84 |
| 2.30 | 5.22 | 10.00 | 2.62 | 5.18 | 10.68 |
| 2.15 | 5.18 | 9.95 | 2.74 | 5.75 | 10.64 |
| 2.16 | 5.12 | 9.94 | 2.42 | 5.75 | 10.87 |
| 2.55 | 5.04 | 9.92 | 2.51 | 5.61 | 10.66 |
| 2.23 | 5.20 | 10.21 | 2.58 | 5.85 | 10.23 |
| 2.17 | 5.06 | 10.06 | 2.49 | 5.55 | 10.58 |
| 2.16 | 4.70 | 10.08 | 2.49 | 5.53 | 10.87 |
| 2.16 | 5.14 | 9.90 | 2.55 | 5.68 | 10.82 |
| 2.13 | 5.13 | 9.92 | 2.82 | 5.43 | 10.16 |
| 2.22 ± 0.12 | 5.09 ± 0.14 | 9.99 ± 0.09 | 2.56 ± 0.13 | 5.59 ± 0.19 | 10.63 ± 0.25 |
Results of UV-Vis analysis (
Figure 3) show an immediate rapid decrease in absorbance, meaning that the degradation of the dye (photobleaching so breaking of the conjugated system) starts immediately after the UV-light has been switched on. After 60 min, only a small amount of the initial dye molecule (16%) is still present in solution and after 120 min, the photobleaching process is almost complete. The obtained TOC profile is however completely different. It can be seen that the total amount of organic carbon in the solution is almost constant during the first 20 min. Longer irradiation times (>20 min), oxidation to CO
2 starts to take place but at a much lower rate. This means that at the starting point of the CO
2 removal, the concentration of the initial dye has already reached very low values. After 60 min of UV irradiation, 36% of the initial amount of carbon has been oxidized to CO
2 (μV-TOC analysis, photomineralization) whereas 91% of the initial dye molecule has been photodegraded into smaller compounds (UV-Vis analysis, photobleaching), indicating a long lifetime of intermediates.
More details on the exact degradation pathway, which is out of the scope of this article, can be found in literature [
14]. The combination of both UV-Vis analysis and μV-TOC analysis indicate that the complete photodegradation of R6G into CO
2 is a stepwise process. Indeed, CO
2 formation occurs only after sufficient formation of certain intermediate products and sufficiently low concentration for R6G. Moreover, the difference in kinetics is clearly visible when comparing the curve of photobleaching (UV-Vis) with photomineralization (μV-TOC). This information would not be obtained with a single point TOC measurement at the end of the experiment as often applied.
Table 2 shows the underlying μV-TOC-analysis data and the standard deviations on the measurement.
Table 2.
Calculated TOC-values, average TOC-value and standard deviation.
Table 2.
Calculated TOC-values, average TOC-value and standard deviation.
| Time (min) | TOC (ppm) | Average TOC (ppm) | Standard deviation |
|---|
| 0 | 11.81 | 11.71 | 11.67 | 11.72 | 0.07 |
| 20 | 11.31 | 11.36 | 11.16 | 11.28 | 0.10 |
| 40 | 9.77 | 9.69 | 9.44 | 9.63 | 0.17 |
| 60 | 7.53 | 7.33 | 7.56 | 7.48 | 0.12 |
| 80 | 6.45 | 6.36 | 6.47 | 6.43 | 0.06 |
| 100 | 4.96 | 4.79 | 4.65 | 4.80 | 0.15 |
| 120 | 2.69 | 2.86 | 2.86 | 2.80 | 0.05 |
The above described example shows the advantages of μV-TOC -analysis compared to standard TOC measurements. It can be clearly seen that UV-Vis spectroscopy and the detailed μV-TOC analysis reveal different, though complementary information concerning the photobleaching and mineralization process. Therefore, μV-TOC analysis can become an important analytical technique to obtain detailed information on the photomineralization profile, e.g., the required illumination time after which CO2 will start being formed, the lifetime of intermediates, possible occurring deactivation of the catalyst, rate limiting steps, differences in kinetics etc. It becomes clear that misinterpretations and severe errors can be made by only relying on UV-Vis analysis and/or a few sample points (1 or 2) using a standard TOC.
In order to compare the automatic and manual μV-injection further and to determine the accuracy of the μV-injections as compared to general TOC measurements at three different concentrations (2, 5 and 10 ppm) were performed. The results of the measurements are presented in
Table 1. Moreover, in order to compare the benefit of μV-TOC analysis with the option of diluting μV samples to obtain the required 5 mL sample amount for general TOC, the results of automatic injection of diluted solutions are compared to original concentrations in the μV sample (
Table 3).
As can be seen from
Table 1 the standard deviations of the manual and automatic injection method are quite similar. Although the accuracy of the automated injection is better, the normal injection still shows good values that seem to have a repeated overestimation of 0.5 ppm. This could be due to the opening of the gas portal causing the systematic error on all the results for manual injection method. This can be possibly attributed to a small amount of air that is measured while the portal is open. The
Table 3 shows the results of automatic injection of diluted solutions. As can be seen from the recalculated results there is a large variation in the data. Comparing these results with those of μV manual injection clearly shows the much higher accuracy compared to automatic injection of diluted solution.
Table 3.
Results of automatic injection of diluted solutions (in brackets results recalculated to the μV amount before fifty times dilution).
Table 3.
Results of automatic injection of diluted solutions (in brackets results recalculated to the μV amount before fifty times dilution).
| Automatic injection |
|---|
| 2 ppm TC | 5 ppm TC | 10 ppm TC |
|---|
| 0.21 (10.8) | 0.28 (14.3) | 0.30 (15.0) |
| 0.17 (8.8) | 0.25 (12.9) | 0.31 (16.0) |
| 0.17 (8.7) | 0.22 (11.9) | 0.30 (15.5) |
| 0.18 (9.0) | 0.24 (12.2) | 0.30 (15.5) |
| 0.17 (8.5) | 0.23 (11.8) | 0.30 (15.4) |
| 0.18 (9.2 ± 0.9) | 0.24 (12.6 ± 1.1) | 0.30 (15.5 ± 0.4) |
2.2. Benefits of Microvolume-TOC Analysis in Screening of Different Photocatalysts
Due to the advantages of μV-TOC analysis, the photocatalytic activity/efficiency of different materials can be compared. The benefit of combining UV-Vis for photobleaching with μV-TOCanalysis for photomineralization, is further shown in the following experiment in which μV-TOC and UV-Vis analysis will be combined to evaluate the photocatalytic efficiency of two synthesized photocatalysts (non-calcined hydrogen trititanate nanotubes (H-TNT nc) [
15] and mesoporous titanium dioxide (EISA NH
4OH C450) [
16] towards the degradation of R6G. Both catalysts have strongly divergent characteristics (specific surface area, crystal phase and pore diameter) as can be seen in
Table 4.
Table 4.
Structural characteristics and data deduced from photocatalytic degradation reactions.
Table 4.
Structural characteristics and data deduced from photocatalytic degradation reactions.
| | S.A. a (m²/g) | Pore b Diam. (nm) | Ads c (%) | UV d (removal %) | k-value e | μV-TOC f (removal%) | Crystal g Phase |
|---|
| EISA C450 NH4OH | 247 | 3.6 | 17 | 98 | 0.0073 | 39 | Anatase (TiO2) |
| H-TNT nc | 320 | 4.0 | 22 | 88 | 0.0025 | 74 | Trititanate H2Ti3O7 |
In the photocatalytic tests 16 mg of catalyst is added to 50 mL 4 × 10
−5 M R6G and later illuminated with UV light. The detailed description of the photocatalytic tests can be found in the
Experimental Section. Results of the photocatalytic experiment as obtained by UV-Vis spectroscopy and after establishing the adsorption-desorption equilibrium are plotted in
Figure 4A. The concentration of the initial dye molecule (R6G) is plotted as function of time.
Figure 4B depicts the results of the μV-TOC measurements. UV-Vis-analysis shows that there is an immediate decrease in dye concentration using both catalysts, as soon as the UV-light has been switched on. However, there is a significant difference in the photodegradation rate (
Table 4 and
Figure 4A). Considering only the results of UV-Vis analysis, this could allow to conclude that both catalysts are very active, but that EISA NH
4OH C450 has faster kinetics. Yet, when looking at the photomineralization (
Figure 4B, μV-TOC) neither the TOC profile of EISA NH
4OH C450 nor H-TNT nc show an immediate decrease. Even more, the TOC amount is constant during the first 60 min of UV irradiation indicating that only intermediate products are formed and no complete mineralization to CO
2 has taken place. If the irradiation time is long enough (>60 min), the intermediate degradation products can be photooxidized further to CO
2 as observed by decreasing TOC concentrations.
Figure 4.
Photodegradation of 50 mL 4 × 10−5 M R6G by 16 mg of catalyst at room temperature and after 40 min of adsorption-desorption equilibrium in dark: (A) UV-Vis-analysis and (B) μV-TOC-analysis.
Figure 4.
Photodegradation of 50 mL 4 × 10−5 M R6G by 16 mg of catalyst at room temperature and after 40 min of adsorption-desorption equilibrium in dark: (A) UV-Vis-analysis and (B) μV-TOC-analysis.
According to UV-Vis analysis (
Figure 4A and
Table 5), H-TNT nc needs a longer irradiation time to have the same percentage of degradation of R6G, as compared to EISA NH
4OH C450 and would therefore be seen as the least interesting catalyst. However, the conversion to CO
2 after 360 min is four times higher in case of H-TNT nc indicating that it is much more efficient in photomineralization (
Figure 4B). After 360 min, 74% of the TOC is removed by H-TNT nc, whereas EISA NH
4OH C450 eliminates only 40% of TOC out of the water. It is now clear, that H-TNT nc would be more interesting for applications as the lifetime of intermediate degradation products is much shorter. One can understand that in the water treatment, it is more important to convert the organic pollutants into CO
2 in a fast and efficient way instead of degrading molecules in only smaller intermediate degradation products that can be as harmful (or worse) than the original pollutant. The stepwise decrease of TOC (
Figure 4B) is assumed to be due to competitive adsorption with other intermediate reactions that go not towards direct photomineralization. However, further research and explanations are ongoing.
Table 5.
Degradation percentage of R6G by H-TNT nc and EISA NH4OH C450 using UV-Vis analysis and μV-TOC analysis.
Table 5.
Degradation percentage of R6G by H-TNT nc and EISA NH4OH C450 using UV-Vis analysis and μV-TOC analysis.
| | H-TNT nc | EISA C450 NH4OH |
|---|
| UV analysis (removal %) | 60 | 60 |
| μV-TOC analysis (removal %) | 47 | 12 |
| Time (min) * | 200 | 140 |
The benefit of combining both techniques (UV-Vis analysis and μV-TOC analysis) is clearly illustrated by this large difference in the degradation mechanism for both catalysts. Moreover, for both catalysts, stepwise TOC removal is observed, similar as observed in case of P25 as catalyst. This type of information could not have been derived based on only UV-Vis analysis and/or a few TOC measuring points as classically applied. Therefore, μV-TOC analysis can be applied as quick screening method unraveling important differences between catalysts. The strongly divergent degradation mechanism can be confirmed by UV-Vis scans (
Figure 5A and
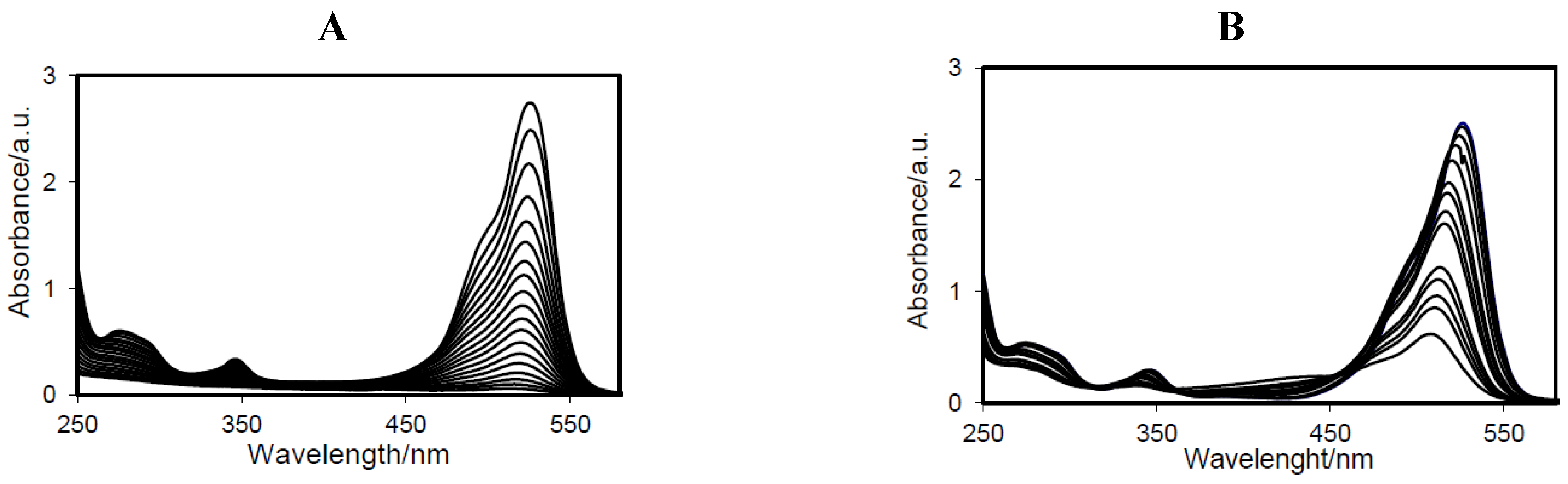
Figure 5B) of the degraded R6G solution by both catalysts in function of time. In
Figure 5B, it can be seen that H-TNT leads to a decrease and hypsochromic shift in absorption maximum whereas for EISA NH
4OH C450 only a decrease in absorption maximum can be observed without a shift of the maximum (
Figure 5A). It has to be noticed that these scans only apply to the photobleaching process.
Figure 5.
Photodegradation of 50 mL 4 × 10−5 M R6G by 16 mg of catalyst at room temperature and after 40 min of adsorption-desorption equilibrium in dark analyzed by means of UV-Vis scan: 16 mg of catalyst in 50 mL 4 × 10−5 M R6G: (A) EISA NH4OH C450 and (B) H-TNT nc.
Figure 5.
Photodegradation of 50 mL 4 × 10−5 M R6G by 16 mg of catalyst at room temperature and after 40 min of adsorption-desorption equilibrium in dark analyzed by means of UV-Vis scan: 16 mg of catalyst in 50 mL 4 × 10−5 M R6G: (A) EISA NH4OH C450 and (B) H-TNT nc.
For a more thorough study of the formed intermediate reaction products, other techniques such as LC-MS are necessary. The reason for the divergent degradation mechanism can be appointed to the structural and/or physicochemical differences, e.g., the crystal phase. However, this does not fall within the scope of this paper though it is part of ongoing research.
In this example, the detailed μV-TOC plots, obtained by the developed μV-TOC analysis method give very valuable synergic information when combined with UV-Vis analysis. The various benefits can be summarized as follows:
(1) Multiple injections in short time intervals using small microliter volumes became possible, revealing important difference in kinetics for photobleaching and photomineralization. Performing only UV-Vis analysis could lead to serious misinterpretations, as has been shown above, since photocatalysts can only be regarded as efficient if both reaction rates (photobleaching and photomineralization) are high.
(2) Due to many sampling points, a detailed TOC profile can be obtained and the time needed for photomineralization to take place can be determined. This offers a huge benefit compared to standard TOC measurements on lab scale applications in which only 1 or 2 sampling points can be measured. Indeed, using standard TOC in this kind of applications, the sampling points have to be chosen arbitrary what could lead to very different and erroneous results depending on the time after which these points are taken.
(3) The combination of μV-TOC analysis with UV-Vis analysis allows for a fast screening of photocatalysts allowing detection of structural and/or physicochemical effects that influence the catalytic efficiency towards CO2. This could be clearly proven by studying the photoeffiency of different catalysts in the degradation of dyes. Effects such as arising deactivation processes or rate limiting steps, etc., which cannot be directly detected via UV-Vis analysis, become very clear in the mineralization profile obtained by μV-TOC analysis. A more detailed study can be performed afterwards to identify the different intermediates and reaction mechanism using more expensive, advanced and time consuming techniques such as LC-MS.