Catalytic Hydrogenation of γ-Butyrolactone to Butanediol over a High-Performance Cu-SiO2 Catalyst
Abstract
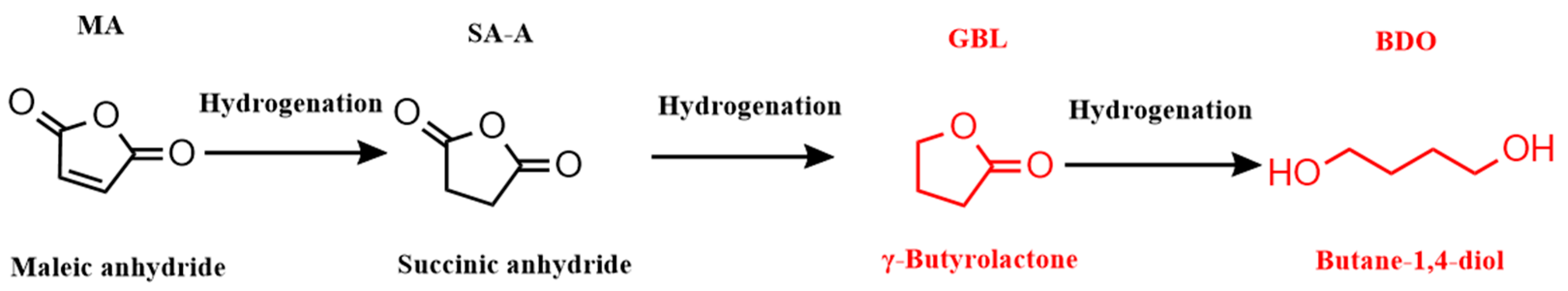
:1. Introduction
2. Results and Discussion
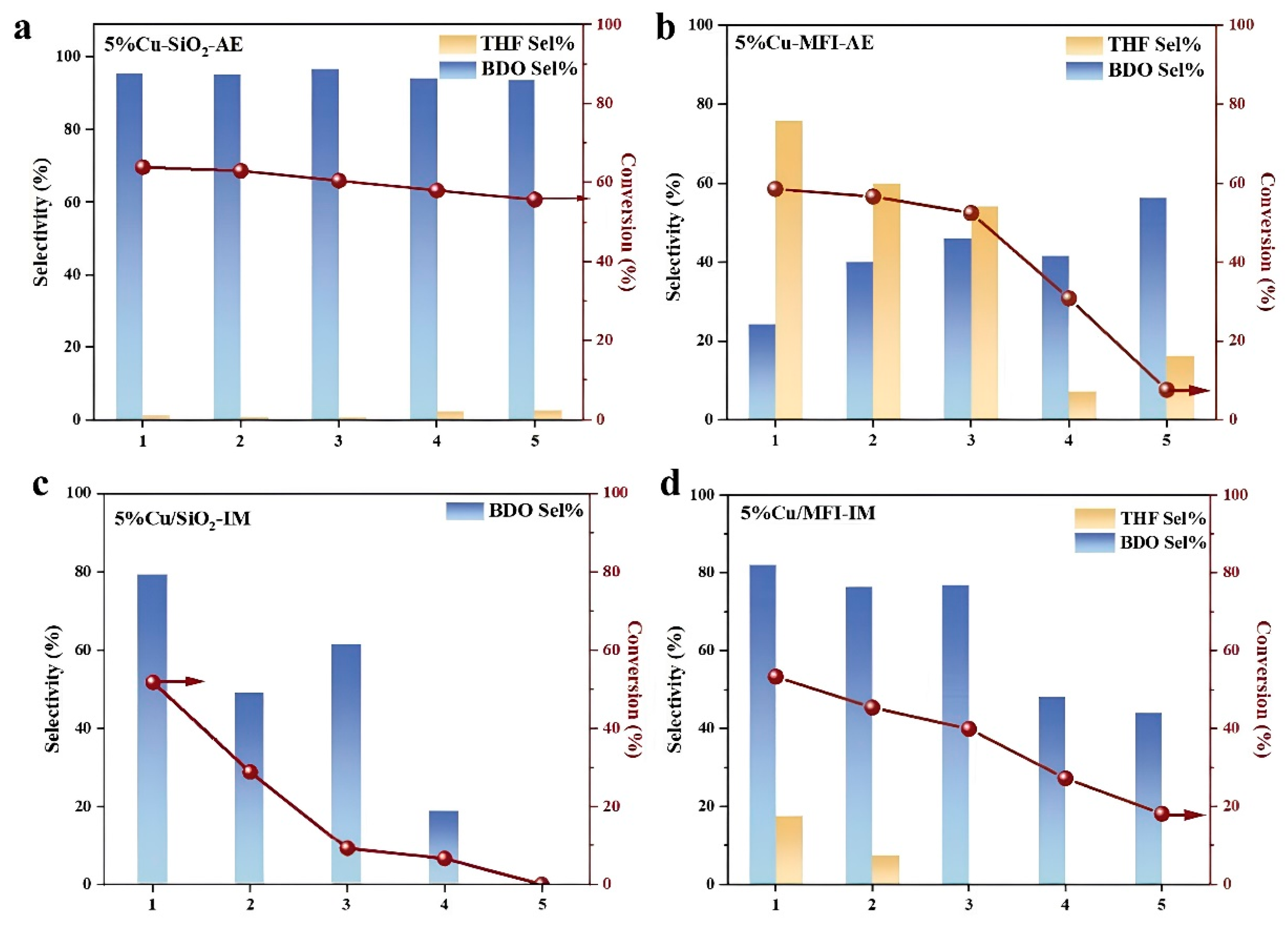
2.1. Catalytic Performance in GBL Hydrogenation
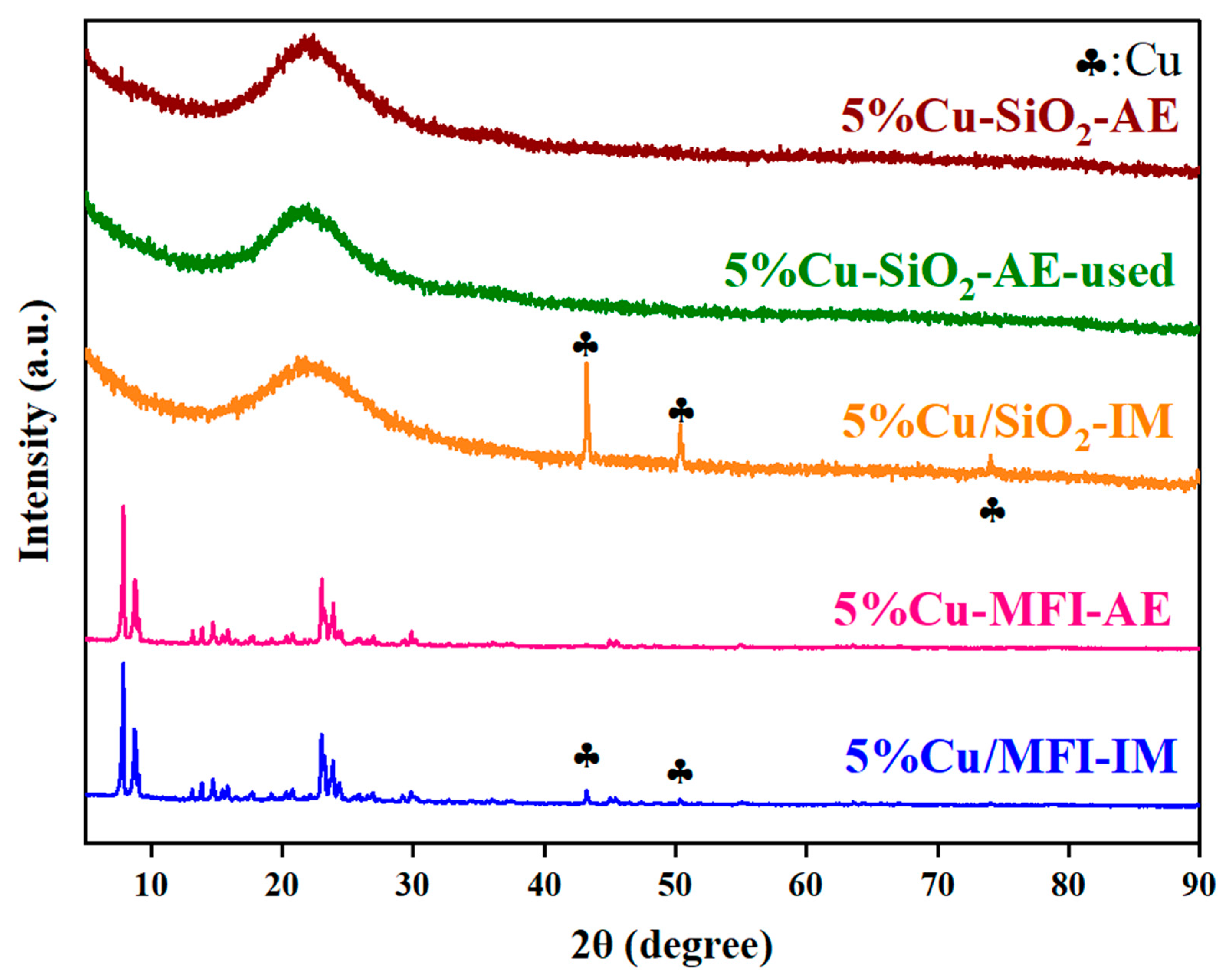
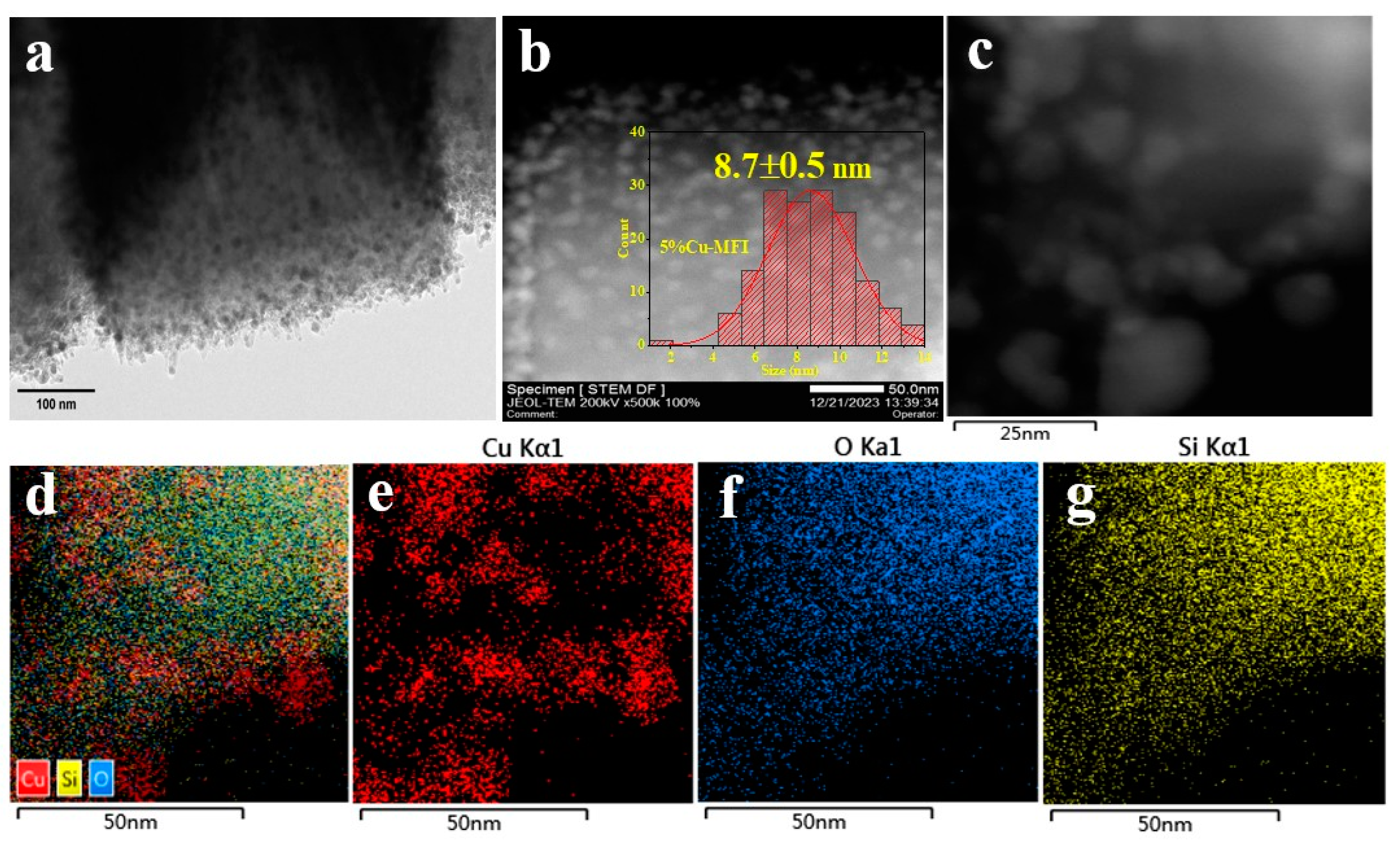
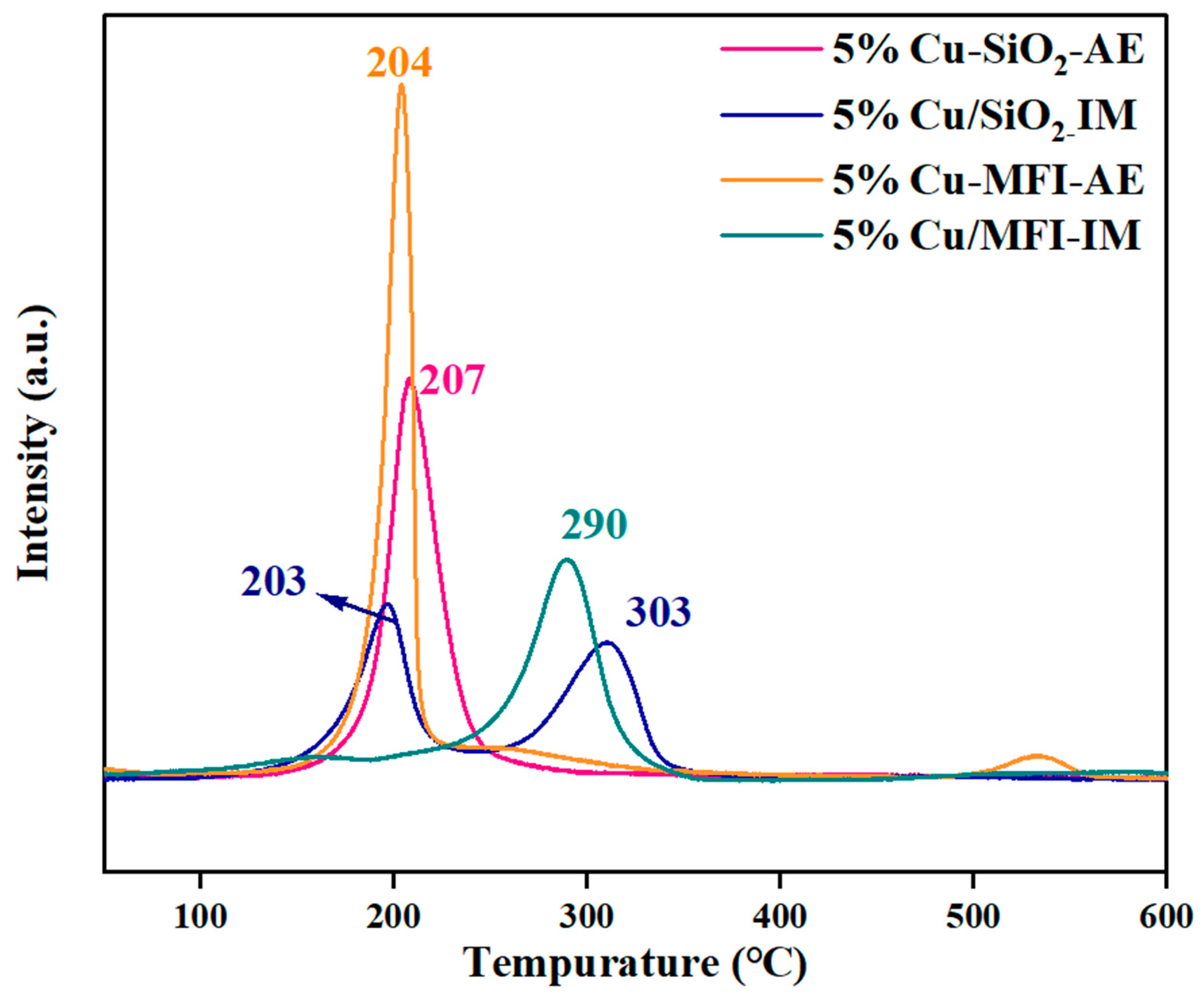
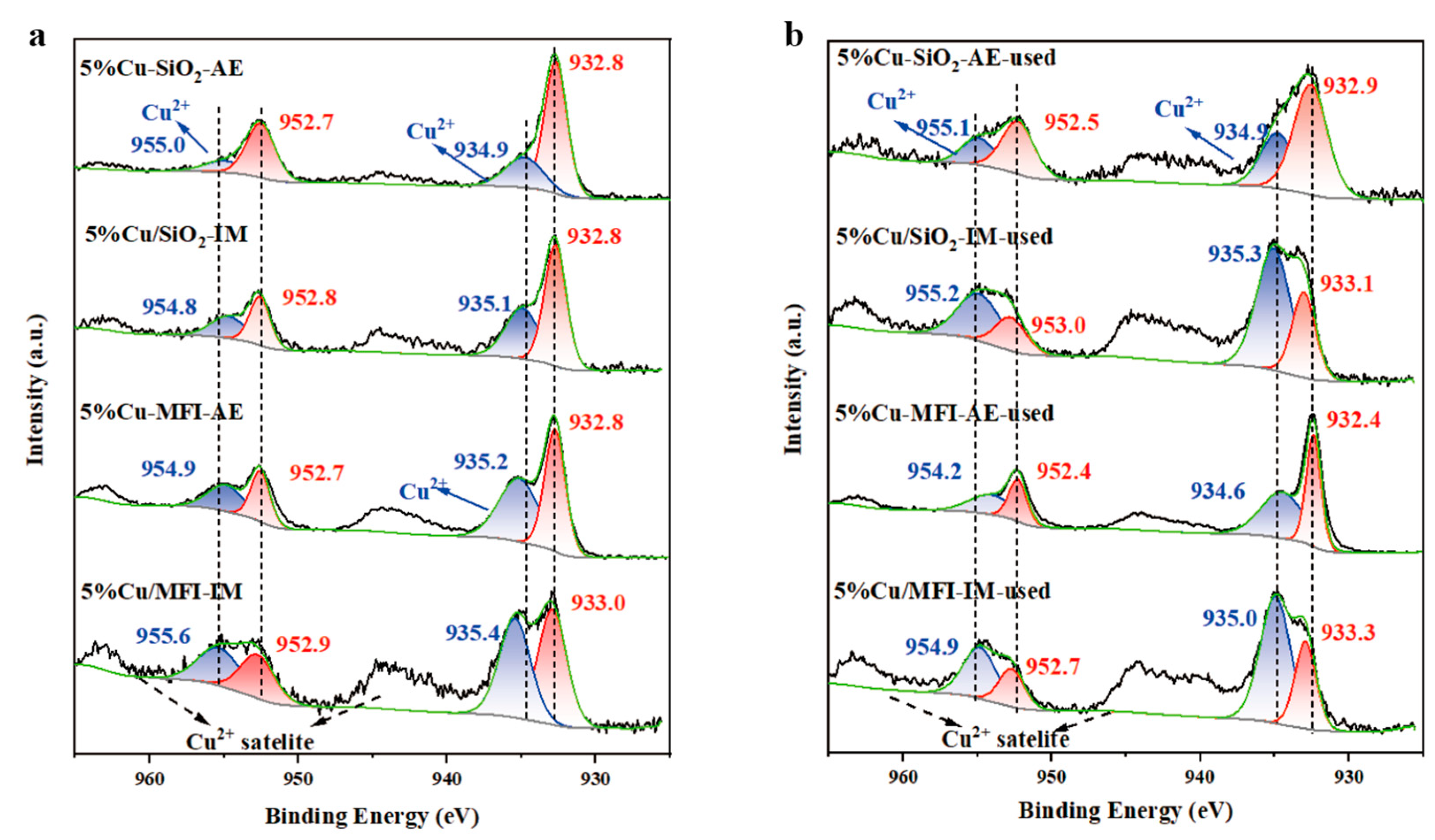
2.2. Catalysts Characterizations and Discussion
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Reaction Process
3.3. Catalyst Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, J.; Gao, G.; Li, Y.; Sun, P.; Wang, J.; Li, F. Highly chemoselective hydrogenation of lactone to diol over efficient copper-based bifunctional nanocatalysts. Appl. Catal. B Environ. 2019, 245, 251–261. [Google Scholar] [CrossRef]
- Zeng, F.; Hohn, K.L. Catalytic Conversion of Biomass-derived Compounds to C4 Chemicals. Catalysis 2019, 31, 346. [Google Scholar] [CrossRef]
- Liu, H.; Liu, S.; Ning, Y.; Zhang, R.; Deng, L.; Wang, F. Metabolic engineering of Escherichia coli for efficient production of 1,4-butanediol from crude glycerol. J. Environ. Chem. Eng. 2024, 12, 111660. [Google Scholar] [CrossRef]
- Le, S.D.; Nishimura, S. Highly Selective Synthesis of 1,4-Butanediol via Hydrogenation of Succinic Acid with Supported Cu–Pd Alloy Nanoparticles. ACS Sustain. Chem. Eng. 2019, 7, 18483–18492. [Google Scholar] [CrossRef]
- Ly, B.K.; Tapin, B.; Epron, F.; Pinel, C.; Especel, C.; Besson, M. In situ preparation of bimetallic ReOx-Pd/TiO2 catalysts for selective aqueous-phase hydrogenation of succinic acid to 1,4-butanediol. Catal. Today 2020, 355, 75–83. [Google Scholar] [CrossRef]
- Zhuang, J.; Yan, S.; Zhang, P.; Liu, X.; Zhao, Y.; Yu, Y.; Wang, Y.; Zhao, Q.; Wu, H.; Zhu, X.; et al. Regulating the states of Ni species by controlling the silanols of MCM-41 support to promote the hydrogenation of maleic anhydride. Fuel 2023, 335, 127030. [Google Scholar] [CrossRef]
- Trotter, C.L.; Babu, G.S.; Wallace, S. Engineering biology for sustainable 1,4-butanediol synthesis. Trends Biotechnol. 2023, 41, 286–288. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Lu, T.; Chen, B.; Huang, Z.; Yuan, G. Pt nanoparticles over TiO2–ZrO2 mixed oxide as multifunctional catalysts for an integrated conversion of furfural to 1,4-butanediol. Appl. Catal. A Gen. 2014, 478, 252–258. [Google Scholar] [CrossRef]
- Li, K.; Yang, J.; Song, T.; Zhu, Z.; Zhao, C.; Wu, P.; Li, X. Synthesis of bio-derived 1,4-butanediol by succinic acid esterification and hydrogenation over CuFeAl catalysts. Green Chem. 2023, 25, 627–638. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, J.; Mei, F.; Li, X.; Zhao, C. Bio-based 1,4-butanediol and tetrahydrofuran synthesis: Perspective. Green Chem. 2022, 24, 6450–6466. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, Y.; Zhao, H.; Hou, Z. Preparation of highly dispersed Cu catalysts from hydrotalcite precursor for the dehydrogenation of 1,4-butanediol. J. Ind. Eng. Chem. 2021, 102, 251–259. [Google Scholar] [CrossRef]
- Patil, K.N.; Prasad, D.; Manoorkar, V.K.; Bhanushali, J.T.; Jadhav, A.H.; Nagaraja, B.M. Selective vapour-phase dehydrocyclization of biomass-derived 1,4-butanediol to γ-butyrolactone over Cu/ZnAl2O4-CeO2 catalyst. J. Ind. Eng. Chem. 2022, 106, 142–151. [Google Scholar] [CrossRef]
- Weidener, D.; Leitner, W.; Dominguez de Maria, P.; Klose, H.; Grande, P.M. Lignocellulose fractionation using recyclable phosphoric acid: Lignin, cellulose, and furfural production. ChemSusChem 2021, 14, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Zuliani, A.; Kikhtyanin, O.; Cova, C.M.; Rodriguez-Padron, D.; Kubička, D.; Luque, R. Boosting the Ni-Catalyzed Hydrodeoxygenation (HDO) of Anisole Using Scrap Catalytic Converters. Adv. Sustain. Syst. 2022, 6, 2100394. [Google Scholar] [CrossRef]
- Weidener, D.; Klose, H.; von Westarp, W.G.; Jupke, A.; Leitner, W.; De Maria, P.D.; Grande, P.M. Selective lignin fractionation using CO2-expanded 2-methyltetrahydrofuran (2-MTHF). Green Chem. 2021, 23, 6330–6336. [Google Scholar] [CrossRef]
- Wang, T.; Liu, S.; Tamura, M.; Nakagawa, Y.; Hiyoshi, N.; Tomishige, K. One-pot catalytic selective synthesis of 1,4-butanediol from 1,4-anhydroerythritol and hydrogen. Green Chem. 2018, 20, 2547–2557. [Google Scholar] [CrossRef]
- Gong, Z.-J.; Li, Y.-R.; Wu, H.-L.; Lin, S.D.; Yu, W.-Y. Direct copolymerization of carbon dioxide and 1,4-butanediol enhanced by ceria nanorod catalyst. Appl. Catal. B Environ. 2020, 265, 118524. [Google Scholar] [CrossRef]
- Duan, H.; Hirota, T.; Ohtsuka, S.; Yamada, Y.; Sato, S. Vapor-phase catalytic dehydration of 1,4-butanediol to 3-buten-1-ol over modified ZrO 2 catalysts. Appl. Catal. A Gen. 2017, 535, 9–16. [Google Scholar] [CrossRef]
- Minh, D.P.; Besson, M.; Pinel, C.; Fuertes, P.; Petitjean, C. Aqueous-Phase Hydrogenation of Biomass-Based Succinic Acid to 1,4-Butanediol Over Supported Bimetallic Catalysts. Top. Catal. 2010, 53, 1270–1273. [Google Scholar] [CrossRef]
- Ly, B.K.; Minh, D.P.; Pinel, C.; Besson, M.; Tapin, B.; Epron, F.; Especel, C. Effect of Addition Mode of Re in Bimetallic Pd–Re/TiO2 Catalysts Upon the Selective Aqueous-Phase Hydrogenation of Succinic Acid to 1,4-Butanediol. Top. Catal. 2012, 55, 466–473. [Google Scholar] [CrossRef]
- Tapin, B.; Epron, F.; Especel, C.; Ly, B.K.; Pinel, C.; Besson, M. Influence of the Re introduction method onto Pd/TiO2 catalysts for the selective hydrogenation of succinic acid in aqueous-phase. Catal. Today 2014, 235, 127–133. [Google Scholar] [CrossRef]
- Kang, K.H.; Hong, U.G.; Bang, Y.; Choi, J.H.; Kim, J.K.; Lee, J.K.; Han, S.J.; Song, I.K. Hydrogenation of succinic acid to 1,4-butanediol over Re–Ru bimetallic catalysts supported on mesoporous carbon. Appl. Catal. A Gen. 2015, 490, 153–162. [Google Scholar] [CrossRef]
- Kang, K.H.; Hong, U.G.; Jun, J.O.; Song, J.H.; Bang, Y.; Choi, J.H.; Han, S.J.; Song, I.K. Hydrogenation of succinic acid to γ-butyrolactone and 1,4-butanediol over mesoporous rhenium–copper–carbon composite catalyst. J. Mol. Catal. A Chem. 2014, 395, 234–242. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Xu, G.; Liu, Q.; Mu, X.; Liu, H. Tuning the catalytic selectivity in biomass-derived succinic acid hydrogenation on FeOx-modified Pd catalysts. J. Mater. Chem. A 2015, 3, 23560–23569. [Google Scholar] [CrossRef]
- Ly, B.K.; Tapin, B.; Aouine, M.; Delichere, P.; Epron, F.; Pinel, C.; Especel, C.; Besson, M. Insights into the Oxidation State and Location of Rhenium in Re-Pd/TiO2 Catalysts for Aqueous-Phase Selective Hydrogenation of Succinic Acid to 1,4-Butanediol as a Function of Palladium and Rhenium Deposition Methods. ChemCatChem 2015, 7, 2161–2178. [Google Scholar] [CrossRef]
- Pary, F.F.; Tirumala, R.T.A.; Andiappan, M.; Nelson, T.L. Copper (i) oxide nanoparticle-mediated C–C couplings for synthesis of polyphenylenediethynylenes: Evidence for a homogeneous catalytic pathway. Catal. Sci. Technol. 2021, 11, 2414–2421. [Google Scholar] [CrossRef]
- Chatterjee, R.; Lidin, S.; Bhattacharyya, S.; Kuila, S.K. Structure, microstructure and photocatalytic properties of embedded spherical Cu nanoparticles on Cu2O–SiO2. Mater. Chem. Phys. 2021, 263, 124360. [Google Scholar]
- Tirumala, R.T.A.; Gyawali, S.; Wheeler, A.; Ramakrishnan, S.B.; Sooriyagoda, R.; Mohammadparast, F.; Khatri, N.; Tan, S.; Kalkan, A.K.; Bristow, A.D. Structure–Property–Performance Relationships of Cuprous Oxide Nanostructures for Dielectric Mie Resonance-Enhanced Photocatalysis. ACS Catal. 2022, 12, 7975–7985. [Google Scholar] [CrossRef]
- Hsieh, M.-H.; Su, Z.-H.; Wu, E.-T.; Huang, M.H. Photocatalytic aryl sulfide oxidation using 4-nitrophenylacetylene-modified Cu2O crystals. ACS Appl. Mater. Interfaces 2023, 15, 11662–11669. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, P.; Li, X.; Pang, J.; Zhai, S.; Zhang, T.; Zheng, M. Catalytic hydrogenation of maleic anhydride to γ-butyrolactone over a high-performance hierarchical Ni-Zr-MFI catalyst. J. Catal. 2022, 410, 69–83. [Google Scholar] [CrossRef]
- Huang, Z.; Barnett, K.J.; Chada, J.P.; Brentzel, Z.J.; Xu, Z.; Dumesic, J.A.; Huber, G.W. Hydrogenation of γ-Butyrolactone to 1,4-Butanediol over CuCo/TiO2 Bimetallic Catalysts. ACS Catal. 2017, 7, 8429–8440. [Google Scholar] [CrossRef]
- Mutschler, R.; Moioli, E.; Luo, W.; Gallandat, N.; Züttel, A. CO2 hydrogenation reaction over pristine Fe, Co, Ni, Cu and Al2O3 supported Ru: Comparison and determination of the activation energies. J. Catal. 2018, 366, 139–149. [Google Scholar] [CrossRef]
- Kacem, S.; Qiao, Y.; Wirtz, C.; Theyssen, N.; Bordet, A.; Leitner, W. Supercritical carbon dioxide as reaction medium for selective hydrogenation of fluorinated arenes. Green Chem. 2022, 24, 8671–8676. [Google Scholar] [CrossRef]
- Pang, J.; Zheng, M.; Wang, C.; Yang, X.; Liu, H.; Liu, X.; Sun, J.; Wang, Y.; Zhang, T. Hierarchical Echinus-like Cu-MFI Catalysts for Ethanol Dehydrogenation. ACS Catal. 2020, 10, 13624–13629. [Google Scholar] [CrossRef]
- Liu, H.; Chang, Z.; Fu, J.; Hou, Z. A CuZn-BTC derived stable Cu/ZnO@SiO2 catalyst for ethanol dehydrogenation. Appl. Catal. B Environ. 2023, 324, 122194. [Google Scholar] [CrossRef]
- Zhao, H.; Yu, R.; Ma, S.; Xu, K.; Chen, Y.; Jiang, K.; Fang, Y.; Zhu, C.; Liu, X.; Tang, Y.; et al. The role of Cu1–O3 species in single-atom Cu/ZrO2 catalyst for CO2 hydrogenation. Nat. Catal. 2022, 5, 818–831. [Google Scholar] [CrossRef]
- Li, X.; Pang, J.; Zhao, Y.; Wu, P.; Yu, W.; Yan, P.; Su, Y.; Zheng, M. Ethanol dehydrogenation to acetaldehyde over a Cuδ+-based Cu-MFI catalyst. Chin. J. Catal. 2023, 49, 91–101. [Google Scholar] [CrossRef]
- Xu, C.; Chen, G.; Zhao, Y.; Liu, P.; Duan, X.; Gu, L.; Fu, G.; Yuan, Y.; Zheng, N. Interfacing with silica boosts the catalysis of copper. Nat. Commun. 2018, 9, 3367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tan, H.-R.; Jaenicke, S.; Chuah, G.-K. Highly efficient and robust Cu catalyst for non-oxidative dehydrogenation of ethanol to acetaldehyde and hydrogen. J. Catal. 2020, 389, 19–28. [Google Scholar] [CrossRef]
- Yuan, X.; Chen, S.; Cheng, D.; Li, L.; Zhu, W.; Zhong, D.; Zhao, Z.J.; Li, J.; Wang, T.; Gong, J. Controllable Cu0-Cu+ Sites for Electrocatalytic Reduction of Carbon Dioxide. Angew. Chem. Int. Ed. 2021, 60, 15344–15347. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, W.; Rehman, M.U.; Liu, W.; Xu, Y.; Huang, H.; Wang, S.; Zhao, Y.; Mei, D.; Ma, X. Copper Phyllosilicate Nanotube Catalysts for the Chemosynthesis of Cyclohexane via Hydrodeoxygenation of Phenol. ACS Catal. 2022, 12, 4724–4736. [Google Scholar] [CrossRef]
- Lan, F.; Zhang, H.; Zhao, C.; Shu, Y.; Guan, Q.; Li, W. Copper Clusters Encapsulated in Carbonaceous Mesoporous Silica Nanospheres for the Valorization of Biomass-Derived Molecules. ACS Catal. 2022, 12, 5711–5725. [Google Scholar] [CrossRef]
- Yu, D.N.; Dai, W.L.; Wu, G.J.; Guan, N.J.; Li, L.D. Stabilizing copper species using zeolite for ethanol catalytic dehydrogenation to acetaldehyde. Chin. J. Catal. 2019, 40, 1375–1384. [Google Scholar] [CrossRef]
- He, X.; Wang, Y.; Zhang, X.; Dong, M.; Wang, G.; Zhang, B.; Niu, Y.; Yao, S.; He, X.; Liu, H. Controllable in Situ Surface Restructuring of Cu Catalysts and Remarkable Enhancement of Their Catalytic Activity. ACS Catal. 2019, 9, 2213–2221. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Wang, C.; Xie, Z.; Guan, N.; Li, L. Water-involved methane-selective catalytic oxidation by dioxygen over copper zeolites. Chem 2021, 7, 1557–1568. [Google Scholar] [CrossRef]
- Luo, H.Y.; Consoli, D.F.; Gunther, W.R.; Román-Leshkov, Y. Investigation of the reaction kinetics of isolated Lewis acid sites in Beta zeolites for the Meerwein–Ponndorf–Verley reduction of methyl levulinate to γ-valerolactone. J. Catal. 2014, 320, 198–207. [Google Scholar] [CrossRef]
- Van de Vyver, S.; Odermatt, C.; Romero, K.; Prasomsri, T.; Román-Leshkov, Y. Solid Lewis Acids Catalyze the Carbon–Carbon Coupling between Carbohydrates and Formaldehyde. ACS Catal. 2015, 5, 972–977. [Google Scholar] [CrossRef]
- Hu, M.; Wang, C.; Chu, Y.; Wang, Q.; Li, S.; Xu, J.; Deng, F. Unravelling the Reactivity of Framework Lewis Acid Sites towards Methanol Activation on H-ZSM-5 Zeolite with Solid-State NMR Spectroscopy. Angew. Chem. Int. Ed. 2022, 61, e202207400. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.-C.; Li, W.-C.; Wang, J.; Sun, D.-H.; Xiang, S.-Y.; Gao, X.-Q.; Lu, A.-H. PO43– coordinated Co2+ species on yttrium phosphate boosting the valorization of ethanol to butadiene. Chin. J. Catal. 2024, 56, 166–175. [Google Scholar] [CrossRef]
- Gao, M.X.; Jiang, H.X.; Zhang, M.H. The influence of calcination temperatures on the acid-based properties and catalytic activity for the 1,3-butadiene synthesis from ethanol/acetaldehyde mixture. Appl. Surf. Sci. 2018, 439, 1072–1078. [Google Scholar] [CrossRef]
- Winoto, H.P.; Fikri, Z.A.; Ha, J.-M.; Park, Y.-K.; Lee, H.; Suh, D.J.; Jae, J. Heteropolyacid supported on Zr-Beta zeolite as an active catalyst for one-pot transformation of furfural to γ-valerolactone. Appl. Catal. B Environ. 2019, 241, 588–597. [Google Scholar] [CrossRef]
- Pang, J.; Yin, M.; Wu, P.; Song, L.; Li, X.; Zhang, T.; Zheng, M. Redispersion and Stabilization of Cu/MFI Catalysts by the Encapsulation Method for Ethanol Dehydrogenation. ACS Sustain. Chem. Eng. 2023, 11, 3297–3305. [Google Scholar] [CrossRef]
- Pampararo, G.; Garbarino, G.; Riani, P.; Vykoukal, V.; Busca, G.; Debecker, D.P. Ethanol dehydrogenation to acetaldehyde with mesoporous Cu-SiO2 catalysts prepared by aerosol-assisted sol–gel. Chem. Eng. J. 2023, 465, 142715. [Google Scholar] [CrossRef]
- Morales, M.V.; Asedegbega-Nieto, E.; Bachiller-Baeza, B.; Guerrero-Ruiz, A. Bioethanol dehydrogenation over copper supported on functionalized graphene materials and a high surface area graphite. Carbon 2016, 102, 426–436. [Google Scholar] [CrossRef]
- Cui, G.; Zhang, X.; Wang, H.; Li, Z.; Wang, W.; Yu, Q.; Zheng, L.; Wang, Y.; Zhu, J.; Wei, M. ZrO2−x modified Cu nanocatalysts with synergistic catalysis towards carbon-oxygen bond hydrogenation. Appl. Catal. B Environ. 2021, 280, 119406. [Google Scholar] [CrossRef]
- Yan, W.-Q.; Zhang, J.-B.; Zhou, R.-J.; Cao, Y.-Q.; Zhu, Y.-A.; Zhou, J.-H.; Sui, Z.-J.; Li, W.; Chen, D.; Zhou, X.-G. Identification of synergistic actions between Cu0 and Cu+ sites in hydrogenation of dimethyl oxalate from microkinetic analysis. Ind. Eng. Chem. Res. 2020, 59, 22451–22459. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, M.Y.; Li, X.S.; Li, X.Q.; Zhang, T. Catalytic conversion of ethanol into butadiene over high performance LiZnHf-MFI zeolite nanosheets. Green Chem. 2019, 21, 1006–1010. [Google Scholar] [CrossRef]
- Li, X.; Pang, J.; Wang, C.; Li, L.; Pan, X.; Zheng, M.; Zhang, T. Conversion of ethanol to 1,3-butadiene over high-performance Mg–ZrOx/MFI nanosheet catalysts via the two-step method. Green Chem. 2020, 22, 2852–2861. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Pang, J.; Wu, P.; Yu, W.; Yan, P.; Su, Y.; Zhai, S.; Zheng, M. Cu-Zr/SiO2 catalysts featured by different Cu-Zr-Si coordinations for ethanol conversion to 1,3-butadiene. Resour. Chem. Mater. 2024, 3, 27–37. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Zhou, M.; Yu, W.; Zheng, M.; An, Q. Catalytic Hydrogenation of γ-Butyrolactone to Butanediol over a High-Performance Cu-SiO2 Catalyst. Catalysts 2024, 14, 297. https://doi.org/10.3390/catal14050297
Ren X, Zhou M, Yu W, Zheng M, An Q. Catalytic Hydrogenation of γ-Butyrolactone to Butanediol over a High-Performance Cu-SiO2 Catalyst. Catalysts. 2024; 14(5):297. https://doi.org/10.3390/catal14050297
Chicago/Turabian StyleRen, Xiaoni, Mo Zhou, Wenguang Yu, Mingyuan Zheng, and Qingda An. 2024. "Catalytic Hydrogenation of γ-Butyrolactone to Butanediol over a High-Performance Cu-SiO2 Catalyst" Catalysts 14, no. 5: 297. https://doi.org/10.3390/catal14050297




