Abstract
A correlation between polyoxotungstate structures and their catalytic performance for oxidative desulfurization processes was investigated. Bridged lanthanopolyoxometalates that incorporate identical metallic centers with Keggin- Eu[PW11O39]11− and Lindqvist-type [Eu(W5O18)2]9− structures were used as catalysts for the oxidation of the most representative refractory sulfur compounds. Both compounds were able to desulfurize a multicomponent model diesel under sustainable conditions, i.e., using ionic liquid as an extraction solvent and hydrogen peroxide as an oxidant. However, the Lindqvist catalyst appeared to achieve complete desulfurization faster than the Keggin catalyst while using a lesser amount of catalyst and oxidant. Furthermore, the reusable capacity of the Lindqvist-type [Eu(W5O18)2]9− was confirmed for consecutive oxidative desulfurization processes. The contribution of the lanthanide metallic center for the catalytic performance of these compounds was investigated by studying the analogous [TB(W5O18)2]9− compound. Identical desulfurization efficiency was obtained, even reusing this catalyst in consecutive reaction cycles. These results indicate that the active catalytic center of these compounds is probably related to the octahedral tungsten centers. However, a higher number of tungsten centers in the polyoxometalate structure did not result in higher catalytic activity.
1. Introduction
Fossil fuels are now, and will be for the next few decades, the major source of energy, especially for maritime, road and air transportation. Therefore, the elimination emissions from sulfur-derived products during fuel combustion continues to be a crucial topic for investigation. This includes the application of sustainable and efficient desulfurization processes, capable of producing fuels that have strict policies for sulfur contents in fuels: ultra-low for road transportation (<10 ppm), and more recently, a global limit of sulfur in fuel ships of 0.5% (m/m) [1], set by the International Marine Organization. Hydrodesulfurization (HDS) is the traditional method used by petroleum industry, which requires severe operational conditions and is less efficient in removing the aromatic sulfur compounds present in fuels [2,3]. Different complementary desulfurization processes have been studied based on extraction, adsorption, oxidation or even biological methods [1,4,5]. The oxidative desulfurization method (ODS) is an alternative process that allows for a highly efficient removal of refractory sulfur compounds under mild and eco-sustainable conditions [1,6,7,8]. Furthermore, the ODS process allows for the treatment of more viscous and less volatile fuels derived from the heavy fuel oils. The ODS method involves three steps: a selective oxidant to convert sulfides into corresponding sulfones or sulfoxides, followed by the extraction of sulfones, and the recovery of the catalyst [9,10].
Polyoxometalates (POMs) are clusters of anionic metal-oxides that are thermal and oxidative stable compounds. The chemical properties of POMs such as redox potential, electron-transfer properties, acidities and solubility, can be finely changed by adjusting the metal ions, the heteroatoms and the counter-cations [11]. These properties make POMs superior catalytic materials for oxidative catalysis. Our research group has been investigating novel POM-based eco-sustainable oxidative desulfurization systems for the production of sulfur-free fuels [7,12,13,14,15]. Most of these studies have been performed with a derived Keggin-type structure [PM12O40]3-, with M = MoIV, WIV or VV. Some of these studies used lanthanopolyoxometalates (LnPOMs) obtained through the coordination of lanthanide ions, such as Eu3+, Tb3+, Sm3+, etc. to coordinative POM lacunary fragments, namely [PM11O39]7-units with M = W and Mo [16]. In general, LnPOMs exhibit interesting luminescent properties and other specific characteristics, which result from the synergy between the properties of the lanthanide ions and POM units. The LnPOMs have a large number of applications: optics, catalysis, electronics, magnetics, biomedicine and luminescence [17,18,19,20,21,22,23]. The Keggin-type ([XM12O40]n−] anions are mainly studied in catalysis and also in the oxidative desulfurization processes. This is not only due to the high catalytic efficiency of these types of POMs, but also to their properties and catalytic activity, which can be modulated by the substitution of the M addenda atoms or the heteroatom X by different metal centers. Moreover, several Keggin-type derivates can be formed by removing one or more MO4+ units, resulting in lacunary compounds which contain free oxygen atoms, that can readily coordinate with transition metals or even lanthanide ions. Sandwich-type lanthanopolyoxotungstates [Ln(PW11O39)2]11- (Ln3+ = Eu3+, Tb3+) are efficient catalysts for ODS [11,12,15,24]. The Lindqvist-type [Ln(W5O18)2]9– (Ln3+ = Eu3+, Tb3+, Gd3+, La3+) has also been used to catalyze the oxidation of sulfur compounds in fuels [11,25,26,27]. The Lindqvist-type is an iso-polyoxometalate with only one type of transition metal atom in its structure and multiwavelength systems that allow the excitation wavelength to be tuned by variation of the lanthanide center or through the coordination of an organic ligand to the POM.
This work presents for the first time a comparative study using Keggin- and Lindqvist-type LnPOMs as catalysts for desulfurization technology. Furthermore, the influence of the number of tungsten centers in the POM structure and the nature of the lanthanide is evaluated here for the first time. The potassium salts of the sandwich monovacant Keggin- and Lindqvist-type phosphotungstates were tested in the desulfurization of the model diesel containing three different sulfur compounds with a total sulfur concentration of 1500 ppm. The reusability and stability of the catalysts have also been investigated.
2. Results
2.1. Catalysts Characterization
The Eu3+ based POMs, Lindqvist-type [Eu(W5O18)2]9− and Keggin-type [Eu(PW11O39)2]9−, are clearly the most studied POMs, and already described in the literature by different techniques, such as elemental and thermal analysis, powder X-ray diffraction, vibrational (FTIR and FT-Raman), 31P NMR and photoluminescence spectroscopy, to prove the authenticity and purity of the compounds [11,28,29,30].
Spectroscopic methods including FTIR and 31P NMR were used to characterize the europium compounds. The infrared spectrum of [Eu(W5O18)2] 9− presents several bands in the range 700–1200 cm–1, the terminal νas(W = Ot) stretch ca.931 cm–1 and the νas(W–O–W) corner or edge-shared stretching modes between 700–900 cm–1 (Figure S1 in Supplementary Materials). The infrared spectrum of Eu(PW11)2 displays four characteristic strong asymmetric vibration bands for the Keggin-type frameworks: νas(P–O) between 1100–1040 cm–1, terminal νas(W–Ot) at ca. 950 cm–1, corner-sharing νas(W–Ob–W) at ca. 850 cm–1, and edge-sharing νas(W–Oc–W) at ca. 800 cm–1. 31P NMR spectroscopy was also used to identify and characterize the potassium salt of Eu(PW11)2 structure in D2O solution, showing as expected, a singlet at 0.36 ppm [28]. These results are in accordance with the literature data and indicate that they were successfully prepared. The thermal behaviour of the lanthanopolyoxotungstates was investigated by thermogravimetric analysis (TGA) and the obtained TGA curves are exhibited in Figure S2 in Supplementary Materials. The Lindqvist-type POMs exhibit a main weight loss until 150 °C that can be assigned to the to the evaporation of physisorbed water (hydration water molecules). An additional weight loss can be observed in the TGA curve of Tb(W5O18)2 in the 150–325 °C range, most likely due to the presence of chemisorbed water. The TGA curve of Keggin-type POM shows a longer initial weight loss step until ca. 200 °C (hydration water molecules) followed by another weight loss in the 350–550 °C range, which suggests the degradation of the sandwich-type structure.
2.2. Desulfurization Studies
The preliminary studies with potassium salts of Keggin- (Eu(PW11)2) and Lindqvist-type (Eu(W5)2) POMs were performed using a model diesel containing three refractory sulfur compounds present in real diesel that are the most difficult to desulfurize: dibenzothiophene (DBT), 4-methyldibenzothiophene (4-MDBT) and 4,6-dimethyldibenzothiophene (4,6-DMDBT), with an individual concentration of approximately 500 ppm of sulfur containing compounds in n-octane. These oxidative desulfurization studies were performed using a biphasic system (1:1 model diesel/[BMIM]PF6, extraction solvent). The process procedures in two main steps: first an initial liquid-liquid extraction in the presence of the catalyst by stirring for 10 min at 70 °C (initial extraction); and then, after the initial extraction equilibrium is reached, the catalytic oxidation stage is initiated by adding the hydrogen peroxide oxidant (promoting the oxidation of sulfur compounds to the corresponding sulfoxides and/or sulfones in the extraction solvent). The oxidation occurs in the extraction phase, since no oxidation products were detected in the model diesel phase. However, when the sulfur compounds are catalytically oxidized, more sulfur compounds from the diesel phase can be transferred to the extraction phase. Therefore, during the catalytic oxidation stage, a continuous extraction of sulfur occurs (ECODS, extraction catalytic oxidative desulfurization system). The comparison of the catalytic performance of the homogeneous Lindqvist and the Keggin catalysts for the ECODS was performed, by varying the catalyst and oxidant amounts. The initial conditions adopted were 0.75 mL of model diesel, 0.75 mL of extraction solvent [BMIM]PF6 and 75 µL of H2O2 at 70 °C. These were optimized conditions previously reported by the research group for similar catalytic systems [31]. Ionic liquids have been demonstrated to have an effective effect as extractive desulfurization solvents. Between these, the [BMIM]PF6 have demonstrated to be most efficient [8,14,16]. The effect of the catalyst amount was studied using 0.3 and 3 µmol of catalyst and the results obtained are displayed in Figure 1. The desulfurization profile using Linqvist-type catalyst Eu(W5)2 is less influenced by the amount of catalyst, and higher catalytic efficiency is achieved using the lower amount of catalyst. Using this catalyst, total desulfurization was achieved after only 1 h of reaction. Slightly higher catalytic efficiency was found using 3 µmol than 0.3 µmol only during the first minutes. The desulfurization profile of the model diesel when catalysed by the Keggin-type catalyst Eu(PW11)2 demonstrates that complete desulfurization was faster achieved using the highest amount of catalyst. Therefore, 3 µmol of Keggin-type catalyst were needed to achieve complete desulfurization after 2 h (instead of 79% obtained using 0.3 µmol of Eu(PW11)2). However, after 3 h, complete desulfurization was achieved using 3 and 0.3 µmol of Eu(PW11)2 catalyst.
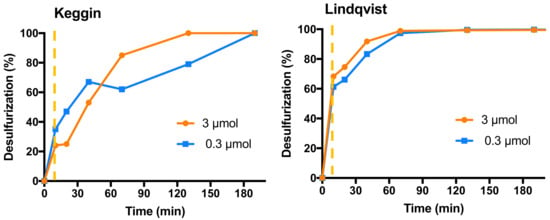
Figure 1.
Desulfurization profile using 0.3 and 3 µmol of Keggin-type [Eu(PW11O39)2]9 (right) and Lindqvist-type [Eu(W5O18)2]9− (left) catalysts to treat the multicomponent model diesel in a biphasic diesel/[BMIM]PF6 (1:1) ECODS system, using and 75 µL of H2O2 at 70 °C. The vertical dashed line indicates the time that the oxidative catalytic reaction was started by the addition of the oxidant.
The amount of oxidant presents in the ECODS system is another important parameter that can have a high influence in the catalyst performance. The H2O2 oxidant is activated by its interaction with the catalyst, forming active species. These are usually hydroperoxy- or peroxo-POM species that are able to oxidize the sulfur compounds into the corresponding sulfoxides through a nucleophilic attack. The initial POM is further regenerated. The subsequent oxidation of the sulfoxides leads to the formation of sulfones. The mechanism involved in ECODS system using polyoxotungstate catalysts is well reported in the literature [12,24,32,33,34,35]. In the oxidant amount study, 75 and 100 µL of H2O2 were used. Results obtained using Keggin and Linqvist catalysts are displayed in Figure 2. Using the Lindqvist catalyst, the increase in oxidant amount decreased the desulfurization efficiency of the ECODS system. This is probably caused by the occurrence of some deactivation of the catalyst or even by the difficulty of sulfur extraction in the presence of a higher amount of water content in the system. Visually, the aqueous oxidant is located between the apolar model diesel phase and the polar ionic liquid [BMIM]PF6 extraction phase. In both Keggin and Lindqvist catalytic systems, it is possible to observe a decrease in the initial extraction data when the oxidant amount is increased, i.e., the extractive desulfurization achieved after the first 10 min. However, using the Keggin catalyst, it is possible to observe an increment in the desulfurization efficiency during the oxidative catalytic step. Therefore, the Keggin catalyst also needs a higher amount of oxidant to achieve the same desulfurization efficiency as the Lindqvist catalyst obtained after 1 h, which corresponds to complete desulfurization of model diesel. When 75 µL of H2O2 was used instead of 100 µL, only 85% of desulfurization was obtained after 1 h. From the catalyst and oxidant amounts study is possible to verify that the Lindqvist catalyst achieved complete desulfurization faster, using less amount of catalyst and less amount of oxidant. This demonstrates that the Lindqvist (Eu(W5)2) compound is a more efficient catalyst than the Keggin (Eu(PW11)2). Therefore, the number of atomic tungsten centers in the POM structure does not have a direct correlation with the activity of the compound. A higher dimensional polyoxotungstate structure containing a higher number of tungsten centers may not be more catalytically active for an oxidative reaction as others with a smaller size and lower number of tungsten centers. Moreover, the primary phosphorus atomic center in the Keggin (Eu(PW11)2) structure does not have an active participation in catalyst activity.
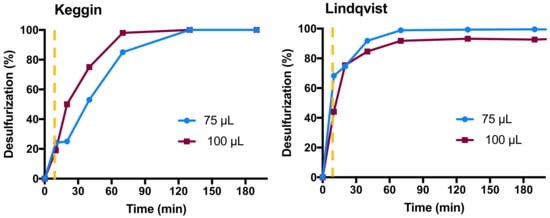
Figure 2.
Desulfurization profile of the multicomponent model diesel in a biphasic diesel/[BMIM]PF6 (1:1) ECODS system, using 3 µmol of catalyst and 100 and 75 µL of H2O2 at 70 °C. The vertical dashed line indicates the time that the oxidative catalytic reaction was started by the addition of the oxidant.
2.2.1. Reusing Homogeneous POMs
The reusability capacity of both the structures, Keggin and Lindqvist POMs, was investigated under the ECODS system, using 3 µmol of catalyst and 75 µL of H2O2. The reusability of both catalysts was evaluated for five consecutive cycles. At the end of each ECODS cycle, the desulfurized model diesel was removed from the system and a new sulfurized model diesel and oxidant sample were added to perform a consecutive ECODS cycle, maintaining the same experimental conditions and the POM/[BMIM]PF6 catalytic active phase. Figure 3 displays the results of desulfurization obtained for five ECODS consecutive cycles, after 1 h of reaction. The Lindqvist catalyst maintained its catalytic performance during the 5 consecutive cycles, whereas the Keggin one slightly increased its activity after the first cycle that was maintained for over 4 consecutive ECODS cycles. This behavior indicates that some catalytic activation must occur using the Keggin POM. In fact, the oxidant needs to be activated by the interaction with the catalyst, forming peroxo compounds as catalytic active intermediates [16]. In the case of the Keggin POM catalyst, a slower interaction with the H2O2 oxidant must have occurred, when compared to the Lindqvist POM which caused the increased catalytic activity observed.
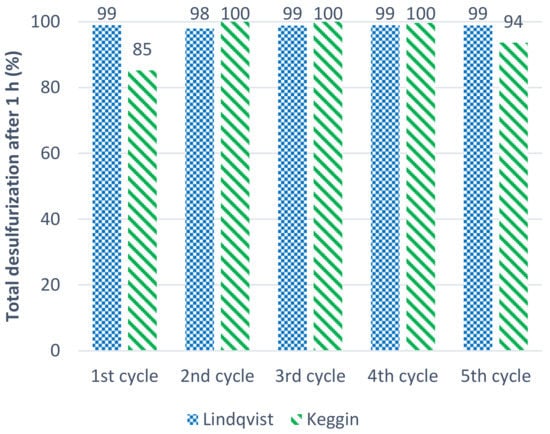
Figure 3.
Desulfurization results obtained after 1 h of 5 consecutive ECODS diesel/[BMIM]PF6 processes, maintaining the same POM/[BMIM]PF6 catalytic phase, using the Keggin and the Lindqvist catalysts (3 µmol) and H2O2 (75 µL) at 70 °C.
2.2.2. Influence of Lanthanide Nature
To investigate the contribution of the lanthanide as a catalytic active center, the catalytic activity of the europium Lindqvist POM Eu(W5)2 was compared with the analogous terbium Tb(W5)2 compound (Figure 4). Similar desulfurization profile of the multicomponent model diesel was obtained between both Lindqvist compounds, which indicates that the bridge lanthanide metallic center should not have an important contribution on the catalytic performance of the POM (Figure 4). Furthermore, Tb(W5)2 was reused for several ECODS cycles and also for this Lindqvist POM, and its activity was maintained for at least five consecutive ECODS cycles (Figure 5).
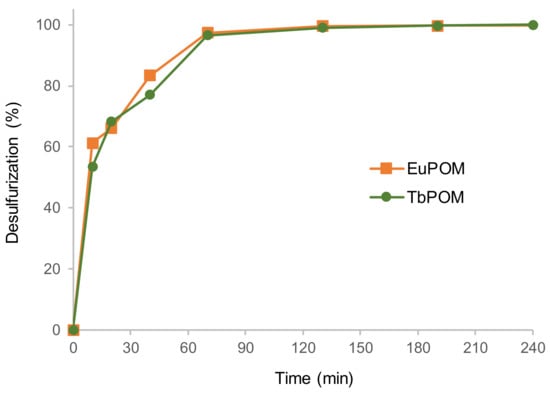
Figure 4.
Desulfurization profile of the multicomponent model diesel in a biphasic diesel/[BMIM]PF6 (1:1) ECODS system, using 3 µmol of Lindqvist catalyst (Eu(W5)2 or Tb(W5)2) and 75 µL of H2O2 at 70 °C.
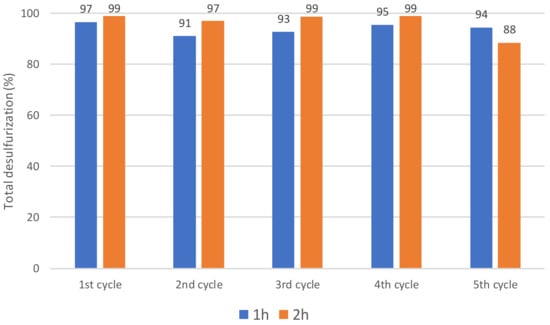
Figure 5.
Desulfurization results obtained after 1 h and 2 h of five consecutive ECODS diesel/[BMIM]PF6 cycles, maintaining the same POM/[BMIM]PF6 catalytic phase, using Tb(W5)2 Lindqvist catalysts (3 µmol) and H2O2 (75 µL) at 70 °C.
2.2.3. Comparison between Different POM Structures
A comparison of the desulfurization efficiency to treat model diesel catalyzed by different POM structures in the presence of H2O2 as oxidant is presented in Table 1. It is possible to observe that the structure of the POM catalysts play an important role in the desulfurization efficiency; however, the extraction solvent also presents an important influence. In general, ionic liquids appeared to promote a higher desulfurization efficiency than the acetonitrile. Different studies using the Keggin-type Eu(PW11)2 catalyst were performed and faster complete desulfurization was achieved in this study using [BMIM]PF6 solvent [15,24]. Furthermore, the lanthano-bridged POM Keggin-type (Eu(PW11)2) presents a higher structural stability than the pristine Keggin [PM12O40]3− (M = W and Mo) and the monovacant [PW11O39]7−, when used as catalysts for oxidative desulfurization of identical multicomponent model diesel [7,12,31]. Only a few examples could be found in the literature using the lanthano-bridged POM with the Lindqvist structure, and these only used single-model diesel containing only DBT, which is more easily oxidized than the BT or the DBT derivatives [25,27]. In this study, identical results were obtained using sulfur multicomponent model diesel.

Table 1.
Extractive/Oxidative Desulfurization efficiency of model diesel, using H2O2 as oxidant and various POM structures as homogeneous catalysts.
2.2.4. Stability of Keggin- and Lindqvist-Type Polyoxometalates
The stability of the Europium Keggin- and Lindqvist-type POMs were evaluated by comparison of the emission spectra, before and after catalysis. The studied EuPOMs exhibit peculiar photoluminescent properties, with an efficient energy transfer process to the lanthanide emitting center, as a result of the coordinated POM moieties [11,36]. For this reason, we prepared aqueous solutions, 1 mM of the as-prepared Lindqvist and Keggin POMs, and studied its emission under excitation into the maximum of the W-O charge transfer band (285 nm). The results confirm the occurrence of the characteristic intramolecular energy transfer to the Ln3+, with both emission spectra displaying the typical 5D0 → 7FJ (J = 2–4) emission peaks of Eu3+ (Figure 6) [28,37]. The EuPOM/[BMIM]PF6 phases were recovered after catalytic use and its emission spectra were acquired under the same experimental conditions for comparison purposes. As expected, the spectra of the recovered phases show peaks with considerably lower emission intensity when compared with the as-prepared samples due to the higher dispersion of the EuPOMs in the [BMIM]PF6. Nevertheless, both spectra are composed by the same Eu3+ emission peaks assigned to the 5D0 → 7FJ (J = 2–4) transitions (Figure 6—insets). Moreover, the relative intensities of the 5D0 → 7FJ transitions are still preserved in the emission spectra of the recovered phases, which could indicate that the Keggin and Lindqvist POM structures are retained after catalytic use since these transitions are known to be extremely sensitive to changes in the local symmetry of Eu3+ [29,38,39].
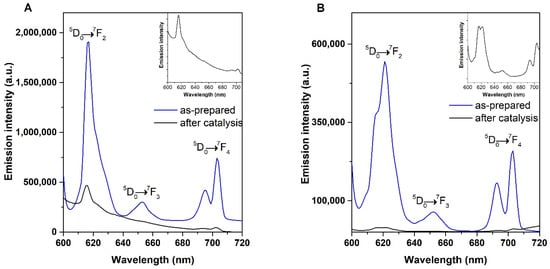
Figure 6.
Emission spectra of (A) Keggin-type and (B) Lindqvist-type POMs as-prepared (blue) and the LnPOM/[BMIM]PF6 phases after catalytic use (black) with an excitation wavelength of 285 nm.
3. Experimental Section
3.1. Materials and Methods
All the reagents used in the preparation of the polyoxometalates, namely sodium tungstate dihydrate (Aldrich, St. Louis, MO, USA, 99%), glacial acetic acid (Merck, Kenilworth, NJ, USA), potassium chloride (Merck), europium chloride hexahydrate (Sigma, St. Louis, MO, USA, 99.9 %,), terbium (III) chloride hexahydrate (Aldrich, St. Louis, MO, USA, 99.90%), hydrochloric acid (Panreac, Barcelona, Spain, 37 %) were used as received without further purification. The reagents for catalytic studies, including dibenzothiophene (DBT, Aldrich, St. Louis, MO, USA), 4-methyldibenzothiophene (4-MDBT, Aldrich, St. Louis, MO, USA), 4,6-dimethyldibenzothiophene (4,6-DMDBT, Alfa Aeser, Haverhill, MA, USA), n-octane (Aldrich), 1-butyl-3-methylimidazolium hexafluorophosphate ([BMIM]PF6, Sigma-Aldrich) and 30% w/w hydrogen peroxide (Aldrich, St. Louis, MO, USA) were purchased from commercial sources. Infrared absorption spectra were recorded for 400–4000 cm−1 regions on a Jasco 460 Plus spectrometer (Jasco, Tokyo, Japan), using KBr pellets. Thermogravimetric analysis was performed using a Hitachi STA 7200 RV equipment (Hitachi, Tokyo, Japan) under inert atmosphere (nitrogen flow of 200 mL/min), at room temperature up to 500 °C with a heating rate of 5 °C/min. 31P NMR spectra for liquid solutions were recorded with a Bruker Avance III 400 spectrometer (Bruker, Billerica, MA, USA), and chemical shifts are given with respect to external 85% H3PO4. Emission spectra were acquired in a Horiba Fluorolog-QM fluorometer (Horiba, Kyoto, Japan) with an excitation wavelength of 285 nm. Catalytic reactions were periodically monitored by GC-FID analysis carried out in a Bruker 430-GC-FID chromatograph (Bruker, Freemont, CA, USA). Hydrogen was used as carrier gas (55 cm s−1) and fused silica Supelco capillary columns SPB-5 (30 m × 0.25 mm i. d.; 25 μm film thickness) were used.
3.2. Synthesis of Lanthanopolyoxometalates
3.2.1. Lindqvist-Type POM
The potassium salts of the [Ln(W5O18)2]9−-type [Ln (III) = Eu and Tb] lanthanopolyoxometalates were prepared using an adaptation of the Weakley et al. method [36,40]. Briefly, an aqueous solution of sodium tungstate dihydrate (15.2 mmol, 7 mL) was prepared and the pH adjusted to 7 by addition of glacial CH3COOH. The solution was heated at 90 °C and a hot aqueous solution of LnCl3·6H2O (1.52 mmol; 2 mL) was added dropwise followed by an aqueous solution of KCl (17.5 mmol; 8 mL). The mixture was allowed to stir at 90 °C for 30 min and stored in the refrigerator for 3 days. The obtained precipitate was filtered, washed with ethanol, and dried in a desiccator over silica gel. Thermogravimetric analyses were performed for the determination of the hydration water molecules.
K9[Eu(W5O18)2]·7H2O (abbreviated as Eu(W5)2). TGA showed a mass loss of 4.2% up to 150 °C (loss of seven H2O hydration molecules). Selected FT-IR (cm−1): 931, 833, 791, 708.
K9[Tb(W5O18)2]∙5H2O (abbreviated as Tb(W5)2). TGA showed a mass loss of 3.3% up to 150 °C (loss of five H2O hydration molecules). Selected FT-IR (cm−1): 933, 843, 793, 706.
3.2.2. Keggin-Type POM
The potassium salt of europium, K11[Eu(PW11O39)2]·5H2O (abbreviated as Eu(PW11)2) was prepared by following a modified literature procedure [28]. A solution of EuCl3·6H2O was added dropwise to an aqueous solution of the precursor ligand (K7[PW11O39]·10H2O; abbreviated as PW11), previously prepared [41]. The mixture was stirred for 1 h at 90 °C. PW11 and Eu3+ were dissolved in the minimum volume of water, and both solutions were added in rigorously stoichiometric amounts to prepare Eu(PW11)2 (1:2). Elemental analysis: calcd (%). Eu 2.5, K 7.1, P 1.1, W 66.9; found (%) Eu 2.9, K 7.5, P 1.8, W 67.7. Selected FT-IR (cm−1): 1096, 1040, 942, 878, 798, 698. TGA showed a mass loss of 1.4% in the range 50–150 °C (loss of five H2O hydration molecules).
3.3. Oxidative Desulfurization Studies
The ODS studies were performed using a model diesel containing three sulfur compounds: dibenzothiophene
(DBT), 4-methyldibenzothiophene (4-MDBT) and 4,6-dimethyldibenzothiophene (4,6-DMDBT), in n
-octane (with a total sulfur concentration of 1500 ppm). The experiments were carried out under air in a
closed borosilicate 5 mL vessel, equipped with a magnetic stirrer and immersed in a thermostatically
controlled liquid paraffin bath at 70 °C. In a typical experiment, 3 µmol of POM was added to 1:1
model diesel/extraction solvent (750 µL each). The ionic liquid, 1-butyl-3-methylimidazolium
hexafluorophosphate ([BMIM]PF6), was used as extraction solvent. The resulting mixture was
stirred for 10 min, after which the catalytic step was initiated by adding 30% w/v
hydrogen peroxide (75 µL, H2O2/S = 14). The sulfur content in the model diesel
was periodically quantified by GC analysis using tetradecane as the external standard. The reusability of
the catalyst was evaluated by removing the desulfurized model diesel at the end of each ODS cycle and
adding a new portion of model diesel and oxidant, maintaining the condition.
4. Conclusions
The catalytic performance of Europium-bridged polyoxometalates with Keggin- Eu[PW11O39]11− and Lindqvist-type [Eu(W5O18)2]9− structures was investigated for the oxidative desulfurization of a multicomponent model diesel. The Lindqvist catalyst showed a slightly higher catalytic performance than the Keggin type, since complete desulfurization was achieved after only 1 h using less amount of oxidant. Furthermore, the catalytic activity of the Lindqvist compounds was investigated using the analogous [Tb(W5O18)2]9− structure. In this case, identical catalytic performance was obtained and this result suggests that the Lanthanide center does not contribute to the catalytic efficiency of the Lindqvist compounds. The reusability of the catalysts was confirmed for at least five desulfurization cycles and the stability of the Keggin and the Lindqvist structures were confirmed by their photoluminescent properties.
Supplementary Materials
The following supporting information can be downloaded at: www.mdpi.com/article/10.3390/catal12060581/s1, Figure S1: FT-IR spectra of Eu(W5)2, Tb(W5)2 and Eu(PW11)2; Figure S2: TGA curves for (A) Eu(W5)2, (B) Tb(W5)2 and (C) Eu(PW11)2.
Author Contributions
Conceptualization, S.S.B. and C.M.G.; methodology, S.F. and F.M.; investigation, S.F. and F.M.; writing—original draft preparation, S.F. and S.S.B.; writing—review and editing, C.M.G.; supervision, B.d.C. and S.S.B.; funding acquisition, S.S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Fundação para a Ciência e Tecnologia (FCT), Portugal, by the strategic project UIDB/50006/2020 for LAQV. S.S.B. thanks FCT/MCTES for funding through the Individual Call to Scientific Employment Stimulus Ref. CEECIND/03877/2018. C.M.G acknowledges FCT/MCTES for funding through DL 57/2016 program contract –Norma transitória.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, J.; Yang, Z.; Li, S.; Jin, Q.; Zhao, J. Review on oxidative desulfurization of fuel by supported heteropolyacid catalysts. J. Ind. Eng. Chem. 2019, 82, 1–16. [Google Scholar] [CrossRef]
- Jiang, W.; Zhu, W.; Li, H.; Wang, X.; Yin, S.; Chang, Y.; Li, H. Temperature-responsive ionic liquid extraction and separation of the aromatic sulfur compounds. Fuel 2015, 140, 590–596. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Mehrvarz, E.; Taghipour, A. Polyoxometalate as an effective catalyst for the oxidative desulfurization of liquid fuels: A critical review. Rev. Chem. Eng. 2019, 36, 831–858. [Google Scholar] [CrossRef]
- Soleimani, M.; Bassi, A.; Margaritis, A. Biodesulfurization of refractory organic sulfur compounds in fossil fuels. Biotechnol. Adv. 2007, 25, 570–596. [Google Scholar] [CrossRef] [PubMed]
- Cychosz, K.A.; Wong-Foy, A.G.; Matzger, A.J. Liquid Phase Adsorption by Microporous Coordination Polymers: Removal of Organosulfur Compounds. J. Am. Chem. Soc. 2008, 130, 6938–6939. [Google Scholar] [CrossRef] [PubMed]
- Julião, D.; Gomes, A.C.; Pillinger, M.; Lopes, A.D.; Valença, R.; Ribeiro, J.C.; Gonçalves, I.S.; Balula, S.S. Desulfurization of diesel by extraction coupled with Mo-catalyzed sulfoxidation in polyethylene glycol-based deep eutectic solvents. J. Mol. Liq. 2020, 309, 113093. [Google Scholar] [CrossRef]
- Mirante, F.; De Castro, B.; Granadeiro, C.M.; Balula, S.S. Solvent-Free Desulfurization System to Produce Low-Sulfur Diesel Using Hybrid Monovacant Keggin-Type Catalyst. Molecules 2020, 25, 4961. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Li, H.; Wei, Y.; Fu, Y.; Liao, W.; Zhu, L.; Chen, G.; Zhu, W.; Li, H. Tuning the electrophilicity of vanadium-substituted polyoxometalate based ionic liquids for high-efficiency aerobic oxidative desulfurization. Appl. Catal. B Environ. 2020, 271, 118936. [Google Scholar] [CrossRef]
- Craven, M.; Xiao, D.; Kunstmann-Olsen, C.; Kozhevnikova, E.F.; Blanc, F.; Steiner, A.; Kozhevnikov, I.V. Oxidative desulfurization of diesel fuel catalyzed by polyoxometalate immobilized on phosphazene-functionalized silica. Appl. Catal. B Environ. 2018, 231, 82–91. [Google Scholar] [CrossRef]
- Ribeiro, S.O.; Julião, D.; Cunha-Silva, L.; Domingues, V.F.; Valença, R.; Ribeiro, J.C.; de Castro, B.; Balula, S.S. Catalytic oxidative/extractive desulfurization of model and untreated diesel using hybrid based zinc-substituted polyoxometalates. Fuel 2016, 166, 268–275. [Google Scholar] [CrossRef]
- Granadeiro, C.M.; de Castro, B.; Balula, S.S.; Cunha-Silva, L. Lanthanopolyoxometalates: From the structure of polyanions to the design of functional materials. Polyhedron 2013, 52, 10–24. [Google Scholar] [CrossRef]
- Ribeiro, S.; Barbosa, A.; Gomes, A.; Pillinger, M.; Gonçalves, I.; Cunha-Silva, L.; Balula, S. Catalytic oxidative desulfurization systems based on Keggin phosphotungstate and metal-organic framework MIL-101. Fuel Process. Technol. 2013, 116, 350–357. [Google Scholar] [CrossRef]
- Granadeiro, C.M.; Barbosa, A.D.S.; Ribeiro, S.; Santos, I.C.M.S.; de Castro, B.; Cunha-Silva, L.; Balula, S.S. Oxidative catalytic versatility of a trivacant polyoxotungstate incorporated into MIL-101(Cr). Catal. Sci. Technol. 2014, 4, 1416–1425. [Google Scholar] [CrossRef]
- Mirante, F.; Dias, L.; Silva, M.; Ribeiro, S.O.; Corvo, M.; de Castro, B.; Granadeiro, C.; Balula, S. Efficient heterogeneous polyoxometalate-hybrid catalysts for the oxidative desulfurization of fuels. Catal. Commun. 2018, 104, 1–8. [Google Scholar] [CrossRef]
- Granadeiro, C.M.; Nogueira, L.S.; Julião, D.; Mirante, F.; Ananias, D.; Balula, S.S.; Cunha-Silva, L. Influence of a porous MOF support on the catalytic performance of Eu-polyoxometalate based materials: Desulfurization of a model diesel. Catal. Sci. Technol. 2016, 6, 1515–1522. [Google Scholar] [CrossRef]
- Mirante, F.; Alves, A.C.; Julião, D.; Almeida, P.L.; Gago, S.; Valença, R.; Ribeiro, J.C.; de Castro, B.; Granadeiro, C.M.; Balula, S.S. Large-pore silica spheres as support for samarium-coordinated undecamolybdophosphate: Oxidative desulfurization of diesels. Fuel 2020, 259, 116213. [Google Scholar] [CrossRef]
- Mizuno, N.; Kamata, K. Catalytic oxidation of hydrocarbons with hydrogen peroxide by vanadium-based polyoxometalates. Co-ord. Chem. Rev. 2011, 255, 2358–2370. [Google Scholar] [CrossRef]
- Wu, H.; Yan, B.; Li, H.; Singh, V.; Ma, P.; Niu, J.; Wang, J. Enhanced Photostability Luminescent Properties of Er3+-Doped Near-White-Emitting DyxEr(1–x)-POM Derivatives. Inorg. Chem. 2018, 57, 7665–7675. [Google Scholar] [CrossRef]
- Sato, R.; Suzuki, K.; Sugawa, M.; Mizuno, N. Heterodinuclear Lanthanoid-Containing Polyoxometalates: Stepwise Synthesis and Single-Molecule Magnet Behavior. Chem. A Eur. J. 2013, 19, 12982–12990. [Google Scholar] [CrossRef]
- Yan, B.; Wu, H.; Ma, P.; Niu, J.; Wang, J. Recent advances in rare earth co-doped luminescent tungsten oxygen complexes. Inorg. Chem. Front. 2021, 8, 4158–4176. [Google Scholar] [CrossRef]
- Viravaux, C.; Oms, O.; Dolbecq, A.; Nassar, E.; Busson, L.; Mellot-Draznieks, C.; Dessapt, R.; Serier-Brault, H.; Mialane, P. Temperature sensors based on europium polyoxometalate and mesoporous terbium metal–organic framework. J. Mater. Chem. C 2021, 9, 8323–8328. [Google Scholar] [CrossRef]
- Fateixa, S.; Carvalho, R.S.; Daniel-Da-Silva, A.L.; Nogueira, H.I.S.; Trindade, T. Luminescent Carrageenan Hydrogels Containing Lanthanopolyoxometalates. Eur. J. Inorg. Chem. 2017, 2017, 4976–4981. [Google Scholar] [CrossRef]
- Lotfian, N.; Heravi, M.M.; Mirzaei, M.; Daraie, M. Investigation of the uncommon basic properties of [Ln(W5O18)2]9– (Ln = La, Ce, Nd, Gd, Tb) by changing central lanthanoids in the syntheses of pyrazolopyranopyrimidines. J. Mol. Struct. 2019, 1199, 126953. [Google Scholar] [CrossRef]
- Ribeiro, S.O.; Nogueira, L.S.; Gago, S.; Almeida, P.L.; Corvo, M.C.; de Castro, B.; Granadeiro, C.M.; Balula, S.S. Desulfurization process conciliating heterogeneous oxidation and liquid extraction: Organic solvent or centrifugation/water? Appl. Catal. A Gen. 2017, 542, 359–367. [Google Scholar] [CrossRef]
- Wang, X.; Chen, W.; Song, Y. Directional Self-Assembly of Exfoliated Layered Europium Hydroxide Nanosheets and Na 9 EuW 10 O 36 ·32H 2 O for Application in Desulfurization. Eur. J. Inorg. Chem. 2014, 2014, 2779–2786. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, S.; Song, Y.-F. An efficient heterogeneous catalyst based on highly dispersed Na7H2LaW10O36·32H2O nanoparticles on mesoporous silica for deep desulfurization. Appl. Catal. A Gen. 2013, 466, 307–314. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, S.; Ji, Y.; Song, Y.-F. Deep Desulfurization by Amphiphilic Lanthanide-Containing Polyoxometalates in Ionic-Liquid Emulsion Systems under Mild Conditions. Chem. A Eur. J. 2012, 19, 709–715. [Google Scholar] [CrossRef]
- Neves, C.S.; Granadeiro, C.M.; Cunha-Silva, L.; Ananias, D.; Gago, S.; Feio, G.; Carvalho, P.A.; Eaton, P.; Balula, S.S.; Pereira, E. Europium Polyoxometalates Encapsulated in Silica Nanoparticles—Characterization and Photoluminescence Studies. Eur. J. Inorg. Chem. 2013, 2013, 2877–2886. [Google Scholar] [CrossRef]
- Sousa, F.L.; Pillinger, M.; Sá Ferreira, R.A.; Granadeiro, C.M.; Cavaleiro, A.M.V.; Rocha, J.; Carlos, L.D.; Trindade, T.; Nogueira, H.I.S. Luminescent Polyoxotungstoeuropate Anion-Pillared Layered Double Hydroxides. Eur. J. Inorg. Chem. 2006, 2006, 726–734. [Google Scholar] [CrossRef] [Green Version]
- Shiozaki, R.; Inagaki, A.; Ozaki, A.; Kominami, H.; Yamaguchi, S.; Ichihara, J.; Kera, Y. Catalytic behavior of a series of lanthanide decatungstates [Ln(III)W10O369; Ln: LaYb] for H2O2-oxidations of alcohols and olefins. Some chemical effects of the 4fn-electron in the lanthanide(III) ion on the catalyses. J. Alloy Compd. 1997, 261, 132–139. [Google Scholar] [CrossRef]
- Silva, D.; Viana, A.; Mirante, F.; de Castro, B.; Cunha-Silva, L.; Balula, S. Removing Simultaneously Sulfur and Nitrogen from Fuel under a Sustainable Oxidative Catalytic System. Sustain. Chem. 2021, 2, 382–391. [Google Scholar] [CrossRef]
- Nogueira, L.S.; Ribeiro, S.; Granadeiro, C.M.; Pereira, E.; Feio, G.; Cunha-Silva, L.; Balula, S.S. Novel polyoxometalate silica nano-sized spheres: Efficient catalysts for olefin oxidation and the deep desulfurization process. Dalton Trans. 2014, 43, 9518–9528. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhao, S.; Chen, W.; Wang, M.; Song, Y.-F. Highly Efficient Extraction and Oxidative Desulfurization System Using Na7H2LaW10O36⋅32 H2O in [bmim]BF4 at Room Temperature. Chem. A Eur. J. 2012, 18, 4775–4781. [Google Scholar] [CrossRef] [PubMed]
- Granadeiro, C.M.; Ferreira, R.A.S.; Soares-Santos, P.C.R.; Carlos, L.D.; Trindade, T.; Nogueira, H.I.S. Lanthanopolyoxotungstates in silica nanoparticles: Multi-wavelength photoluminescent core/shell materials. J. Mater. Chem. 2010, 20, 3313–3318. [Google Scholar] [CrossRef] [Green Version]
- Rezvani, M.A.; Afshari, P.; Aghmasheh, M. Deep catalytic oxidative desulfurization process catalyzed by TBA-PWFe@NiO@BNT composite material as an efficient and recyclable phase-transfer nanocatalyst. Mater. Chem. Phys. 2021, 267, 124662. [Google Scholar] [CrossRef]
- Granadeiro, C.M.; Ribeiro, S.O.; Kaczmarek, A.M.; Cunha-Silva, L.; Almeida, P.L.; Gago, S.; Van Deun, R.; de Castro, B.; Balula, S.S. A novel red emitting material based on polyoxometalate@periodic mesoporous organosilica. Microporous Mesoporous Mater. 2016, 234, 248–256. [Google Scholar] [CrossRef]
- Ali Rezvani, M.; Shaterian, M.; Aghbolagh, Z.S.; Akbarzadeh, F. Synthesis and Characterization of New Inorganic-Organic Hybrid Nanocomposite PMo11Cu@MgCu2O4@CS as an Efficient Heterogeneous Nanocatalyst for ODS of Real Fuel. ChemistrySelect 2019, 4, 6370–6376. [Google Scholar] [CrossRef]
- Binnemans, K. Interpretation of europium(III) spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef] [Green Version]
- Granadeiro, C.M.; Ferreira, R.A.S.; Soares-Santos, P.C.; Carlos, L.D.; Nogueira, H.I. Photoluminescent hybrid materials based on lanthanopolyoxotungstates and 3-hydroxypicolinic acid. J. Alloy. Compd. 2008, 451, 422–425. [Google Scholar] [CrossRef]
- Peacock, R.D.; Weakley, T.J.R. Heteropolytungstate complexes of the lanthanide elements. Part I. Preparation and reactions. J. Chem. Soc. A 1971, 1836–1839. [Google Scholar] [CrossRef]
- Brevard, C.; Schimpf, R.; Tourne, G.; Tourne, C.M. W-183 NMR—A complete and unequivocal assignment of the tungsten-tungsten connectivities in heteropolytungstates via two-dimensional W-183 NMR techniques. J. Am. Chem. Soc. 1983, 105, 7059–7063. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).