Na2WO4/Mn/SiO2 Catalyst Pellets for Upgrading H2S-Containing Biogas via the Oxidative Coupling of Methane
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Catalyst Pellets and Their Physical Properties
2.2. Biogas Upgrading Using Catalyst Pellets
2.3. Catalyst Characterization
2.3.1. O2 Temperature-Programmed Desorption (O2 TPD)
2.3.2. Powder X-ray Diffraction (Powder XRD)
2.3.3. Electronic Structures
2.4. Reaction Condition Optimization
3. Experimental Section
3.1. Materials
3.2. Catalyst Preparation
3.2.1. Na2WO4/Mn/SiO2 (NWM) Catalyst
3.2.2. Catalyst Pellets
3.3. Catalytic Activity Measurement
3.4. Catalyst Characterization
3.5. Calculation of Higher Heat Values (HHV)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holm-Nielsen, J.B.; Al Seadi, T.; Oleskowicz-Popiel, P. The future of anaerobic digestion and biogas utilization. Bioresour. Technol. 2009, 100, 5478–5484. [Google Scholar] [CrossRef]
- Hoyer, K.; Hulteberg, C.; Svensson, M.; Jernberg, J.; Nörregård, Ö. Biogas Upgrading-Technical Review; Report; Energiforsk: Stockholm, Sweden, 2016; Available online: http://vav.griffel.net/filer/C_Energiforsk2016-275.pdf (accessed on 6 October 2021).
- Scarlat, N.; Dallemand, J.-F.; Fahl, F. Biogas: Developments and perspectives in Europe. Renew. Energy 2018, 129, 457–472. [Google Scholar] [CrossRef]
- Achinas, S.; Achinas, V.; Euverink, G.J.W. A Technological Overview of Biogas Production from Biowaste. Engineering 2017, 3, 299–307. [Google Scholar] [CrossRef]
- Paolini, V.; Petracchini, F.; Segreto, M.; Tomassetti, L.; Naja, N.; Cecinato, A. Environmental impact of biogas: A short review of current knowledge. J. Environ. Sci. Health Part A 2018, 53, 899–906. [Google Scholar] [CrossRef] [Green Version]
- Adnan, A.I.; Ong, M.Y.; Nomanbhay, S.; Chew, K.W.; Show, P.L. Technologies for biogas upgrading to biomethane: A review. Bioengineering 2019, 6, 92. [Google Scholar] [CrossRef] [Green Version]
- Gustafsson, M.; Ammenberg, J.; Murphy, J.D. IEA Bioenergy Task 37—Country Reports Summaries 2019; Report; IEA Bioenergy: Paris, France, 2020; Available online: https://task37.ieabioenergy.com/country-reports.html (accessed on 6 October 2021).
- Association, E.B. EBA Statistical Report 2018; Annual Report; European Biogas Association: Brussels, Belgium, 2018. [Google Scholar]
- Bharathiraja, B.; Sudharsanaa, T.; Bharghavi, A.; Jayamuthunagai, J.; Praveenkumar, R. Biohydrogen and Biogas—An overview on feedstocks and enhancement process. Fuel 2016, 185, 810–828. [Google Scholar] [CrossRef]
- Anukam, A.; Mohammadi, A.; Naqvi, M.; Granström, K. A review of the chemistry of anaerobic digestion: Methods of accelerating and optimizing process efficiency. Processes 2019, 7, 504. [Google Scholar] [CrossRef] [Green Version]
- Petersson, A.; Wellinger, A. Biogas upgrading technologies–developments and innovations. IEA Bioenergy 2009, 20, 1–19. [Google Scholar]
- Angelidaki, I.; Treu, L.; Tsapekos, P.; Luo, G.; Campanaro, S.; Wenzel, H.; Kougias, P.G. Biogas upgrading and utilization: Current status and perspectives. Biotechnol. Adv. 2018, 36, 452–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penteado, A.T.; Kim, M.; Godini, H.R.; Esche, E.; Repke, J.-U. Biogas as a renewable feedstock for green ethylene production via oxidative coupling of methane: Preliminary feasibility study. Chem. Eng. Trans. 2017, 61, 589–594. [Google Scholar]
- Penteado, A.T.; Kim, M.; Godini, H.R.; Esche, E.; Repke, J.-U. Techno-economic evaluation of a biogas-based oxidative coupling of methane process for ethylene production. Front. Chem. Sci. Eng. 2018, 12, 598–618. [Google Scholar] [CrossRef]
- Sokolov, S.; Seeburg, D.; Wohlrab, S.; Friedel, M.; Nitzsche, J.; Kondratenko, E.V. An Approach Using Oxidative Coupling of Methane for Converting Biogas and Acid Natural Gas into High-Calorific Fuels. Ind. Eng. Chem. Res. 2019, 58, 2454–2459. [Google Scholar] [CrossRef]
- Gu, S.; Choi, J.-W.; Suh, D.J.; Park, Y.-K.; Choi, J.; Ha, J.-M. Upgrading of sulfur-containing biogas into high quality fuel via oxidative coupling of methane. Int. J. Energy Res. 2021, 45, 19363–19377. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Schluter, M.; Baerns, M.; Linke, D.; Holena, M. Developing catalytic materials for the oxidative coupling of methane through statistical analysis of literature data. Catal. Sci. Technol. 2015, 5, 1668–1677. [Google Scholar] [CrossRef] [Green Version]
- Gambo, Y.; Jalil, A.A.; Triwahyono, S.; Abdulrasheed, A.A. Recent advances and future prospect in catalysts for oxidative coupling of methane to ethylene: A review. J. Ind. Eng. Chem. 2018, 59, 218–229. [Google Scholar] [CrossRef]
- Fang, X.; Li, S.; Lin, J.; Chu, Y. Oxidative coupling of methane on W-Mn catalysts. J. Mol. Catal. 1992, 6, 427–433. [Google Scholar]
- Pak, S.; Lunsford, J.H. Thermal effects during the oxidative coupling of methane over Mn/Na2WO4/SiO2 and Mn/Na2WO4/MgO catalysts. Appl. Catal. A Gen. 1998, 168, 131–137. [Google Scholar] [CrossRef]
- Malekzadeh, A.; Khodadadi, A.; Abedini, M.; Amini, M.; Bahramian, A.; Dalai, A.K. Correlation of electrical properties and performance of OCM MOx/Na2WO4/SiO2 catalysts. Catal. Commun. 2001, 2, 241–247. [Google Scholar] [CrossRef]
- Arndt, S.; Otremba, T.; Simon, U.; Yildiz, M.; Schubert, H.; Schomäcker, R. Mn–Na2WO4/SiO2 as catalyst for the oxidative coupling of methane. What is really known? Appl. Catal. A Gen. 2012, 425–426, 53–61. [Google Scholar] [CrossRef]
- Blanksby, S.J.; Ellison, G.B. Bond Dissociation Energies of Organic Molecules. Acc. Chem. Res. 2003, 36, 255–263. [Google Scholar] [CrossRef]
- Takanabe, K.; Iglesia, E. Mechanistic aspects and reaction pathways for oxidative coupling of methane on Mn/Na2WO4/SiO2 catalysts. J. Phys. Chem. C 2009, 113, 10131–10145. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Park, M.-J.; Jeon, W.; Choi, J.-W.; Suh, Y.-W.; Suh, D.J. PLS-based kinetics modeling and optimization of the oxidative coupling of methane over Na2WO4/Mn/SiO2 catalyst. Korean J. Chem. Eng. 2011, 28, 2142–2147. [Google Scholar] [CrossRef]
- Lee, M.R.; Park, M.J.; Jeon, W.; Choi, J.W.; Suh, Y.W.; Suh, D.J. A kinetic model for the oxidative coupling of methane over Na2WO4/Mn/SiO2. Fuel Process. Technol. 2012, 96, 175–182. [Google Scholar] [CrossRef]
- Kiani, D.; Sourav, S.; Wachs, I.E.; Baltrusaitis, J. Synthesis and molecular structure of model silica-supported tungsten oxide catalysts for oxidative coupling of methane (OCM). Catal. Sci. Technol. 2020, 10, 3334–3345. [Google Scholar] [CrossRef]
- Lee, J.Y.; Jeon, W.; Choi, J.-W.; Suh, Y.-W.; Ha, J.-M.; Suh, D.J.; Park, Y.-K. Scaled-up production of C2 hydrocarbons by the oxidative coupling of methane over pelletized Na2WO4/Mn/SiO2 catalysts: Observing hot spots for the selective process. Fuel 2013, 106, 851–857. [Google Scholar] [CrossRef]
- Zhao, X.; Stachurski, P.; Shah, S.; Maiti, D.; Ramani, S.; Wright, A.; Walker, D.; Joseph, B.; Kuhn, J.N. Design and optimization of NiMg/ceria-zirconia catalyst pellets. Powder Technol. 2019, 357, 214–222. [Google Scholar] [CrossRef]
- Baldovino-Medrano, V.G.; Le, M.T.; Van Driessche, I.; Bruneel, E.; Alcázar, C.; Colomer, M.T.; Moreno, R.; Florencie, A.; Farin, B.; Gaigneaux, E.M. Role of shaping in the preparation of heterogeneous catalysts: Tableting and slip-casting of oxidation catalysts. Catal. Today 2015, 246, 81–91. [Google Scholar] [CrossRef]
- Hernando, H.; Ochoa-Hernández, C.; Shamzhy, M.; Moreno, I.; Fermoso, J.; Pizarro, P.; Coronado, J.M.; Čejka, J.; Serrano, D.P. The crucial role of clay binders in the performance of ZSM-5 based materials for biomass catalytic pyrolysis. Catal. Sci. Technol. 2019, 9, 789–802. [Google Scholar] [CrossRef]
- Nevalainen, P.; Kinnunen, N.; Suvanto, M. Developmental Study of Soot-Oxidation Catalysts for Fireplaces: The Effect of Binder and Preparation Techniques on Catalyst Texture and Activity. Catalysts 2019, 9, 957. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.-H.; Chae, H.-J.; Kim, T.-W.; Jeong, K.-E.; Kim, C.-U.; Lee, S.-B.; Jeong, S.-Y. Influence of Binder on Fe-based Extrudate as Fischer-Tropsch Catalysts. Korean Chem. Eng. Res. 2011, 49, 726–731. [Google Scholar] [CrossRef]
- Palermo, A.; Holgado Vazquez, J.P.; Lee, A.F.; Tikhov, M.S.; Lambert, R.M. Critical influence of the amorphous silica-to-cristobalite phase transition on the performance of Mn/Na2WO4/SiO2 catalysts for the oxidative coupling of methane. J. Catal. 1998, 177, 259–266. [Google Scholar] [CrossRef]
- Ji, S.-F.; Xiao, T.-C.; Li, S.-B.; Xu, C.-Z.; Hou, R.-L.; Coleman, K.S.; Green, M.L.H. The relationship between the structure and the performance of Na-W-Mn/SiO2 catalysts for the oxidative coupling of methane. Appl. Catal. A Gen. 2002, 225, 271–284. [Google Scholar] [CrossRef]
- Nipan, G.D. Melt-assisted phase transformations of A/W/Mn/SiO2 (A = Li, Na, K, Rb, Cs) composite catalysts. Inorg. Mater. 2017, 53, 553–559. [Google Scholar] [CrossRef]
- Jeon, W.; Lee, J.Y.; Lee, M.; Choi, J.-W.; Ha, J.-M.; Suh, D.J.; Kim, I.W. Oxidative coupling of methane to C2 hydrocarbons on the Mg–Ti mixed oxide-supported catalysts at the lower reaction temperature: Role of surface oxygen atoms. Appl. Catal. A Gen. 2013, 464–465, 68–77. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, G.; Liu, Y.; Lu, Y. TiO2-doped Mn2O3-Na2WO4/SiO2 catalyst for oxidative coupling of methane: Solution combustion synthesis and MnTiO3-dependent low-temperature activity improvement. Appl. Catal. A Gen. 2017, 544, 77–83. [Google Scholar] [CrossRef]
- Gu, S.; Oh, H.-S.; Choi, J.-W.; Suh, D.J.; Jae, J.; Choi, J.; Ha, J.-M. Effects of metal or metal oxide additives on oxidative coupling of methane using Na2WO4/SiO2 catalysts: Reducibility of metal additives to manipulate the catalytic activity. Appl. Catal. A Gen. 2018, 562, 114–119. [Google Scholar] [CrossRef]
- Lim, S.; Choi, J.-W.; Suh, D.J.; Song, K.H.; Ham, H.C.; Ha, J.-M. Combined experimental and density functional theory (DFT) studies on the catalyst design for the oxidative coupling of methane. J. Catal. 2019, 375, 478–492. [Google Scholar] [CrossRef]
- Fleischer, V.; Simon, U.; Parishan, S.; Colmenares, M.G.; Görke, O.; Gurlo, A.; Riedel, W.; Thum, L.; Schmidt, J.; Risse, T.; et al. Investigation of the role of the Na2WO4/Mn/SiO2 catalyst composition in the oxidative coupling of methane by chemical looping experiments. J. Catal. 2018, 360, 102–117. [Google Scholar] [CrossRef]
- Werny, M.J.; Wang, Y.; Girgsdies, F.; Schlögl, R.; Trunschke, A. Fluctuating storage of the active phase in a Mn-Na2WO4/SiO2 catalyst for the oxidative coupling of methane. Angew. Chem. Int. Ed. 2020, 59, 14921–14926. [Google Scholar] [CrossRef] [PubMed]
- Vandewalle, L.A.; Van de Vijver, R.; Van Geem, K.M.; Marin, G.B. The role of mass and heat transfer in the design of novel reactors for oxidative coupling of methane. Chem. Eng. Sci. 2019, 198, 268–289. [Google Scholar] [CrossRef]
- Yoon, S.; Lim, S.; Choi, J.-W.; Suh, D.J.; Song, K.H.; Ha, J.-M. Study on the unsteady state oxidative coupling of methane: Effects of oxygen species from O2, surface lattice oxygen, and CO2 on the C2+ selectivity. RSC Adv. 2020, 10, 35889–35897. [Google Scholar] [CrossRef]
- Mleczko, L.; Baerns, M. Catalytic oxidative coupling of methane—Reaction engineering aspects and process schemes. Fuel Process. Technol. 1995, 42, 217–248. [Google Scholar] [CrossRef]
- Ohyama, J.; Nishimura, S.; Takahashi, K. Data Driven Determination of Reaction Conditions in Oxidative Coupling of Methane via Machine Learning. ChemCatChem 2019, 11, 4307–4313. [Google Scholar] [CrossRef]
- Hayek, N.S.; Khlief, G.J.; Horani, F.; Gazit, O.M. Effect of reaction conditions on the oxidative coupling of methane over doped MnOx-Na2WO4/SiO2 catalyst. J. Catal. 2019, 376, 25–31. [Google Scholar] [CrossRef]
- Damby, D.E.; Llewellin, E.W.; Horwell, C.J.; Williamson, B.J.; Najorka, J.; Cressey, G.; Carpenter, M. The α-β phase transition in volcanic cristobalite. J. Appl. Cryst. 2014, 47, 1205–1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linstrom, P.; Mallard, W. NIST Chemistry Webbook. NIST Standard Reference Database. 2021. Available online: http://webbook.nist.gov/chemistry (accessed on 18 August 2021).
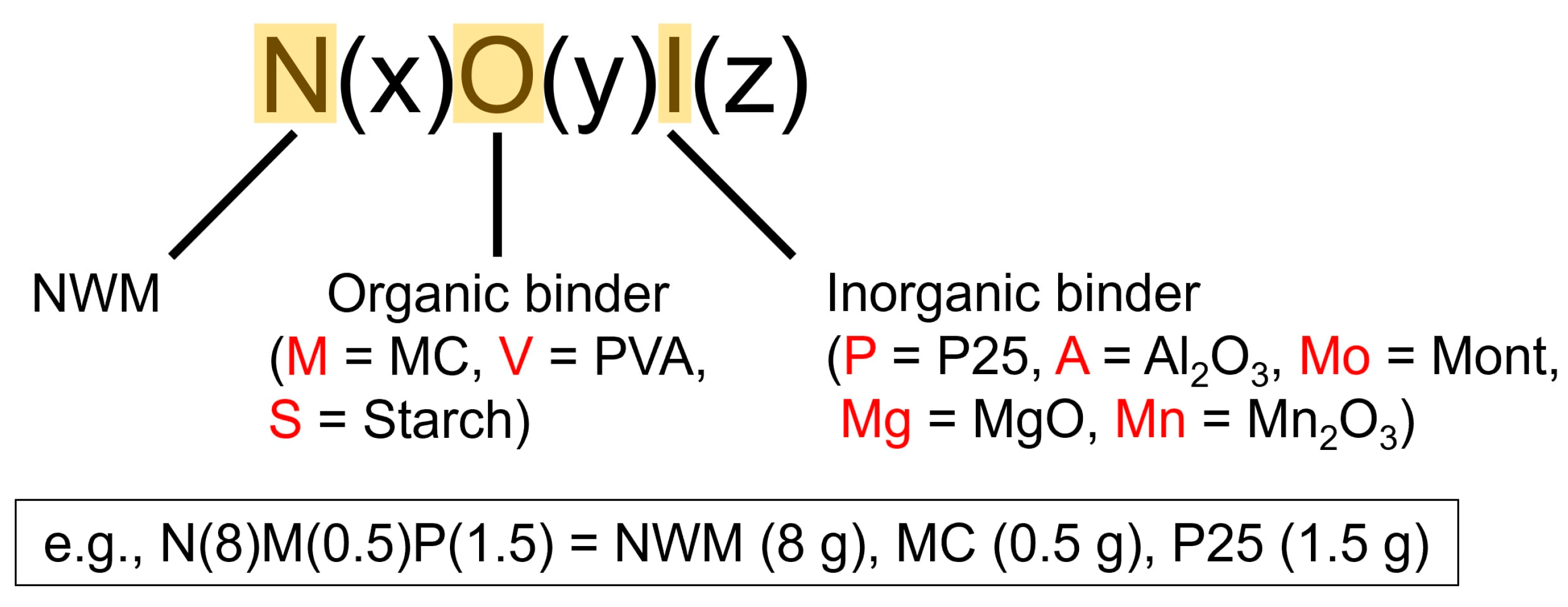

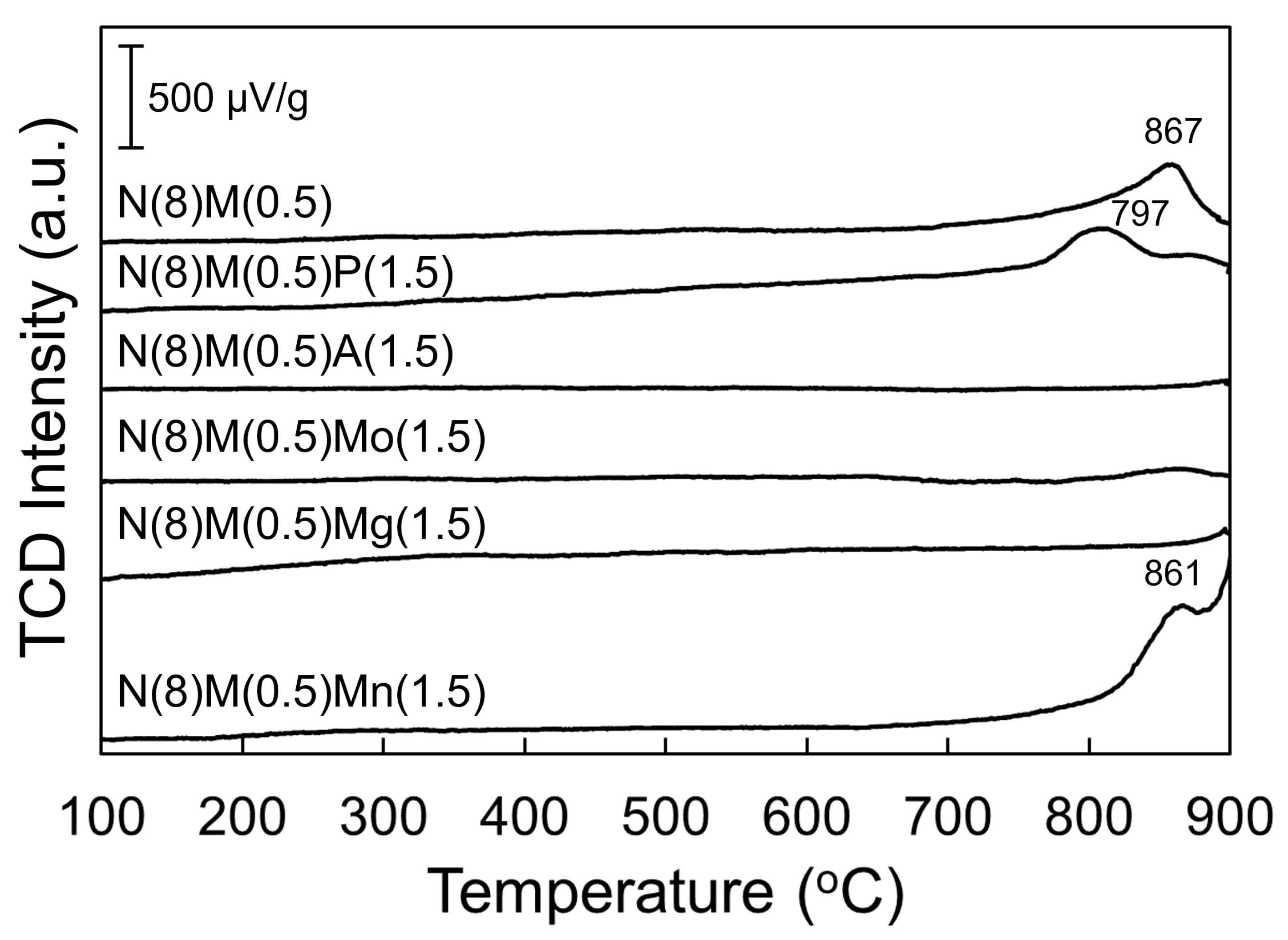



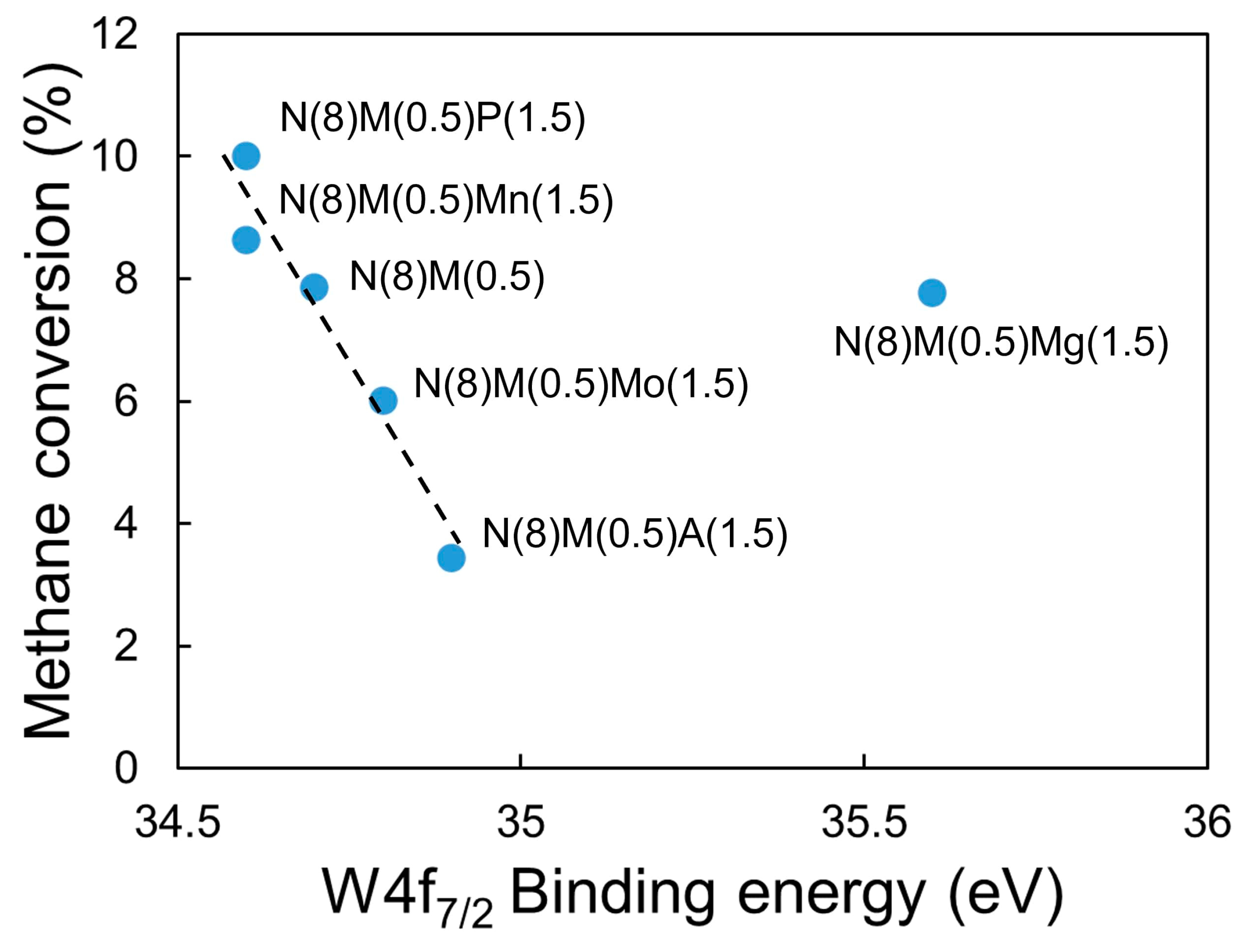



| Catalyst | Fracture Strength (MPa) | BET Surface Area (m2/g) |
|---|---|---|
| NWM (Powder) | n.a. | 3 |
| Using organic binder of MC | ||
| N(8)M(0.5) | 1.45 | 2 |
| N(8)M(0.5)P(1.5) | 2.79 | 2 |
| N(80)M(5)A(5) | 0.54 | 8 |
| N(8)M(0.5)Mo(1.5) | 0.19 | 2 |
| N(8)M(0.5)Mg(1.5) | 0.42 | 2 |
| N(8)M(0.5)Mn(1.5) | 0.40 | 1 |
| Using inorganic binder of P25 | ||
| N(8)M(0.5)P(1.5) | 2.79 | 2 |
| N(8)V(0.5)P(1.5) | 2.65 | 2 |
| N(8)S(0.5)P(1.5) | 2.52 | 1 |
| Catalyst | Fracture Strength (MPa) | BET Surface Area (m2/g) |
|---|---|---|
| N(8)M(0.5) | 1.45 | 2 |
| M(8)M(0.5)P(0.75) | 1.60 | 2 |
| N(8)M(0.5)P(1.5) | 2.79 | 2 |
| N(8)M(0.5)P(3) | 2.57 | 3 |
| N(8)M(0.5)P(6) | 2.23 | 3 |
| N(8)P(1.5) | 0.29 | 1 |
| N(8)M(0.25)P(1.5) | 1.72 | 2 |
| N(8)M(0.5)P(1.5) | 2.79 | 2 |
| N(8)M(1)P(1.5) | 0.43 | 2 |
| N(8)M(2)P(1.5) | 0.29 | 2 |
| Catalyst | Methane Conversion (%) | C2+ Selectivity (%) b | Olefin Selectivity (%) c | C2+ Yield (%) b | Olefin/Paraffin (mol/mol) d | O2 Conversion (%) | HHV (MJ/Nm3) e |
|---|---|---|---|---|---|---|---|
| NWM (Powder) | 8.20 | 63.5 | 28.5 | 5.09 | 0.82 | 66.7 | 40.6 |
| N(8)M(0.5) | 7.85 | 51.0 | 21.0 | 3.99 | 0.70 | 65.4 | 40.4 |
| N(8)M(0.5)P(1.5) | 10.0 | 83.9 | 44.4 | 8.39 | 1.12 | 77.8 | 41.0 |
| N(8)M(0.5)A(1.5) | 3.43 | 49.0 | 16.7 | 1.68 | 0.52 | 59.2 | 40.1 |
| N(8)M(0.5)Mo(1.5) | 6.00 | 33.3 | 11.0 | 1.95 | 0.50 | 56.6 | 40.1 |
| N(8)M(0.5)Mg(1.5) | 7.76 | 28.9 | 10.5 | 2.19 | 0.58 | 65.9 | 40.2 |
| N(8)M(0.5)Mn(1.5) | 8.63 | 71.7 | 34.9 | 6.19 | 0.95 | 70.6 | 40.7 |
| Catalyst | Methane Conversion (%) | C2+ Selectivity (%) b | Olefin Selectivity (%) c | C2+ Yield (%) b | Olefin/Paraffin (mol/mol) d | O2 Conversion (%) | HHV (MJ/Nm3) e |
|---|---|---|---|---|---|---|---|
| N(8)M(0.5) | 7.85 | 51.0 | 21.0 | 3.99 | 0.70 | 65.4 | 40.4 |
| M(8)M(0.5)P(0.75) | 9.51 | 81.5 | 42.6 | 7.75 | 1.10 | 79.2 | 40.9 |
| N(8)M(0.5)P(1.5) | 10.0 | 83.9 | 44.4 | 8.39 | 1.12 | 77.8 | 41.0 |
| N(8)M(0.5)P(3) | 11.5 | 77.2 | 41.9 | 8.80 | 1.18 | 85.4 | 41.1 |
| N(8)M(0.5)P(6) | 12.8 | 73.6 | 40.6 | 9.36 | 1.23 | 95.5 | 41.2 |
| N(8)P(1.5) | 5.72 | 67.7 | 29.0 | 3.83 | 0.75 | 65.7 | 40.4 |
| N(8)M(0.25)P(1.5) | 10.3 | 80.3 | 42.4 | 8.26 | 1.12 | 86.8 | 41.0 |
| N(8)M(0.5)P(1.5) | 10.0 | 83.9 | 44.4 | 8.39 | 1.12 | 77.8 | 41.0 |
| N(8)M(1)P(1.5) | 11.5 | 75.2 | 40.7 | 8.66 | 1.18 | 78.2 | 41.1 |
| N(8)M(2)P(1.5) | 10.7 | 73.1 | 38.2 | 7.81 | 1.09 | 74.3 | 41.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, S.; Choi, J.-W.; Suh, D.J.; Yoo, C.-J.; Choi, J.; Ha, J.-M. Na2WO4/Mn/SiO2 Catalyst Pellets for Upgrading H2S-Containing Biogas via the Oxidative Coupling of Methane. Catalysts 2021, 11, 1301. https://doi.org/10.3390/catal11111301
Gu S, Choi J-W, Suh DJ, Yoo C-J, Choi J, Ha J-M. Na2WO4/Mn/SiO2 Catalyst Pellets for Upgrading H2S-Containing Biogas via the Oxidative Coupling of Methane. Catalysts. 2021; 11(11):1301. https://doi.org/10.3390/catal11111301
Chicago/Turabian StyleGu, Sangseo, Jae-Wook Choi, Dong Jin Suh, Chun-Jae Yoo, Jungkyu Choi, and Jeong-Myeong Ha. 2021. "Na2WO4/Mn/SiO2 Catalyst Pellets for Upgrading H2S-Containing Biogas via the Oxidative Coupling of Methane" Catalysts 11, no. 11: 1301. https://doi.org/10.3390/catal11111301







