Preclinical Activity of the Vascular Disrupting Agent OXi4503 against Head and Neck Cancer
Abstract
:1. Introduction
2. Results
2.1. Acute Response of Subcutaneous FaDu-luc HNSCC Xenografts to OXi4503
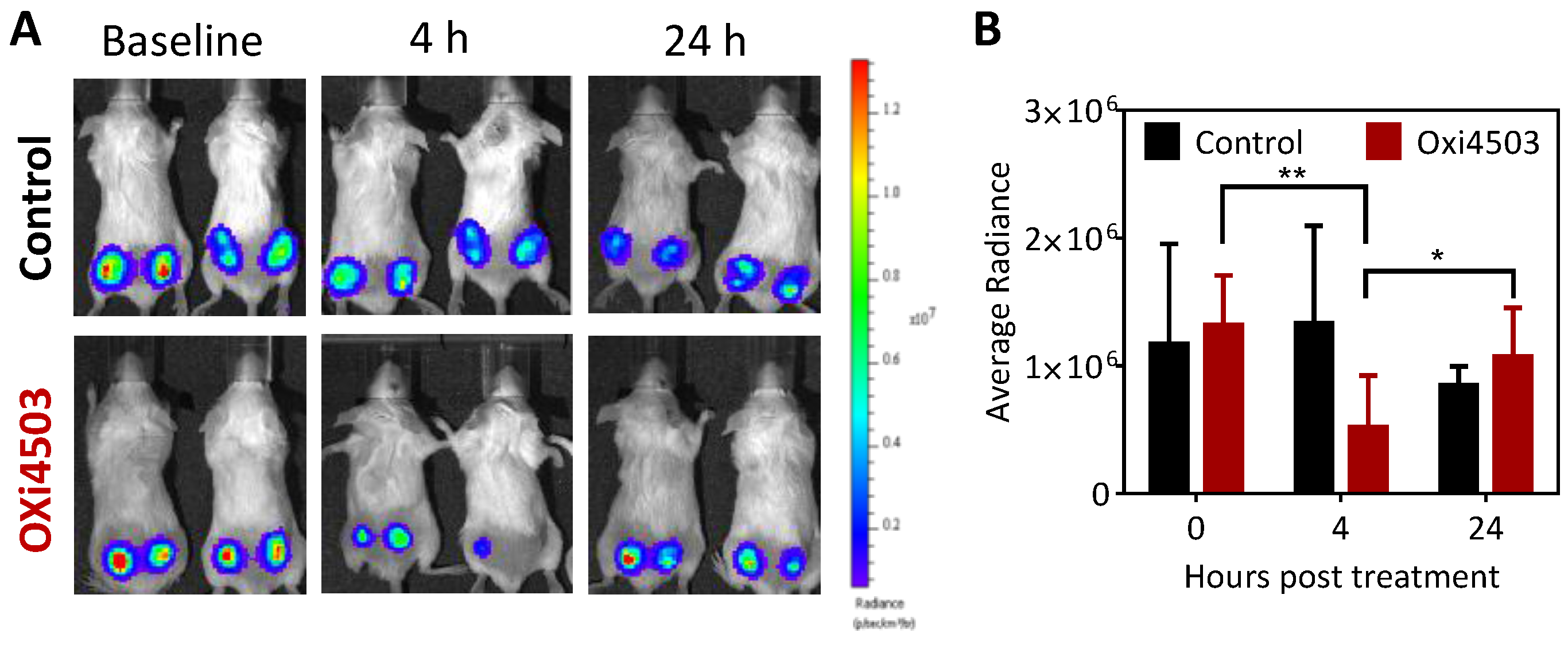
2.2. Antitumor Activity of OXi4503 Against Subcutaneous FaDu-luc HNSCC Xenografts

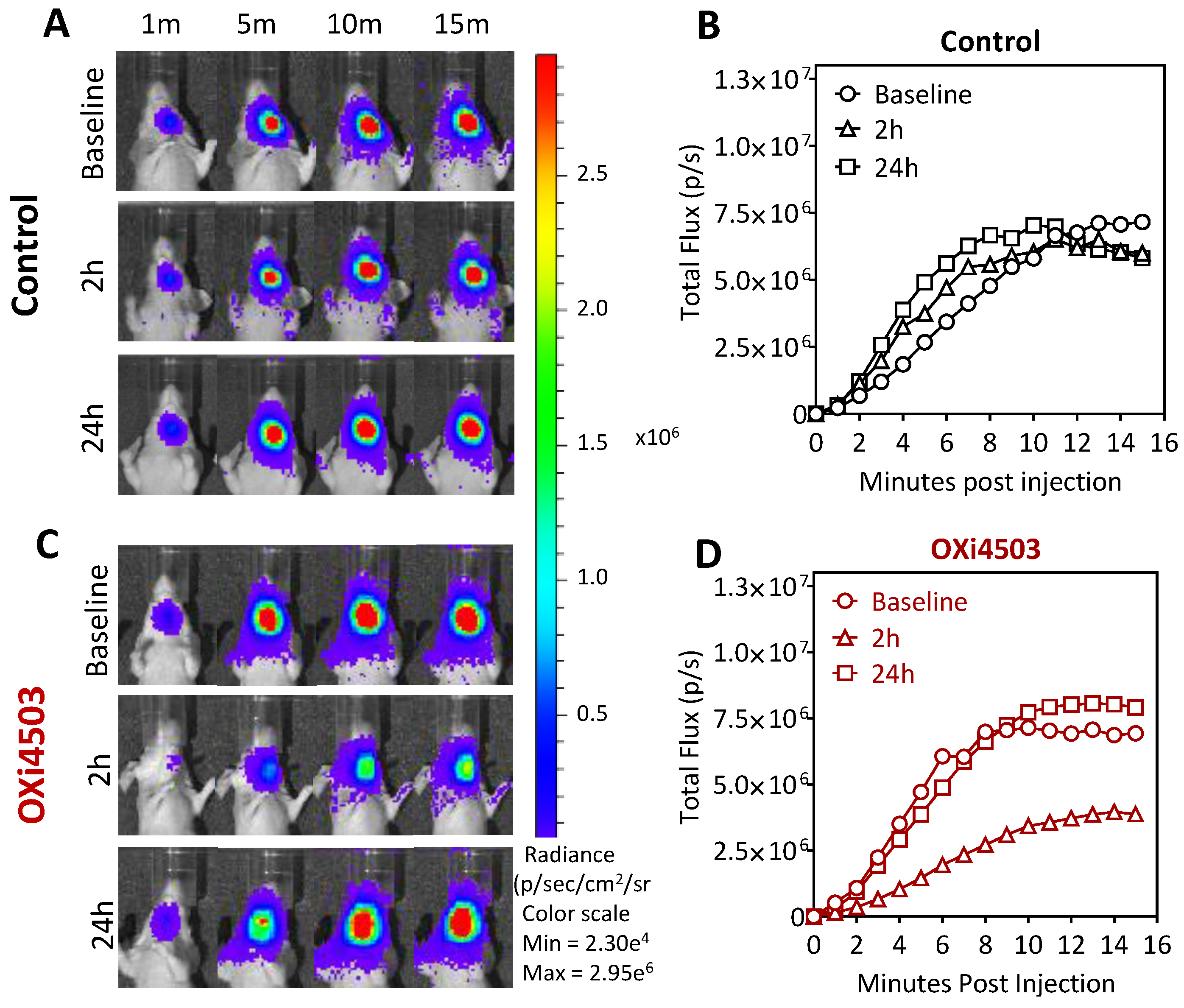
2.3. Dynamic Bioluminescence Imaging (dBLI) of Orthotopic FaDu-luc Tumor Vascular Response to OXi4503
2.4. Magnetic Resonance Imaging and Histopathologic Assessment of HNSCC Response to OXi4503

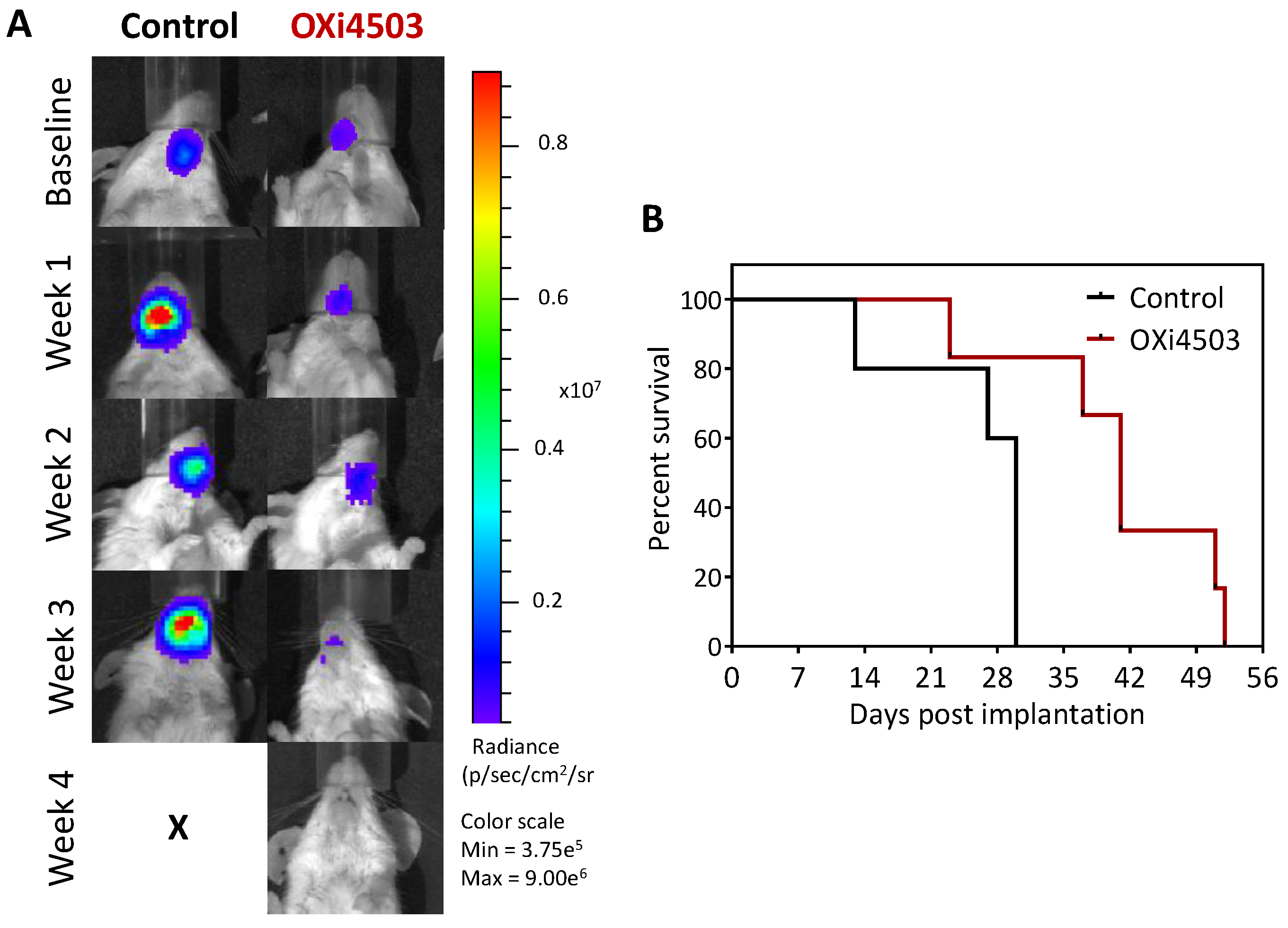
2.5. Therapeutic Efficacy of OXi4503 in the Orthotopic FaDu-luc Xenograft Model of HNSCC
3. Discussion
4. Materials and Methods
4.1. Tumor Model
4.2. Drug Treatment
4.3. Bioluminescence Imaging
4.4. Magnetic Resonance Imaging
4.5. Histopathology
4.6. Therapeutic Response Assessment
4.7. Statistical Considerations
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Patel, S.G.; Shah, J.P. TNM staging of cancers of the head and neck: Striving for uniformity among diversity. CA Cancer J. Clin. 2005, 55, 242–258. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, M. Cancer statistics. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Bernier, J.; Cooper, J.S. Chemoradiation after surgery for high-risk head and neck cancer patients: How strong is the evidence? Oncologist 2005, 10, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Cognetti, D.M.; Weber, R.S.; Lai, S.Y. Head and neck cancer: An evolving treatment paradigm. Cancer 2008, 113, 1911–1932. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, P.A.; Cunha, I.W.; Loannidis, J.P.A. Prognostic significance of vascular endothelial growth factor immunohistochemical expression in head and neck squamous cell carcinoma: A meta-analysis. Clin. Cancer Res. 2005, 11, 1434–1440. [Google Scholar] [CrossRef] [PubMed]
- Bozec, A.; Sudaka, A.; Fischel, J.L.; Brunstein, M.C.; Etienne-Grimaldi, M.C.; Milano, G. Combined effects of bevacizumab with erlotinib and irradiation: A preclinical study on a head and neck cancer orthotopic model. Br. J. Cancer 2008, 99, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Argiris, A.; Kotsakis, A.; Hoang, T.; Worden, F.P.; Savvides, P.; Gibson, M.K.; Gyanchandani, R.; Blumenschein, G.R., Jr.; Chen, H.X.; Grandis, J.R.; et al. Cetuximab and bevacizumab: preclinical data and phase II trial in recurrent or metastatic squamous cell carcinoma of the head and neck. Ann. Oncol. 2013, 24, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Machiels, J.; Henry, S.; Zanetta, S.; Kaminsky, M.C.; Michoux, N.; Rommel, D.; Schmitz, S.; Bompas, E.; Dillies, A.F.; Faivre, S.; et al. Phase II study of sunitinib in recurrent or metastatic squamous cell carcinoma of the head and neck: GORTEC 2006–01. J. Clin. Oncol. 2010, 28, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, M.; Mazurchuk, R.; Spernyak, J.A.; Bhattacharya, A.; Rustum, Y.A.; Bellnier, D.A. Activity of the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid against human head and neck carcinoma xenografts. Neoplasia 2006, 8, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.D.; Dougherty, G.L.; Blakey, D.C.; Galbraith, S.M.; Tozer, G.M.; Holder, A.L.; Naylor, M.A.; Nolan, J.; Stratford, M.R.; Chaplin, D.J.; et al. ZD6126 A novel vascular-targeting agent that causes selective destruction of tumor vasculature. Cancer Res. 2002, 62, 7247–7253. [Google Scholar] [PubMed]
- Clemenson, C.; Jouannot, E.; Merino-Trigo, A.; Rubin-Carrez, C.; Deutsch, E. The vascular disrupting agent ombrabulin (AVE8062) enhances the efficacy of standard therapies in head and neck squamous cell carcinoma xenograft models. Investig. New Drugs 2013, 31, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Tozer, G.M.; Prise, V.E.; Wilson, J.; Locke, R.J.; Vojnovic, B.; Stratford, M.R.L.; Dennis, M.F.; Chaplin, D.J. Combretastatin A-4 phosphate as a tumor vascular-targeting agent: Early effects in tumors and normal tissues. Cancer Res. 1999, 59, 1626–1634. [Google Scholar] [PubMed]
- Kanthou, C.; Tozer, G.M. The tumor vascular targeting agent combretastatin A-4-phosphate induces reorganization of the actin cytoskeleton and early membrane blebbing in human endothelial cells. Blood 2002, 99, 2060–2069. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Sheng, Y.; Pinney, K.G.; Garner, C.M.; Kane, R.R.; Prezioso, J.A.; Pettit, G.R.; Chaplin, D.J.; Edvardsen, K. Oxi4503, a novel vascular targeting agent: effects on blood flow and antitumor activity in comparison to combretastatin A-4 phosphate. Anticancer Res. 2003, 23, 1433–1440. [Google Scholar] [PubMed]
- Chan, L.S.; Macontenti-Wilson, C.; Muralidharan, V.; Christophi, C. Effect of vascular targeting agent OXi4503 on tumor cell kinetics in a mouse model of colorectal liver metastasis. Anticancer Res. 2007, 27, 2317–2324. [Google Scholar] [PubMed]
- Siemann, D.W.; Shi, W. Dual targeting of tumor vasculature: combining avastin and vascular disrupting agents (CA4P or OXi4503). Anticancer Res. 2008, 28, 2027–2031. [Google Scholar] [PubMed]
- Benezra, M.; Phillips, E.; Tilki, D.; Ding, B.S.; Butler, J.; Dobrenkov, K.; Siim, B.; Chaplin, D.; Rafii, S.; Rabbany, S.; Bradbury, M.S. Serial monitoring of human systemic and xenograft models of leukemia using a novel vascular disrupting agent. Leukemia 2012, 26, 1771–1778. [Google Scholar] [CrossRef] [PubMed]
- Clémenson, C.; Chargari, C.; Deutsch, E. Combination of vascular disrupting agents and ionizing radiation. Crit. Rev. Oncol. Hematol. 2013, 86, 143–160. [Google Scholar]
- Sun, A.; Hou, L.; Prugpichailers, T.; Dunkel, J.; Kalani, M.A.; Chen, X.; Kalani, M.Y.; Tse, V. Firefly luciferase-based dynamic bioluminescence imaging: A noninvasive technique to assess tumor angiogenesis. Neurosurgery 2010, 66, 751–777. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Richer, E.; Antich, P.P.; Mason, R.P. Antivascular effects of combretastatin A4 phosphate in breast cancer xenograft assessed using dynamic bioluminescence imaging and confirmed by MRI. FASEB 2008, 22, 2445–2451. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, K.; Alhasan, L.; Liu, L.; Lewis, M.A.; Magnusson, J.; Mason, R.P. Comparison of optical and power Doppler ultrasound imaging for non-invasive evaluation of arsenic trioxide as a vascular disrupting agent in tumors. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Liu, L.; Mason, R.P.; Gimi, B. Dynamic and fluorescence imaging of the effects of the antivascular agent Combretastatin-A4P (CA4P) on brain tumor xenografts. Cancer Lett. 2015, 356, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, M.; Spernyak, J.A.; Maiery, P.G.; Cheney, R.T.; Mazurchuk, R.; Bellnier, D.A. Visualizing the acute effects of vascular-targeted therapy in vivo using intravital microscopy and magnetic resonance imaging: Correlation with endothelial apoptosis, cytokine induction and treatment outcome. Neoplasia 2007, 9, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Folaron, M.; Kalmuk, J.; Lockwood, J.; Frangou, C.; Vokes, J.; Turowski, S.G.; Merzianu, M.; Rigual, N.R.; Sullivan-Nasca, M.; Kuriakose, M.A.; et al. Vascular priming enhances chemotherapeutic efficacy against head and neck cancer. Oral Oncol. 2013, 49, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, M.; Spernyak, J.A.; Mazurchuk, R.; Camacho, S.H.; Cheney, R.T.; Bellnier, D.A. Tumor vascular response to photodynamic therapy and the anti-vascular agent 5,6-dimethylxanthenone-4-acetic acid: Implications for combination therapy. Clin. Cancer Res. 2005, 11, 4241–4250. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.A.; Tozer, G.M.; Pettit, G.R.; Chaplin, D.J. Preclinical evaluation of the antitumor activity of the novel vascular targeting agent OXi4503. Anticancer Res. 2002, 22, 1453–1458. [Google Scholar] [PubMed]
- Salmon, H.; Siemenn, D.W. Effect of the second-generation vascular disrupting agent OXi4503 on tumor vascularity. Clin. Cancer Res. 2006, 12, 4090–4094. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Hua, J.; Pinney, K.G.; Garner, C.M.; Kane, R.R.; Prezioso, J.A.; Chaplin, D.J.; Edvardsen, K. Combretastatin family member OXi4503 induces vascular collapse through the induction of endothelial apoptosis. Int. J. Cancer 2004, 11, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Madlambayan, G.J.; Meacham, A.M.; Hosaka, K.; Mir, S.; Jorgensen, M.; Scott, E.W.; Siemann, D.W.; Cogle, C.R. Leukemia regression by vascular disruption and antiangiogenic therapy. Blood 2010, 116, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Rice, L.; Pampo, C.; Lepler, S.; Rojiani, A.M.; Siemann, D.W. Support of a free radical mechanism for enhanced antitumor efficacy of the microtubule disruptor OXi4503. Microvasc. Res 2011, 81, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Malcontenti-Wilson, C.; Chan, L.; Nikfarjam, M.; Muralidharan, V.; Christophi, C. Vascular targeting agent Oxi4503 inhibits tumor growth in a colorectal liver metastases model. J. Gastroenterol. Hepatol. 2008, 23, e96–e104. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Ansari, R.; Bustin, F.; Flynn, P.; Otterson, G.A.; Vlahovic, G.; Soh, C.H.; O’Connor, P.; Hainsworth, J. Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small cell lung cancer after failure of standard first-line chemotherapy (BeTa): A double-blind, placebo-controlled phase 3 trial. Lancet 2011, 377, 1846–1854. [Google Scholar] [CrossRef]
- Lara, P.N., Jr.; Douillard, J.Y.; Nakagawa, K.; von Pawel, J.; McKeage, M.J.; Albert, I.; Losonczy, G.; Reck, M.; Heo, D.S.; Fan, X.; et al. Randomized phase III placebo-controlled trial of carboplatin and paclitaxel with or without the vascular disrupting agent vadimezan (ASA404) in advanced non-small-cell lung cancer. J. Clin. Oncol. 2010, 29, 2965–2971. [Google Scholar] [CrossRef] [PubMed]
- Sosa, J.A.; Elisei, R.; Jarzab, B.; Bal, C.S.; Koussis, H.; Gramza, A.W.; Ben-Yosef, R.; Gitilitz, B.J.; Haugen, B.; Karandikar, S.M.; et al. A randomized phase II/III trial of a tumor vascular disrupting agent fosbretabulin tromethamine (CA4P) with carboplatin (C) and paclitaxel (P) in anaplastic thyroid cancer (ATC): Final survival analysis for the FACT trial. J. Clin. Oncol. 2011, 29, 5502. [Google Scholar]
- A Phase I/II Trial of Crolibulin (EPC2407) Plus Cisplatin in Adults with Solid Tumors With a Focus on Anaplastic Thyroid Cancer (ATC). In: ClinicalTrials.gov [Internet].Bethesda (MD): National Library of Medicine (US). 2000. Available online: https://clinicaltrials.gov/ct2/show/NCT01240590 (accessed on 23 October 2015).
- Shaked, Y.; Ciarrocchi, A.; Franco, M.; Lee, C.R.; Man, S.; Cheung, A.M.; Hicklin, D.J.; Chaplin, D.; Foster, F.S.; Benezra, R.; et al. Therapy-induced recruitment of circulating endothelial progenitor cells to tumors. Science 2006, 313, 1785–1787. [Google Scholar] [CrossRef] [PubMed]
- Fifis, T.; Nguyen, L.; Macontenti-Wilson, C.; Chan, L.S.; Nunes Costa, P.L.; Daruwalla, J.; Nikfarjam, M.; Muralidharan, V.; Waltham, M.; Thompson, E.W.; et al. Treatment with the vascular disruptive agent OXi4503 induces an immediate and widespread epithelial to mesenchymal transition in the surviving tumor. Cancer Med. 2013, 2, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Siemann, D.W.; Mercer, E.; Lepler, S.; Rojiani, A.M. Vascular targeting agents enhance chemotherapeutic agent activities in solid tumor therapy. Int. J. Cancer 2002, 99, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Horsman, M.R.; Siemann, D.W. Pathophysiological effects of vascular targeting agents and the implications for combination with conventional therapies. Cancer Res. 2006, 66, 11520–11539. [Google Scholar] [CrossRef] [PubMed]
- Del Conte, G.; Bahleda, R.; Moreno, V.; Damian, S.; Perotti, A.; Lassau, N.; Farace, F.; Ong, M.; Stimpson, S.J.; Tunariu, N.; et al. A phase I study of ombrabulin (O) combined with bevacizumab (B) in patients with advanced solid tumors. In Proceedings of the ASCO Annual Meeting, Chicago, IL, USA, 4 June 2013.
- Martinelli, M.; Bonezzi, K.; Riccardi, E.; Kuhn, E.; Frapolli, R.; Zucchetti, M.; Ryan, A.J.; Taraboletti, G.; Giavazzi, R. Sequence dependent antitumour efficacy of the vascular disrupting agent ZD6126 in combination with paclitaxel. Br. J. Cancer 2007, 97, 888–894. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marysael, T.; Ni, Y.; Lerut, E.; de Witte, P. Influence of the vascular damaging agents DMXAA and ZD6126 on hypericin distribution and accumulation in RIF-1 tumors. J. Cancer Res. Clin. Oncol. 2011, 137, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.S.; Pili, R.; Seshadri, M. Modulation of chemotherapeutic efficacy by vascular disrupting agents: Optimizing the sequence and the schedule. J. Clin. Oncol. 2012, 30, 760–761. [Google Scholar] [CrossRef] [PubMed]
- Bahleda, R.; Sessa, C.; Del Conte, G.; Gianni, L.; Capri, G.; Varga, A.; Oprea, C.; Daglish, B.; Hospitel, M.; Soria, J.C. Phase I clinical and pharmacokinetic study of ombrabulin (AVE8062) combined with cisplatin/docetaxel or carboplatin/paclitaxel in patients with advanced solid tumors. Investig. New Drugs 2014, 32, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bothwell, K.D.; Folaron, M.; Seshadri, M. Preclinical Activity of the Vascular Disrupting Agent OXi4503 against Head and Neck Cancer. Cancers 2016, 8, 11. https://doi.org/10.3390/cancers8010011
Bothwell KD, Folaron M, Seshadri M. Preclinical Activity of the Vascular Disrupting Agent OXi4503 against Head and Neck Cancer. Cancers. 2016; 8(1):11. https://doi.org/10.3390/cancers8010011
Chicago/Turabian StyleBothwell, Katelyn D., Margaret Folaron, and Mukund Seshadri. 2016. "Preclinical Activity of the Vascular Disrupting Agent OXi4503 against Head and Neck Cancer" Cancers 8, no. 1: 11. https://doi.org/10.3390/cancers8010011
APA StyleBothwell, K. D., Folaron, M., & Seshadri, M. (2016). Preclinical Activity of the Vascular Disrupting Agent OXi4503 against Head and Neck Cancer. Cancers, 8(1), 11. https://doi.org/10.3390/cancers8010011





