Postoperative Radiation Therapy for Non-Small Cell Lung Cancer and Thymic Malignancies
Abstract
:1. Introduction
2. Non-Small Cell Lung Cancer
2.1. Indications and Results—Surgical Margins
2.2. Indications and Results—Stage and Nodal Status
| Author/Name of Study | Year | No. of Patients | Inclusion Criteria | Survival Outcomes | Conclusions |
|---|---|---|---|---|---|
| Lung Cancer Study Group [7] (4) | 1986 | 230 (110 with PORT) | Stage I–III | 3 vs. 41% LRR with PORT (p = 0.001) 40 vs. 40% 5-year OS with PORT (p = NS) | PORT improved recurrence rate, no effect on OS. |
| Dautzenberg et al. [8] (5) | 1999 | 728 (373 with PORT) | Stage I–III | 5-year OS 30% vs. 43% with PORT (p = 0.002) Intercurrent death 31 vs. 8% with PORT | PORT detrimental in survival. |
| Douilliard et al. [9] (ANITA) (6) | 2008 | 840 (232 with PORT) | I B–III A | MS–N1 Obs—50 vs. 26 months N1 Chemo—47 vs. 94 months N2 Obs—47 vs. 24 months N2 Chemo—23 vs. 13 months | Positive effect of PORT in pN2 disease and pN1 disease without chemotherapy. |
| Feng et al. [10] (7) | 2000 | 366 (183 with PORT) | N1 and N2 | 13 vs. 33% thoracic failure with PORT (p < 0.01) 5-year OS 43 vs. 41% (p = NS) | PORT improved LRR but no impact on survival. |
| Mayer et al. [11] (8) | 1997 | 155 (83 with PORT) | pT1–T3 pN0–N2 | 27 vs. 16% 5-year DFS with PORT (p = 0.07) 30 vs. 20% 5-year OS with PORT (p > 0.05) | PORT improved recurrence rate, no effect on OS. |
| Trodella et al. [12] (9) | 2002 | 104 (51 with PORT) | Stage I | 2 vs. 23% local recurrence with PORT. 71 vs. 60% 5-year DFS with PORT (p = 0.039). 67 vs. 58% OS with PORT (p = 0.048) | Improvement in local control with PORT, “promising trend” in 5-year OS and DFS. |
2.3. Indications and Results—Radiation Therapy vs. Chemoradiation Postoperatively and Sequencing of Chemotherapy and Radiation Therapy
2.4. Radiation Technique, Dose, and Fields

2.5. Postoperative Radiation Therapy—Superior Sulcus Tumors
| Outcome Variables | Preoperative Concurrent Chemoradiation | Postoperative Concurrent Chemoradiation Current Study | |
|---|---|---|---|
| SWOG 94-16 | JCOG 9806 | ||
| Adherence to treatment regimen | 75% * | 76% | 78% |
| Operative mortality rates | 2.4% | 3.5% | 0 |
| R0/R1 resection rates | 92% ** | 95% | 100% |
| Locoregional control rates | 85% *** | 87% † | 76% |
| 5-year overall survival rates | 44% | 56% | 50% |
3. Thymoma
3.1. Indications and Results
3.2. Radiation Dose and Fields
| Author/Name of Study | Year | No. of Patients | Masaoka Stage | Median (Range) RT Dose | Survival Outcomes | Conclusions |
|---|---|---|---|---|---|---|
| Berman et al. [53] (45) | 2011 | 175 | II | 50.4 Gy (not reported) | Local recurrence rate 0% with PORT, 8% without PORT (p = 0.15) | PORT not beneficial in controlling local recurrence in Stage II disease. |
| Chang et al. [54] (46) | 2011 | 76 | II and III | 50 Gy (43.2–66 Gy) | 5-year DFS—98% with PORT, 80% without PORT 10-year DFS—93% with PORT, 70% without PORT (p = 0.043) | PORT beneficial in prolonging time to disease recurrence in Stage II and III thymoma. |
| Curran et al. [55] (47) | 1988 | 57 | II and III | 50 Gy (32–60 Gy) | 5-year DFS—100% with PORT, 45% with PORT (p = 0.12). | Nonsignificant trend towards improvement in DFS with PORT. |
| Forquer et al. [56] (48) | 2010 | 901 | Local and Regional Disease (SEER) | Not reported | Localized—5-year DSS 91% with PORT, 98% without PORT (p = 0.03). No difference in OS (p > 0.05) Regional—5-year OS 76% with PORT vs. 66% without PORT (p = 0.01). No difference in CSS. | PORT not beneficial in localized disease, may be beneficial in regional disease. |
| Kondo et al. [57] (49) | 2003 | 1,320 | II and III | Median not reported (<40–53.8 Gy) | II—Local recurrence 0% with PORT, 1.6% without PORT (p > 0.05) III—5.1% with PORT, 3.1% without PORT (p > 0.05) | PORT not beneficial in completely resected stage II or III disease. |
| Rena et al. [58] (50) | 2007 | 197 | II | Median not reported (45–54 Gy) | Five intrathoracic recurrences total, 3 with PORT and II without PORT (p = 0.432) | PORT not beneficial in stage II disease. |
| Utsumi et al. [59] (51) | 2009 | 159 | II and III | 40 Gy (10–50 Gy) | II—100% DSS in all patients. III—88% DSS with PORT, 85% without PORT (p > 0.05). | PORT not beneficial in stage II or III disease. |
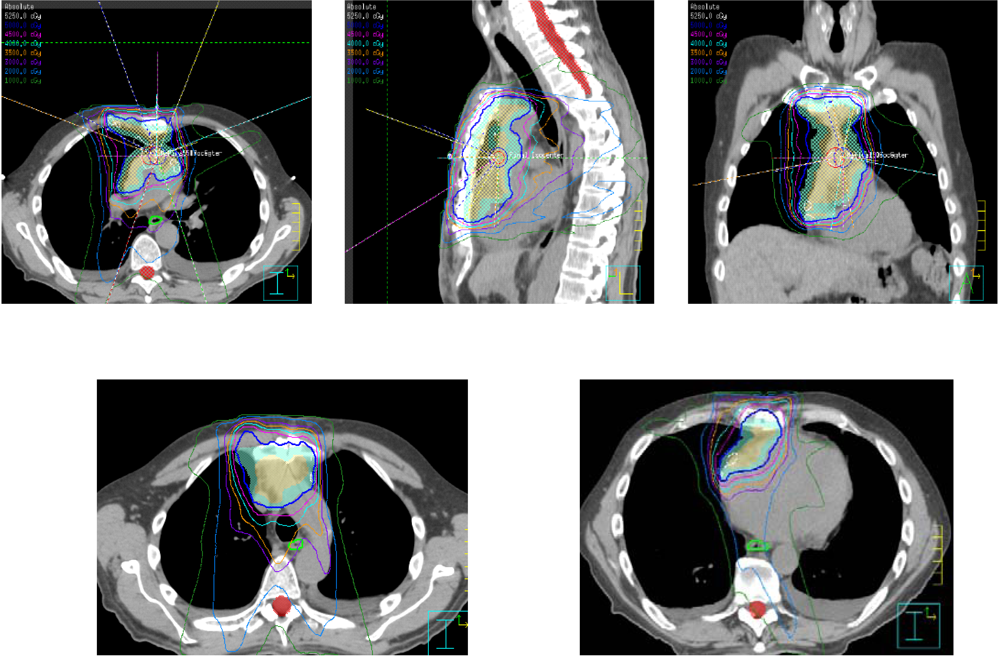
4. Conclusions
Acknowledgements
References
- Lee, J.H.; Machtay, M.; Kaiser, L.R.; Friedberg, J.S.; Hahn, S.M.; McKenna, M.G.; McKenna, W.G. Non-small cell lung cancer: Prognostic factors in patients treated with surgery and postoperative radiation therapy. Radiology 1999, 213, 845–852. [Google Scholar]
- Lee, S.W.; Choi, E.K.; Chung, W.K.; Shin, K.H.; Ahn, S.D.; Kim, J.H.; Kim, S.W.; Suh, C.; Lee, J.S.; Kim, W.S.; et al. Postoperative adjuvant chemotherapy and radiotherapy for stage II and III non-small cell lung cancer (NSCLC). Lung Cancer 2002, 37, 65–71. [Google Scholar] [CrossRef]
- Rodrigus, P. The impact of surgical adjuvant thoracic radiation for different stages of non-small cell lung cancer: The experience from a single institution. Lung Cancer 1999, 23, 11–17. [Google Scholar] [CrossRef]
- El-Sherif, A.; Fernando, H.C.; Santos, R.; Pettiford, B.; Luketich, J.D.; Close, J.M.; Landreneau, R.J. Margin and local recurrence after sublobar resection of non-small cell lung cancer. Ann. Surg. Oncol. 2007, 14, 2400–2405. [Google Scholar] [CrossRef]
- Sawabata, N.; Maeda, H.; Matsumura, A.; Ohta, M.; Okumura, M. Clinical implications of the margin cytology findings and margin/tumor size ratio in patients who underwent pulmonary excision for peripheral non-small cell lung cancer. Surg. Today 2012, 42, 238–244. [Google Scholar] [CrossRef]
- Tomaszek, S.C.; Kim, Y.; Cassivi, S.D.; Jensen, M.R.; Shen, K.H.; Nichols, F.C.; Deschamps, C.; Wigle, D.A. Bronchial resection margin length and clinical outcome in non-small cell lung cancer. Eur. J. Cardiothorac. Surg. 2011, 40, 1151–1156. [Google Scholar]
- The Lung Cancer Study Group. Effects of postoperative mediastinal radiation on completely resected stage II and stage III epidermoid cancer of the lung. N. Engl. J. Med. 1986, 315, 1377–1381. [CrossRef]
- Dautzenberg, B.; Arriagada, R.; Chammard, A.B.; Jarema, A.; Mezzetti, M.; Mattson, K.; Lagrange, J.L.; Le Pechoux, C.; Lebeau, B.; Chastang, C. A controlled study of postoperative radiotherapy for patients with completely resected nonsmall cell lung carcinoma. Groupe d'Etude et de Traitement des Cancers Bronchiques. Cancer 1999, 86, 265–273. [Google Scholar]
- Douillard, J.Y.; Rosell, R.; de Lena, M.; Riggi, M.; Hurteloup, P.; Mahe, M.A. Impact of postoperative radiation therapy on survival in patients with complete resection and stage I, II, or IIIA non-small-cell lung cancer treated with adjuvant chemotherapy: The adjuvant Navelbine International Trialist Association (ANITA) Randomized Trial. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 695–701. [Google Scholar]
- Feng, Q.F.; Wang, M.; Wang, L.J.; Yang, Z.Y.; Zhang, Y.G.; Zhang, D.W.; Yin, W.B. A study of postoperative radiotherapy in patients with non-small-cell lung cancer: A randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 925–929. [Google Scholar] [CrossRef]
- Mayer, R.; Smolle-Juettner, F.M.; Szolar, D.; Stuecklschweiger, G.F.; Quehenberger, F.; Friehs, G.; Hackl, A. Postoperative radiotherapy in radically resected non-small cell lung cancer. Chest 1997, 112, 954–959. [Google Scholar]
- Trodella, L.; Granone, P.; Valente, S.; Valentini, V.; Balducci, M.; Mantini, G.; Turriziani, A.; Margaritora, S.; Cesario, A.; Ramella, S.; et al. Adjuvant radiotherapy in non-small cell lung cancer with pathological stage I: Definitive results of a phase III randomized trial. Radiother. Oncol. 2002, 62, 11–19. [Google Scholar] [CrossRef]
- Lally, B.E.; Zelterman, D.; Colasanto, J.M.; Haffty, B.G.; Detterbeck, F.C.; Wilson, L.D. Postoperative radiotherapy for stage II or III non-small-cell lung cancer using the surveillance, epidemiology, and end results database. J. Clin. Oncol. 2006, 24, 2998–3006. [Google Scholar]
- PORT Meta-analysis Trialists Group. Postoperative radiotherapy in non-small-cell lung cancer: Systematic review and meta-analysis of individual patient data from nine randomised controlled trials. Lancet 1998, 352, 257–263.
- Burdett, S.; Stewart, L. Postoperative radiotherapy in non-small-cell lung cancer: Update of an individual patient data meta-analysis. Lung Cancer 2005, 47, 81–83. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network Guidelines. Available online: http://www.nccn.org/ (accessed on 3 January 2012).
- Le Pechoux, C.; Dunant, A.; Pignon, J.P.; de Ruysscher, D.; Mornex, F.; Senan, S.; Casas, F.; Price, A.; Milleron, B. Need for a new trial to evaluate adjuvant postoperative radiotherapy in non-small-cell lung cancer patients with N2 mediastinal involvement. J. Clin. Oncol. 2007, 25, e10–e11. [Google Scholar]
- Matsuguma, H.; Nakahara, R.; Ishikawa, Y.; Suzuki, H.; Inoue, K.; Katano, S.; Yokoi, K. Postoperative radiotherapy for patients with completely resected pathological stage IIIA-N2 non-small cell lung cancer: Focusing on an effect of the number of mediastinal lymph node stations involved. Interact. Cardiovasc. Thorac. Surg. 2008, 7, 573–577. [Google Scholar] [CrossRef]
- Saji, H.; Tsuboi, M.; Yoshida, K.; Kato, Y.; Nomura, M.; Matsubayashi, J.; Nagao, T.; Kakihana, M.; Usuda, J.; Kajiwara, N.; et al. Prognostic impact of number of resected and involved lymph nodes at complete resection on survival in non-small cell lung cancer. J. Thorac. Oncol. 2011, 6, 1865–1871. [Google Scholar] [CrossRef]
- Moretti, L.; Yu, D.S.; Chen, H.; Carbone, D.P.; Johnson, D.H.; Keedy, V.L.; Putnam, J.B., Jr.; Sandler, A.B.; Shyr, Y.; Lu, B. Prognostic factors for resected non-small cell lung cancer with pN2 status: Implications for use of postoperative radiotherapy. Oncologist 2009, 14, 1106–1115. [Google Scholar] [CrossRef]
- Keller, S.M.; Adak, S.; Wagner, H.; Herskovic, A.; Komaki, R.; Brooks, B.J.; Perry, M.C.; Livingston, R.B.; Johnson, D.H. A randomized trial of postoperative adjuvant therapy in patients with completely resected stage II or IIIA non-small-cell lung cancer. Eastern Cooperative Oncology Group. N. Engl. J. Med. 2000, 343, 1217–1222. [Google Scholar] [CrossRef]
- Bradley, J.D.; Paulus, R.; Graham, M.V.; Ettinger, D.S.; Johnstone, D.W.; Pilepich, M.V.; Machtay, M.; Komaki, R.; Atkins, J.; Curran, W.J. Phase II trial of postoperative adjuvant paclitaxel/carboplatin and thoracic radiotherapy in resected stage II and IIIA non-small-cell lung cancer: Promising long-term results of the Radiation Therapy Oncology Group—RTOG 9705. J. Clin. Oncol. 2005, 23, 3480–3487. [Google Scholar]
- Feigenberg, S.J.; Hanlon, A.L.; Langer, C.; Goldberg, M.; Nicolaou, N.; Millenson, M.; Coia, L.R.; Lanciano, R.; Movsas, B. A phase II study of concurrent carboplatin and paclitaxel and thoracic radiotherapy for completely resected stage II and IIIA non-small cell lung cancer. J. Thorac. Oncol. 2007, 2, 287–292. [Google Scholar]
- Arriagada, R.; Auperin, A.; Burdett, S.; Higgins, J.P.; Johnson, D.H.; Le Chevalier, T.; Le Pechoux, C.; Parmar, M.K.; Pignon, J.P.; Souhami, R.L.; et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: Two meta-analyses of individual patient data. Lancet 2010, 375, 1267–1277. [Google Scholar]
- Decker, R.H.; Langer, C.J.; Rosenzweig, K.E.; Chang, J.Y.; Gewanter, R.M.; Ginsburg, M.E.; Kong, F.M.; Lally, B.E.; Videtic, G.M.; Movsas, B. ACR Appropriateness Criteria® postoperative adjuvant therapy in non-small cell lung cancer. Am. J. Clin. Oncol. 2011, 34, 537–544. [Google Scholar] [CrossRef]
- Gagliardi, G.; Constine, L.S.; Moiseenko, V.; Correa, C.; Pierce, L.J.; Allen, A.M.; Marks, L.B. Radiation dose-volume effects in the heart. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S77–S85. [Google Scholar]
- Jackson, A.; Marks, L.B.; Bentzen, S.M.; Eisbruch, A.; Yorke, E.D.; Ten Haken, R.K.; Constine, L.S.; Deasy, J.O. The lessons of QUANTEC: Recommendations for reporting and gathering data on dose-volume dependencies of treatment outcome. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S155–S160. [Google Scholar] [CrossRef]
- Marks, L.B.; Bentzen, S.M.; Deasy, J.O.; Kong, F.M.; Bradley, J.D.; Vogelius, I.S.; El Naqa, I.; Hubbs, J.L.; Lebesque, J.V.; Timmerman, R.D.; et al. Radiation dose-volume effects in the lung. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S70–S76. [Google Scholar]
- Werner-Wasik, M.; Yorke, E.; Deasy, J.; Nam, J.; Marks, L.B. Radiation dose-volume effects in the esophagus. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S86–S93. [Google Scholar] [CrossRef]
- Chapet, O.; Kong, F.M.; Quint, L.E.; Chang, A.C.; Ten Haken, R.K.; Eisbruch, A.; Hayman, J.A. CT-based definition of thoracic lymph node stations: An atlas from the University of Michigan. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 170–178. [Google Scholar]
- AJCC, American Joint Committee on Cancer Staging Manual, 7th ed; Springer-Verlag: New York, NY, USA, 2010.
- Spoelstra, F.O.; Senan, S.; Le Pechoux, C.; Ishikura, S.; Casas, F.; Ball, D.; Price, A.; de Ruysscher, D.; van Sornsen de Koste, J.R. Variations in target volume definition for postoperative radiotherapy in stage III non-small-cell lung cancer: Analysis of an international contouring study. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 1106–1113. [Google Scholar] [CrossRef]
- Miles, E.F.; Kelsey, C.R.; Kirkpatrick, J.P.; Marks, L.B. Estimating the magnitude and field-size dependence of radiotherapy-induced mortality and tumor control after postoperative radiotherapy for non-small-cell lung cancer: Calculations from clinical trials. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 1047–1052. [Google Scholar] [CrossRef]
- Machtay, M.; Lee, J.H.; Shrager, J.B.; Kaiser, L.R.; Glatstein, E. Risk of death from intercurrent disease is not excessively increased by modern postoperative radiotherapy for high-risk resected non-small-cell lung carcinoma. J. Clin. Oncol. 2001, 19, 3912–3917. [Google Scholar]
- Lally, B.E.; Detterbeck, F.C.; Geiger, A.M.; Thomas, C.R., Jr.; Machtay, M.; Miller, A.A.; Wilson, L.D.; Oaks, T.E.; Petty, W.J.; Robbins, M.E.; et al. The risk of death from heart disease in patients with nonsmall cell lung cancer who receive postoperative radiotherapy: Analysis of the Surveillance, Epidemiology, and End Results database. Cancer 2007, 110, 911–917. [Google Scholar] [CrossRef]
- Masson-Cote, L.; Couture, C.; Fortin, A.; Dagnault, A. Postoperative radiotherapy for lung cancer: Improvement in locoregional control using three-dimensional compared with two-dimensional technique. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 686–691. [Google Scholar] [CrossRef]
- Pancoast, H.K. Superior pulmonary sulcus tumor: Tumor characterized by pain, Horner’s syndrome, destruction of the bone, and atrophy of hand muscles. JAMA 1932, 99, 1391–1396. [Google Scholar] [CrossRef]
- Rusch, V.W.; Parekh, K.R.; Leon, L.; Venkatraman, E.; Bains, M.S.; Downey, R.J.; Boland, P.; Bilsky, M.; Ginsberg, R.J. Factors determining outcome after surgical resection of T3 and T4 lung cancers of the superior sulcus. J. Thorac. Cardiovasc. Surg. 2000, 119, 1147–1153. [Google Scholar] [CrossRef]
- Rusch, V.W.; Giroux, D.J.; Kraut, M.J.; Crowley, J.; Hazuka, M.; Johnson, D.; Goldberg, M.; Detterbeck, F.; Shepherd, F.; Burkes, R.; et al. Induction chemoradiation and surgical resection for non-small cell lung carcinomas of the superior sulcus: Initial results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160). J. Thorac. Cardiovasc. Surg. 2001, 121, 472–483. [Google Scholar] [CrossRef]
- Rusch, V.W.; Giroux, D.J.; Kraut, M.J.; Crowley, J.; Hazuka, M.; Winton, T.; Johnson, D.H.; Shulman, L.; Shepherd, F.; Deschamps, C.; et al. Induction chemoradiation and surgical resection for superior sulcus non-small-cell lung carcinomas: Long-term results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160). J. Clin. Oncol. 2007, 25, 313–318. [Google Scholar]
- Favaretto, A.; Pasello, G.; Loreggian, L.; Breda, C.; Braccioni, F.; Marulli, G.; Stragliotto, S.; Magro, C.; Sotti, G.; Rea, F. Preoperative concomitant chemo-radiotherapy in superior sulcus tumour: A mono-institutional experience. Lung Cancer 2010, 68, 228–233. [Google Scholar] [CrossRef]
- Kappers, I.; Klomp, H.M.; Koolen, M.G.; Uitterhoeve, L.J.; Kloek, J.J.; Belderbos, J.S.; Burgers, J.A.; Koning, C.C. Concurrent high-dose radiotherapy with low-dose chemotherapy in patients with non-small cell lung cancer of the superior sulcus. Radiother. Oncol. 2011, 101, 278–283. [Google Scholar] [CrossRef]
- Kappers, I.; van Sandick, J.W.; Burgers, J.A.; Belderbos, J.S.; Wouters, M.W.; van Zandwijk, N.; Klomp, H.M. Results of combined modality treatment in patients with non-small-cell lung cancer of the superior sulcus and the rationale for surgical resection. Eur.J. Cardiothorac. Surg. 2009, 36, 741–746. [Google Scholar] [CrossRef]
- Kunitoh, H.; Kato, H.; Tsuboi, M.; Shibata, T.; Asamura, H.; Ichonose, Y.; Katakami, N.; Nagai, K.; Mitsudomi, T.; Matsumura, A.; et al. Phase II trial of preoperative chemoradiotherapy followed by surgical resection in patients with superior sulcus non-small-cell lung cancers: Report of Japan Clinical Oncology Group trial 9806. J. Clin. Oncol. 2008, 26, 644–649. [Google Scholar]
- Kwong, K.F.; Edelman, M.J.; Suntharalingam, M.; Cooper, L.B.; Gamliel, Z.; Burrows, W.; Hausner, P.; Doyle, L.A.; Krasna, M.J. High-dose radiotherapy in trimodality treatment of Pancoast tumors results in high pathologic complete response rates and excellent long-term survival. J. Thorac. Cardiovasc. Surg. 2005, 129, 1250–1257. [Google Scholar] [CrossRef]
- Suntharalingam, M.; Sonett, J.R.; Haas, M.L.; Doyle, L.A.; Hausner, P.F.; Schuetz, J.; Krasna, M.J. The use of concurrent chemotherapy with high-dose radiation before surgical resection in patients presenting with apical sulcus tumors. Cancer J. 2000, 6, 365–371. [Google Scholar]
- Demir, A.; Sayar, A.; Kocaturk, C.I.; Gunluoglu, M.Z.; Akyn, H.; Metin, M.; Cansever, L.; Olcmen, A.; Dincer, S.I.; Bedirhan, M.A.; et al. Surgical treatment of superior sulcus tumors: Results and prognostic factors. Thorac. Cardiovasc. Surg. 2009, 57, 96–101. [Google Scholar] [CrossRef]
- Bolton, W.D.; Rice, D.C.; Goodyear, A.; Correa, A.M.; Erasmus, J.; Hofstetter, W.; Komaki, R.; Mehran, R.; Pisters, K.; Roth, J.A.; et al. Superior sulcus tumors with vertebral body involvement: A multimodality approach. J. Thorac. Cardiovasc. Surg. 2009, 137, 1379–1387. [Google Scholar] [CrossRef]
- Baumann, M.; Herrmann, T.; Koch, R.; Matthiessen, W.; Appold, S.; Wahlers, B.; Kepka, L.; Marschke, G.; Feltl, D.; Fietkau, R.; et al. Final results of the randomized phase III CHARTWEL-trial (ARO 97-1) comparing hyperfractionated-accelerated vs. conventionally fractionated radiotherapy in non-small cell lung cancer (NSCLC). Radiother. Oncol. 2011, 100, 76–85. [Google Scholar] [CrossRef]
- Lee, J.S.; Komaki, R.; Fossella, F.V.; Glisson, B.S.; Hong, W.K.; Cox, J.D. A pilot trial of hyperfractionated thoracic radiation therapy with concurrent cisplatin and oral etoposide for locally advanced inoperable non-small-cell lung cancer: A 5-year follow-up report. Int. J. Radiat. Oncol. Biol. Phys. 1998, 42, 479–486. [Google Scholar] [CrossRef]
- Gomez, D.R.; Cox, J.D.; Roth, J.A.; Allen, P.K.; Wei, X.; Mehran, R.J.; Kim, J.Y.; Swisher, S.G.; Rice, D.C.; Komaki, R. A prospective phase 2 study of surgery followed by chemotherapy and radiation for superior sulcus tumors. Cancer 2012, 118, 444–451. [Google Scholar]
- Kim, J.H.; Kim, B.H.; Kim, Y.W.; Park, J.C.; Jung, Y.H.; Lee, B.O.; Han, Y.S.; Dong, S.H.; Kim, H.J.; Chang, Y.W.; et al. Autoimmune cholangitis in a patient with thymoma. J. Gastroenterol. Hepatol. 2004, 19, 1324–1327. [Google Scholar] [CrossRef]
- Berman, A.T.; Litzky, L.; Livolsi, V.; Singhal, S.; Kucharczuk, J.C.; Cooper, J.D.; Friedberg, J.R.; Evans, T.L.; Stevenson, J.P.; Metz, J.M.; et al. Adjuvant radiotherapy for completely resected stage 2 thymoma. Cancer 2011, 117, 3502–3508. [Google Scholar]
- Chang, J.H.; Kim, H.J.; Wu, H.G.; Kim, J.H.; Kim, Y.T. Postoperative radiotherapy for completely resected stage II or III thymoma. J. Thorac. Oncol. 2011, 6, 1282–1286. [Google Scholar] [CrossRef]
- Curran, W.J., Jr.; Kornstein, M.J.; Brooks, J.J.; Turrisi, A.T., 3rd. Invasive thymoma: The role of mediastinal irradiation following complete or incomplete surgical resection. J. Clin. Oncol. 1988, 6, 1722–1727. [Google Scholar]
- Forquer, J.A.; Rong, N.; Fakiris, A.J.; Loehrer, P.J., Sr.; Johnstone, P.A. Postoperative radiotherapy after surgical resection of thymoma: Differing roles in localized and regional disease. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 440–445. [Google Scholar] [CrossRef]
- Kondo, K.; Monden, Y. Therapy for thymic epithelial tumors: A clinical study of 1,320 patients from Japan. Ann. Thorac. Surg. 2003, 76, 878–884. [Google Scholar] [CrossRef]
- Rena, O.; Papalia, E.; Oliaro, A.; Ruffini, E.; Filosso, P.; Novero, D.; Maggi, G.; Casadio, C. Does adjuvant radiation therapy improve disease-free survival in completely resected Masaoka stage II thymoma? Eur. J. Cardiothorac. Surg. 2007, 31, 109–113. [Google Scholar] [CrossRef]
- Utsumi, T.; Shiono, H.; Kadota, Y.; Matsumura, A.; Maeda, H.; Ohta, M.; Yoshioka, Y.; Koizumi, M.; Inoue, T.; Okumura, M. Postoperative radiation therapy after complete resection of thymoma has little impact on survival. Cancer 2009, 115, 5413–5420. [Google Scholar]
- Kundel, Y.; Yellin, A.; Popovtzer, A.; Pfeffer, R.; Symon, Z.; Simansky, D.A.; Oberman, B.; Sadezki, S.; Brenner, B.; Catane, R.; et al. Adjuvant radiotherapy for thymic epithelial tumor: Treatment results and prognostic factors. Am. J. Clin. Oncol. 2007, 30, 389–394. [Google Scholar] [CrossRef]
- Sugie, C.; Shibamoto, Y.; Ikeya-Hashizume, C.; Ogino, H.; Ayakawa, S.; Tomita, N.; Baba, F.; Iwata, H.; Ito, M.; Oda, K. Invasive thymoma: Postoperative mediastinal irradiation, and low-dose entire hemithorax irradiation in patients with pleural dissemination. J.Thorac. Oncol. 2008, 3, 75–81. [Google Scholar] [CrossRef]
- Yoshida, H.; Uematsu, M.; Itami, J.; Kondo, M.; Ito, H.; Kubo, A.; Aburano, T. The role of low-dose hemithoracic radiotherapy for thoracic dissemination of thymoma. Radiat. Med. 1997, 15, 399–403. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gomez, D.R.; Komaki, R. Postoperative Radiation Therapy for Non-Small Cell Lung Cancer and Thymic Malignancies. Cancers 2012, 4, 307-322. https://doi.org/10.3390/cancers4010307
Gomez DR, Komaki R. Postoperative Radiation Therapy for Non-Small Cell Lung Cancer and Thymic Malignancies. Cancers. 2012; 4(1):307-322. https://doi.org/10.3390/cancers4010307
Chicago/Turabian StyleGomez, Daniel R., and Ritsuko Komaki. 2012. "Postoperative Radiation Therapy for Non-Small Cell Lung Cancer and Thymic Malignancies" Cancers 4, no. 1: 307-322. https://doi.org/10.3390/cancers4010307




