Effects of Combinatory In Vitro Treatment with Immune Checkpoint Inhibitors and Cytarabine on the Anti-Cancer Immune Microenvironment in De Novo AML Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients’ Description
2.2. AML Patients PBMC Culture with Immune Checkpoint Inhibitors and Cytarabine Presence
2.3. Flow Cytometric Evaluation of Changes Induced In Vitro by Immune Checkpoint Blockers, with/without Cytarabine in AML Blasts
2.4. Cytometric Analysis of AML Lymphocytes Response to In Vitro Culture with Immune Checkpoint Inhibitors with/without Cytarabine
2.5. Evaluation of Cytokines Released by AML PBMC Incubated with Immune Checkpoint Inhibitors with/without Cytarabine
2.6. RT-PCR Analysis of Immune Checkpoint Proteins and Immune Cells-Related Transcription Factors in PBMC of AML Patients
2.7. Statistical Analysis
3. Results
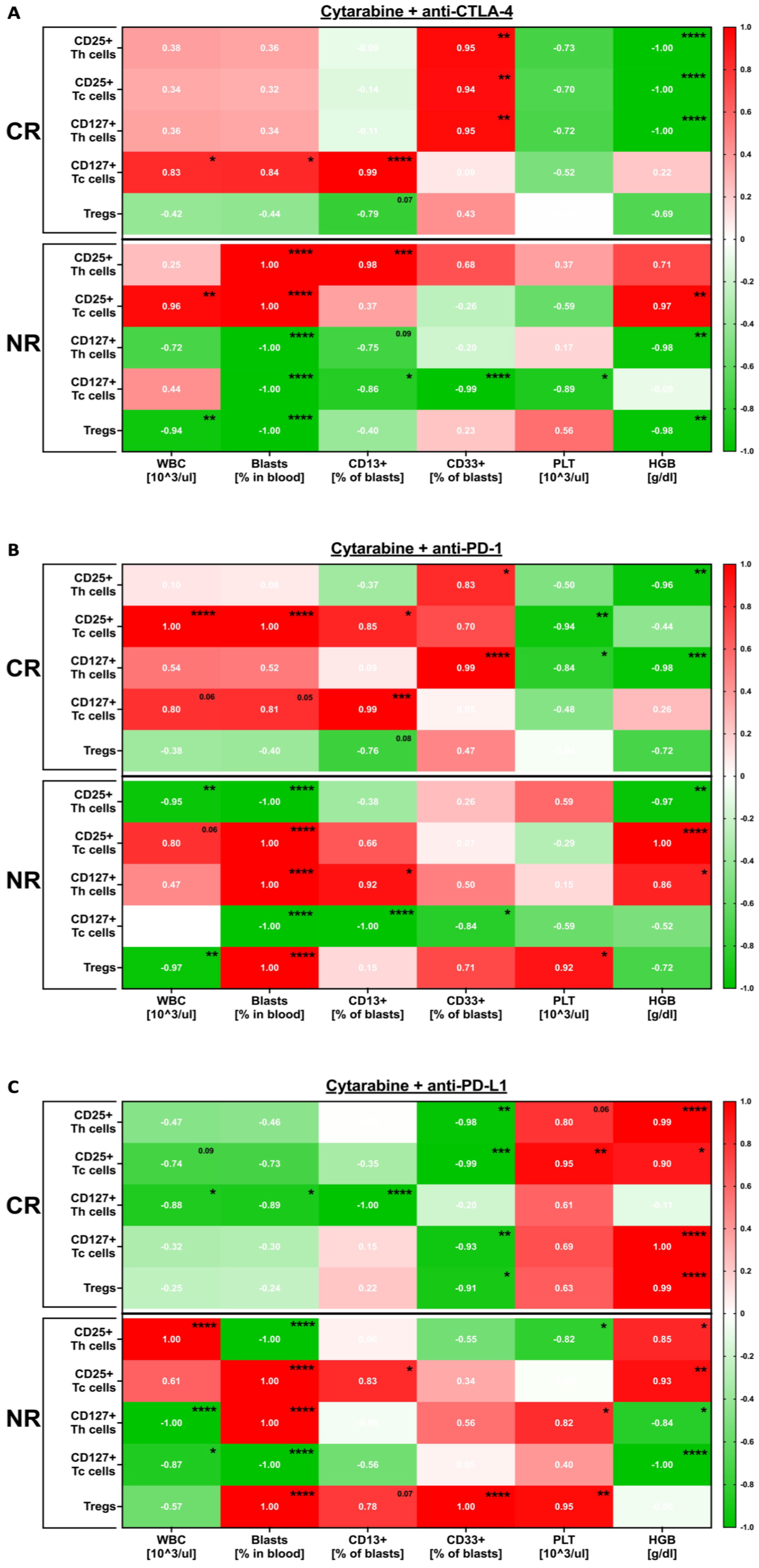
3.1. Immune-Related Changes Occurring in the Course of In Vitro Treatment of AML PBMC with Immune Checkpoint Inhibitors in the Presence of Cytarabine
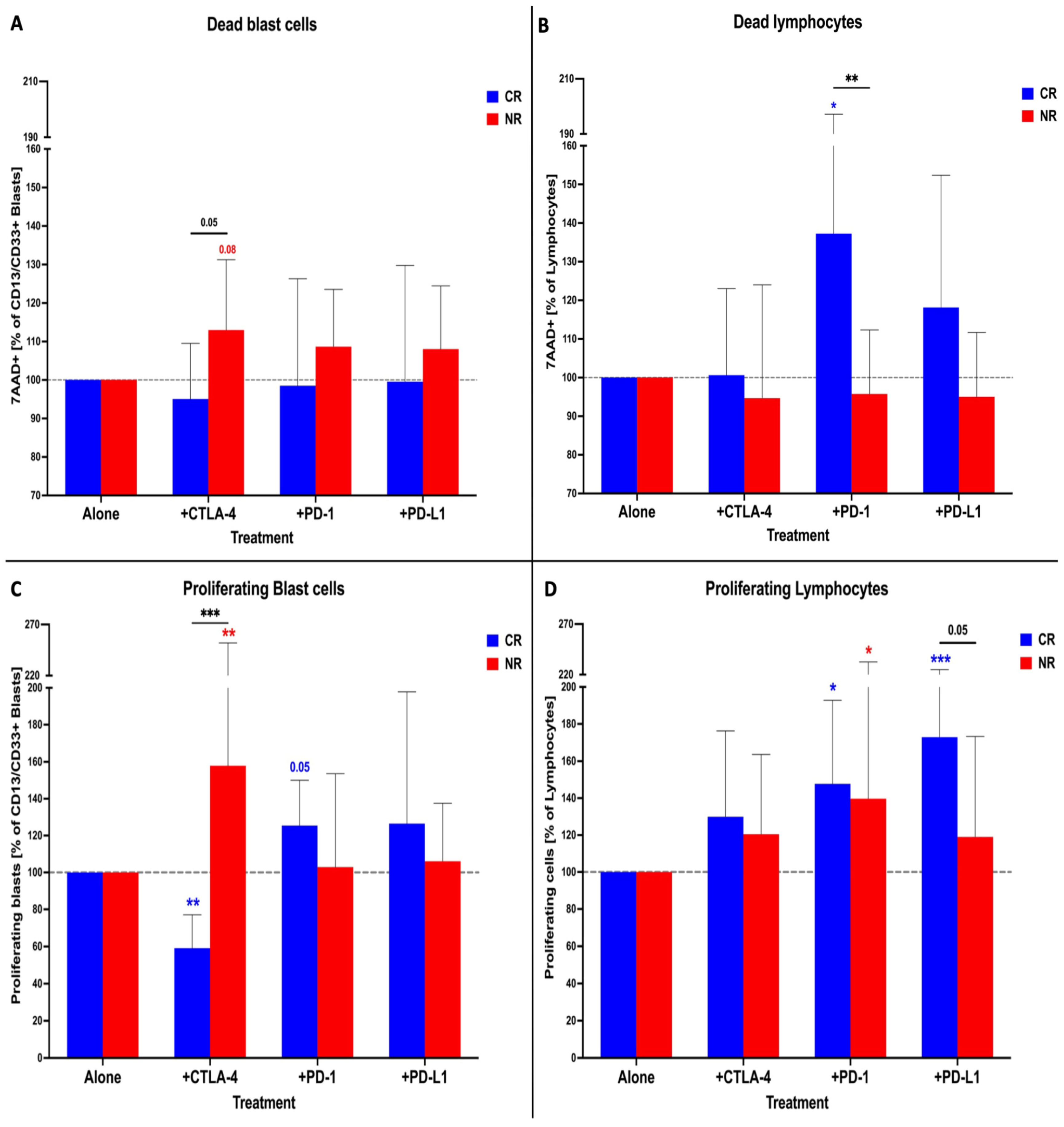
3.2. Effects Exerted In Vitro by Immune Checkpoint Inhibitors on AML Patients’ Blasts and Lymphocytes in the Presence of Cytarabine
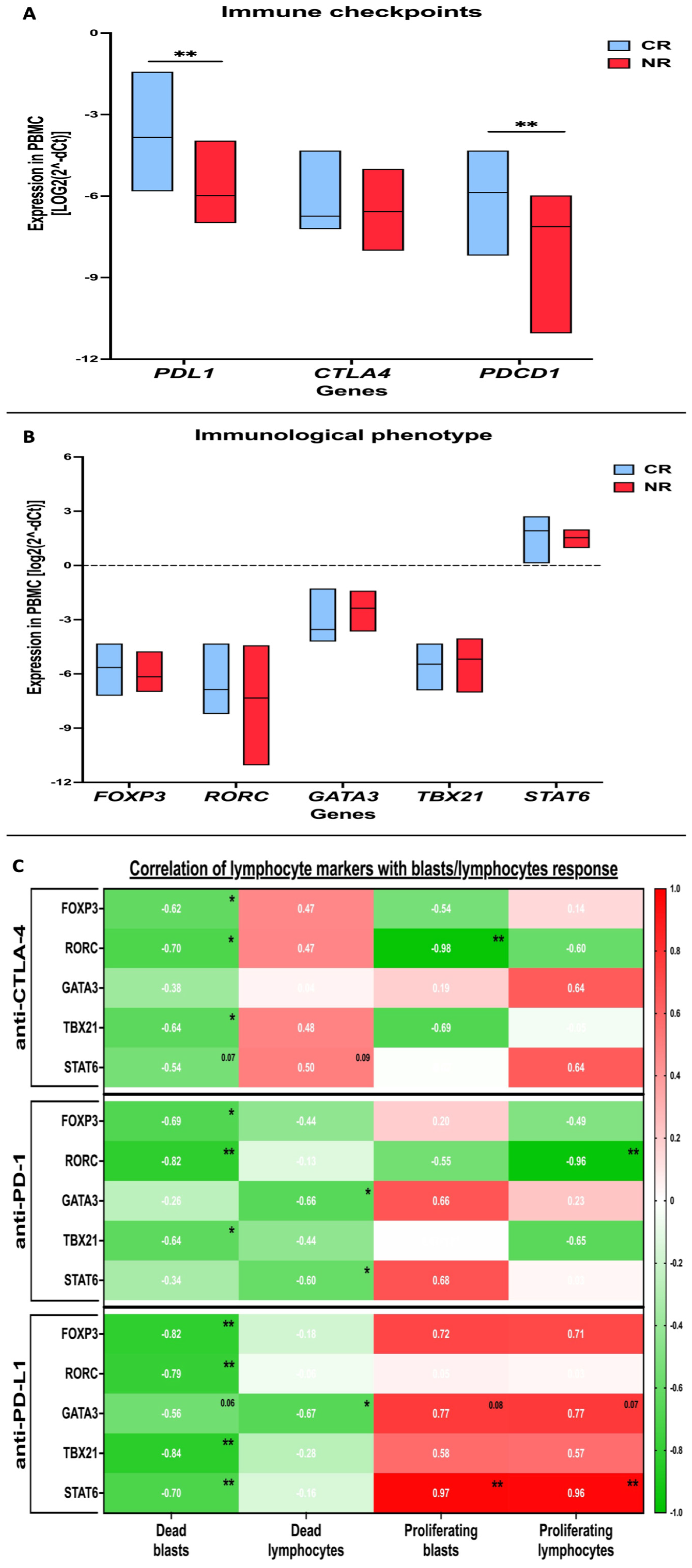
3.3. Evaluation of Selected Immune Checkpoint Proteins in AML Patients and Profiling of Immune Cells Phenotype-Related Markers
3.4. Cytokine Profile and Its Association with AML Patients’ PBMC Response to In Vitro Treatment with Immune Checkpoint Protein Inhibitors in the Presence of Cytarabine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valent, P.; Sadovnik, I.; Eisenwort, G.; Bauer, K.; Herrmann, H.; Gleixner, K.V.; Schulenburg, A.; Rabitsch, W.; Sperr, W.R.; Wolf, D. Immunotherapy-Based Targeting and Elimination of Leukemic Stem Cells in AML and CML. Int. J. Mol. Sci. 2019, 20, 4233. [Google Scholar] [CrossRef] [PubMed]
- Perna, F.; Espinoza-Gutarra, M.R.; Bombaci, G.; Farag, S.S.; Schwartz, J.E. Immune-Based Therapeutic Interventions for Acute Myeloid Leukemia. Cancer Treat Res. 2022, 183, 225–254. [Google Scholar] [PubMed]
- Swaminathan, M.; Wang, E.S. Novel therapies for AML: A round-up for clinicians. Expert Rev. Clin. Pharmacol. 2020, 13, 1389–1400. [Google Scholar] [CrossRef] [PubMed]
- Abaza, Y.; Zeidan, A.M. Immune Checkpoint Inhibition in Acute Myeloid Leukemia and Myelodysplastic Syndromes. Cells 2022, 11, 2249. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar]
- Gao, Z.; Ling, X.; Shi, C.; Wang, Y.; Lin, A. Tumor immune checkpoints and their associated inhibitors. J. Zhejiang Univ. B 2022, 23, 823–843. [Google Scholar] [CrossRef]
- Zhou, Q.; Munger, M.E.; Highfill, S.L.; Tolar, J.; Weigel, B.J.; Riddle, M.; Sharpe, A.H.; Vallera, D.A.; Azuma, M.; Levine, B.L.; et al. Program death-1 signaling and regulatory T cells collaborate to resist the function of adoptively transferred cytotoxic T lymphocytes in advanced acute myeloid leukemia. Blood 2010, 116, 2484–2493. [Google Scholar] [CrossRef]
- Williams, P.; Basu, S.; Garcia-Manero, G.; Hourigan, C.S.; Oetjen, K.A.; Cortes, J.E.; Ravandi, F.; Jabbour, E.J.; Al-Hamal, Z.; Konopleva, M.; et al. The distribution of T-cell subsets and the expression of immune checkpoint receptors and ligands in patients with newly diagnosed and relapsed acute myeloid leukemia. Cancer 2019, 125, 1470–1481. [Google Scholar] [CrossRef]
- Knaus, H.A.; Kanakry, C.G.; Luznik, L.; Gojo, I. Immunomodulatory Drugs: Immune Checkpoint Agents in Acute Leukemia. Curr. Drug Targets 2017, 18, 315–331. [Google Scholar] [CrossRef]
- Liao, D.; Wang, M.; Liao, Y.; Li, J.; Niu, T. A Review of Efficacy and Safety of Checkpoint Inhibitor for the Treatment of Acute Myeloid Leukemia. Front. Pharmacol. 2019, 10, 609. [Google Scholar] [CrossRef]
- Ghosh, A.; Barba, P.; Perales, M.A. Checkpoint inhibitors in AML: Are we there yet? Br. J. Haematol. 2020, 188, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Stahl, M.; Goldberg, A.D. Immune Checkpoint Inhibitors in Acute Myeloid Leukemia: Novel Combinations and Therapeutic Targets. Curr. Oncol. Rep. 2019, 21, 37. [Google Scholar] [CrossRef] [PubMed]
- Davids, M.S.; Kim, H.T.; Bachireddy, P.; Costello, C.; Liguori, R.; Savell, A.; Lukez, A.P.; Avigan, D.; Chen, Y.-B.; McSweeney, P.; et al. Ipilimumab for Patients with Relapse after Allogeneic Transplantation. N. Engl. J. Med. 2016, 375, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liang, C.; Wang, S.; Chio, C.L.; Zhang, Y.; Zeng, C.; Chen, S.; Wang, C.; Li, Y. Expression patterns of immune checkpoints in acute myeloid leukemia. J. Hematol. Oncol. 2020, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Whiteway, A.; Corbett, T.; Anderson, R.; Macdonald, I.; Prentice, H.G. Expression of co-stimulatory molecules on acute myeloid leukaemia blasts may effect duration of first remission. Br. J. Haematol. 2003, 120, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Hock, B.D.; McKenzie, J.L.; Patton, W.N.; Haring, L.F.; Yang, Y.; Shen, Y.; Estey, E.H.; Albitar, M. The clinical significance of soluble CD86 levels in patients with acute myeloid leukemia and myelodysplastic syndrome. Cancer 2003, 98, 1681–1688. [Google Scholar] [CrossRef] [PubMed]
- Löwenberg, B.; Pabst, T.; Vellenga, E.; van Putten, W.; Schouten, H.C.; Graux, C.; Ferrant, A.; Sonneveld, P.; Biemond, B.J.; Gratwohl, A.; et al. Cytarabine dose for acute myeloid leukemia. N. Engl. J. Med. 2011, 364, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Bradstock, K.F.; Matthews, J.P.; Lowenthal, R.M.; Baxter, H.; Catalano, J.; Brighton, T.; Gill, D.; Eliadis, P.; Joshua, D.; Cannell, P.; et al. A randomized trial of high-versus conventional-dose cytarabine in consolidation chemotherapy for adult de novo acute myeloid leukemia in first remission after induction therapy containing high-dose cytarabine. Blood 2005, 105, 481–488. [Google Scholar] [CrossRef]
- Daver, N.; Boddu, P.; Garcia-Manero, G.; Yadav, S.S.; Sharma, P.; Allison, J.; Kantarjian, H. Hypomethylating agents in combination with immune checkpoint inhibitors in acute myeloid leukemia and myelodysplastic syndromes. Leukemia 2018, 32, 1094–1105. [Google Scholar] [CrossRef]
- Heo, S.-K.; Noh, E.-K.; Yu, H.-M.; Kim, D.K.; Seo, H.J.; Lee, Y.J.; Cheon, J.; Koh, S.J.; Min, Y.J.; Choi, Y.; et al. Radotinib enhances cytarabine (Ara-C)-induced acute myeloid leukemia cell death. BMC Cancer 2020, 20, 1193. [Google Scholar] [CrossRef]
- Liu, H. Emerging agents and regimens for AML. J. Hematol. Oncol. 2021, 14, 49. [Google Scholar] [CrossRef]
- Ravandi, F.; Assi, R.; Daver, N.; Benton, C.B.; Kadia, T.; Thompson, P.A.; Borthakur, G.; Alvarado, Y.; Jabbour, E.J.; Konopleva, M.; et al. Idarubicin, cytarabine, and nivolumab in patients with newly diagnosed acute myeloid leukaemia or high-risk myelodysplastic syndrome: A single-arm, phase 2 study. Lancet Haematol. 2019, 6, e480–e488. [Google Scholar] [CrossRef]
- Zeidner, J.F.; Vincent, B.G.; Ivanova, A.; Moore, D.; McKinnon, K.P.; Wilkinson, A.D.; Mukhopadhyay, R.; Mazziotta, F.; Knaus, H.A.; Foster, M.C.; et al. Phase II Trial of Pembrolizumab after High-Dose Cytarabine in Relapsed/Refractory Acute Myeloid Leukemia. Blood Cancer Discov. 2021, 2, 616–629. [Google Scholar] [CrossRef]
- Vago, L.; Gojo, I. Immune escape and immunotherapy of acute myeloid leukemia. J. Clin. Investig. 2020, 130, 1552–1564. [Google Scholar] [CrossRef]
- Aru, B.; Pehlivanoğlu, C.; Dal, Z.; Dereli-Çalışkan, N.N.; Gürlü, E.; Yanıkkaya-Demirel, G. A potential area of use for immune checkpoint inhibitors: Targeting bone marrow microenvironment in acute myeloid leukemia. Front. Immunol. 2023, 14, 1108200. [Google Scholar] [CrossRef]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef]
- Holowiecki, J.; Grosicki, S.; Giebel, S.; Robak, T.; Kyrcz-Krzemien, S.; Kuliczkowski, K.; Skotnicki, A.B.; Hellmann, A.; Sulek, K.; Dmoszynska, A.; et al. Cladribine, but not fludarabine, added to Daunorubicin and cytarabine during induction prolongs survival of patients with acute myeloid leukemia: A multicenter, randomized phase III study. J. Clin. Oncol. 2012, 30, 2441–2448. [Google Scholar] [CrossRef]
- Grubczak, K.; Kretowska-Grunwald, A.; Groth, D.; Poplawska, I.; Eljaszewicz, A.; Bolkun, L.; Starosz, A.; Holl, J.M.; Mysliwiec, M.; Kruszewska, J.; et al. Differential response of MDA-MB-231 and MCF-7 breast cancer cells to in vitro inhibition with CTLA-4 and PD-1 through cancer-immune cells modified interactions. Cells 2021, 10, 2044. [Google Scholar] [CrossRef]
- Savoia, P.; Astrua, C.; Fava, P. Ipilimumab (Anti-Ctla-4 Mab) in the treatment of metastatic melanoma: Effectiveness and toxicity management. Hum. Vaccines Immunother. 2016, 12, 1092–1101. [Google Scholar] [CrossRef]
- Bewersdorf, J.P.; Zeidan, A.M. Randomized trials with checkpoint inhibitors in acute myeloid leukaemia and myelodysplastic syndromes: What have we learned so far and where are we heading? Best Pract. Res. Clin. Haematol. 2020, 33, 101222. [Google Scholar] [CrossRef]
- Kayser, S.; Levis, M.J. Advances in targeted therapy for acute myeloid leukaemia. Br. J. Haematol. 2018, 180, 484–500. [Google Scholar] [CrossRef]
- Yang, X.; Ma, L.; Zhang, X.; Huang, L.; Wei, J. Targeting PD-1/PD-L1 pathway in myelodysplastic syndromes and acute myeloid leukemia. Exp. Hematol. Oncol. 2022, 11, 11. [Google Scholar] [CrossRef]
- Lecciso, M.; Ocadlikova, D.; Sangaletti, S.; Trabanelli, S.; De Marchi, E.; Orioli, E.; Pegoraro, A.; Portararo, P.; Jandus, C.; Bontadini, A.; et al. ATP release from chemotherapy-treated dying leukemia cells elicits an immune suppressive effect by increasing regulatory T cells and tolerogenic dendritic cells. Front. Immunol. 2017, 8, 1918. [Google Scholar] [CrossRef]
- Zeidner, J.F.; Vincent, B.G.; Esparza, S.; Ivanova, A.; Moore, D.T.; Foster, M.C.; Coombs, C.C.; Jamieson, K.; Van Deventer, H.W.; Blanchard, L.; et al. Final clinical results of a phase II study of high dose cytarabine followed by pembrolizumab in relapsed/refractory AML. Blood 2019, 134, 831. [Google Scholar] [CrossRef]
- Greiner, J.; Götz, M.; Hofmann, S.; Schrezenmeier, H.; Wiesneth, M.; Bullinger, L.; Döhner, H.; Schneider, V. Specific T-cell immune responses against colony-forming cells including leukemic progenitor cells of AML patients were increased by immune checkpoint inhibition. Cancer Immunol. Immunother. 2020, 69, 629–640. [Google Scholar] [CrossRef]
- Dong, Y.; Han, Y.; Huang, Y.; Jiang, S.; Huang, Z.; Chen, R.; Yu, Z.; Yu, K.; Zhang, S. PD-L1 Is Expressed and Promotes the Expansion of Regulatory T Cells in Acute Myeloid Leukemia. Front. Immunol. 2020, 11, 1710. [Google Scholar] [CrossRef]
- Radwan, S.M.; Elleboudy, N.S.; Nabih, N.A.; Kamal, A.M. The immune checkpoints Cytotoxic T lymphocyte antigen-4 and Lymphocyte activation gene-3 expression is up-regulated in acute myeloid leukemia. HLA 2020, 96, 3–12. [Google Scholar] [CrossRef]
- Xu, Q.; Cao, D.; Fang, B.; Yan, S.; Hu, Y.; Guo, T. Immune-related gene signature predicts clinical outcomes and immunotherapy response in acute myeloid leukemia. Cancer Med. 2022, 11, 3364–3380. [Google Scholar] [CrossRef]
- Antohe, I.; Dǎscǎlescu, A.; Dǎnǎilǎ, C.; Titieanu, A.; Zlei, M.; Ivanov, I.; Sireteanu, A.; Pavel, M.; Cianga, P. B7-Positive and B7-Negative Acute Myeloid Leukemias Display Distinct T Cell Maturation Profiles, Immune Checkpoint Receptor Expression, and European Leukemia Net Risk Profiles. Front. Oncol. 2020, 10, 264. [Google Scholar] [CrossRef]
- Kang, S.H.; Hwang, H.J.; Yoo, J.W.; Kim, H.; Choi, E.S.; Hwang, S.-H.; Cho, Y.-U.; Jang, S.; Park, C.-J.; Im, H.J.; et al. Expression of Immune Checkpoint Receptors on T-Cells and Their Ligands on Leukemia Blasts in Childhood Acute Leukemia. Anticancer. Res. 2019, 39, 5531–5539. [Google Scholar] [CrossRef]
- Jiang, F.; Wang, X.-Y.; Wang, M.-Y.; Mao, Y.; Miao, X.-L.; Wu, C.-Y.; Zhou, G.-P. An Immune Checkpoint-Related Gene Signature for Predicting Survival of Pediatric Acute Myeloid Leukemia. J. Oncol. 2021, 2021, 5550116. [Google Scholar] [CrossRef]
- Zhong, F.-M.; Yao, F.-Y.; Liu, J.; Zhang, H.-B.; Li, M.-Y.; Jiang, J.-Y.; Xu, Y.-M.; Yang, W.-M.; Li, S.-Q.; Zhang, J.; et al. Inflammatory response mediates cross-talk with immune function and reveals clinical features in acute myeloid leukemia. Biosci. Rep. 2022, 42, BSR20220647. [Google Scholar] [CrossRef]
- Sanchez-Correa, B.; Bergua, J.M.; Campos, C.; Gayoso, I.; Arcos, M.J.; Bañas, H.; Morgado, S.; Casado, J.G.; Solana, R.; Tarazona, R. Cytokine profiles in acute myeloid leukemia patients at diagnosis: Survival is inversely correlated with IL-6 and directly correlated with IL-10 levels. Cytokine 2013, 61, 885–891. [Google Scholar] [CrossRef]
- Luciano, M.; Krenn, P.W.; Horejs-Hoeck, J. The cytokine network in acute myeloid leukemia. Front. Immunol. 2022, 13, 1000996. [Google Scholar] [CrossRef]
- Akdis, M.; Aab, A.; Altunbulakli, C.; Azkur, K.; Costa, R.A.; Crameri, R.; Duan, S.; Eiwegger, T.; Eljaszewicz, A.; Ferstl, R.; et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factors β, and TNF-α: Receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 2016, 138, 984–1010. [Google Scholar] [CrossRef]
- Abdel-Wahab, N.; Shah, M.; Suarez-Almazor, M.E. Adverse Events Associated with Immune Checkpoint Blockade in Patients with Cancer: A Systematic Review of Case Reports. PLoS ONE 2016, 11, e0160221. [Google Scholar] [CrossRef]
- Jimbu, L.; Mesaros, O.; Popescu, C.; Neaga, A.; Berceanu, I.; Dima, D.; Gaman, M.; Zdrenghea, M. Is There a Place for PD-1-PD-L Blockade in Acute Myeloid Leukemia? Pharmaceuticals 2021, 14, 288. [Google Scholar] [CrossRef]
- Gómez-Llobell, M.; Raíndo, A.P.; Medina, J.C.; Centurión, I.G.; Orgueira, A.M. Immune Checkpoint Inhibitors in Acute Myeloid Leukemia: A Meta-Analysis. Front. Oncol. 2022, 12, 882531. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bołkun, Ł.; Starosz, A.; Krętowska-Grunwald, A.; Wasiluk, T.; Walewska, A.; Wierzbowska, A.; Moniuszko, M.; Grubczak, K. Effects of Combinatory In Vitro Treatment with Immune Checkpoint Inhibitors and Cytarabine on the Anti-Cancer Immune Microenvironment in De Novo AML Patients. Cancers 2024, 16, 462. https://doi.org/10.3390/cancers16020462
Bołkun Ł, Starosz A, Krętowska-Grunwald A, Wasiluk T, Walewska A, Wierzbowska A, Moniuszko M, Grubczak K. Effects of Combinatory In Vitro Treatment with Immune Checkpoint Inhibitors and Cytarabine on the Anti-Cancer Immune Microenvironment in De Novo AML Patients. Cancers. 2024; 16(2):462. https://doi.org/10.3390/cancers16020462
Chicago/Turabian StyleBołkun, Łukasz, Aleksandra Starosz, Anna Krętowska-Grunwald, Tomasz Wasiluk, Alicja Walewska, Agnieszka Wierzbowska, Marcin Moniuszko, and Kamil Grubczak. 2024. "Effects of Combinatory In Vitro Treatment with Immune Checkpoint Inhibitors and Cytarabine on the Anti-Cancer Immune Microenvironment in De Novo AML Patients" Cancers 16, no. 2: 462. https://doi.org/10.3390/cancers16020462





