Amplified Cell Cycle Genes Identified in High-Grade Serous Ovarian Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Expression Profiles
2.2. Differential Gene Expression Analysis
2.3. Hierarchical Clustering and Visualization
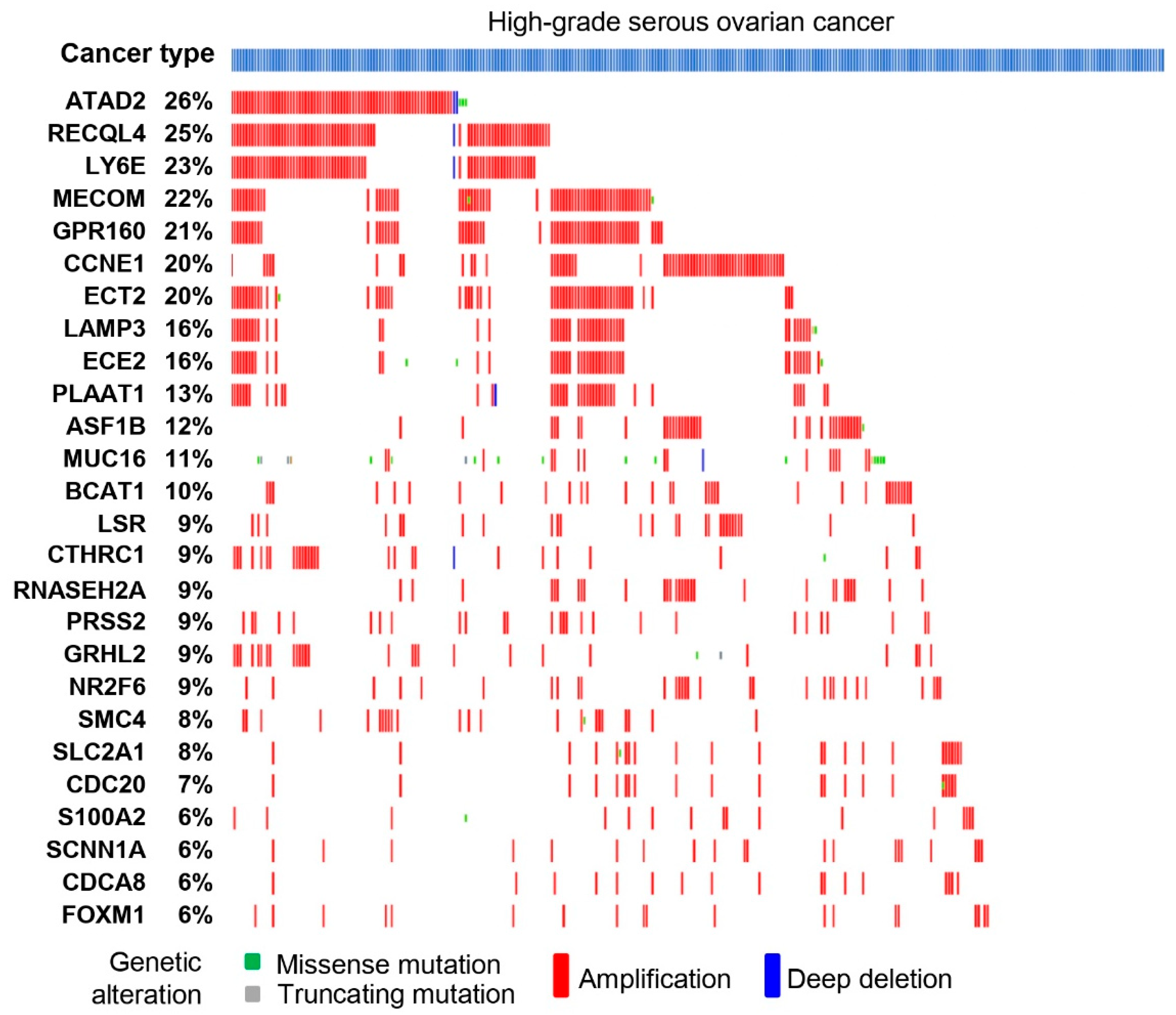
2.4. Mutation Analysis
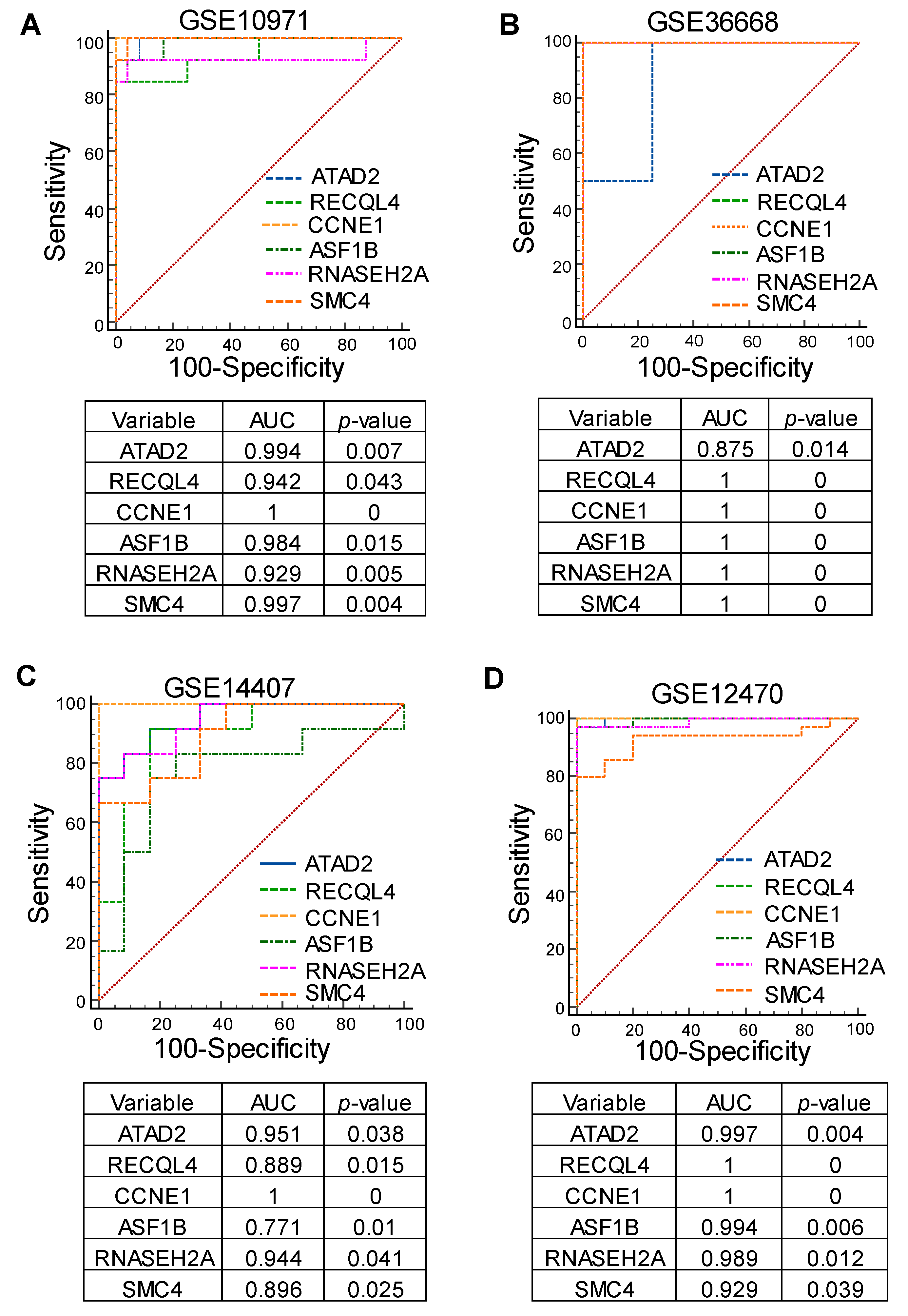
2.5. ROC Curve
2.6. Survival Analysis
2.7. Western Blotting Analysis
3. Results
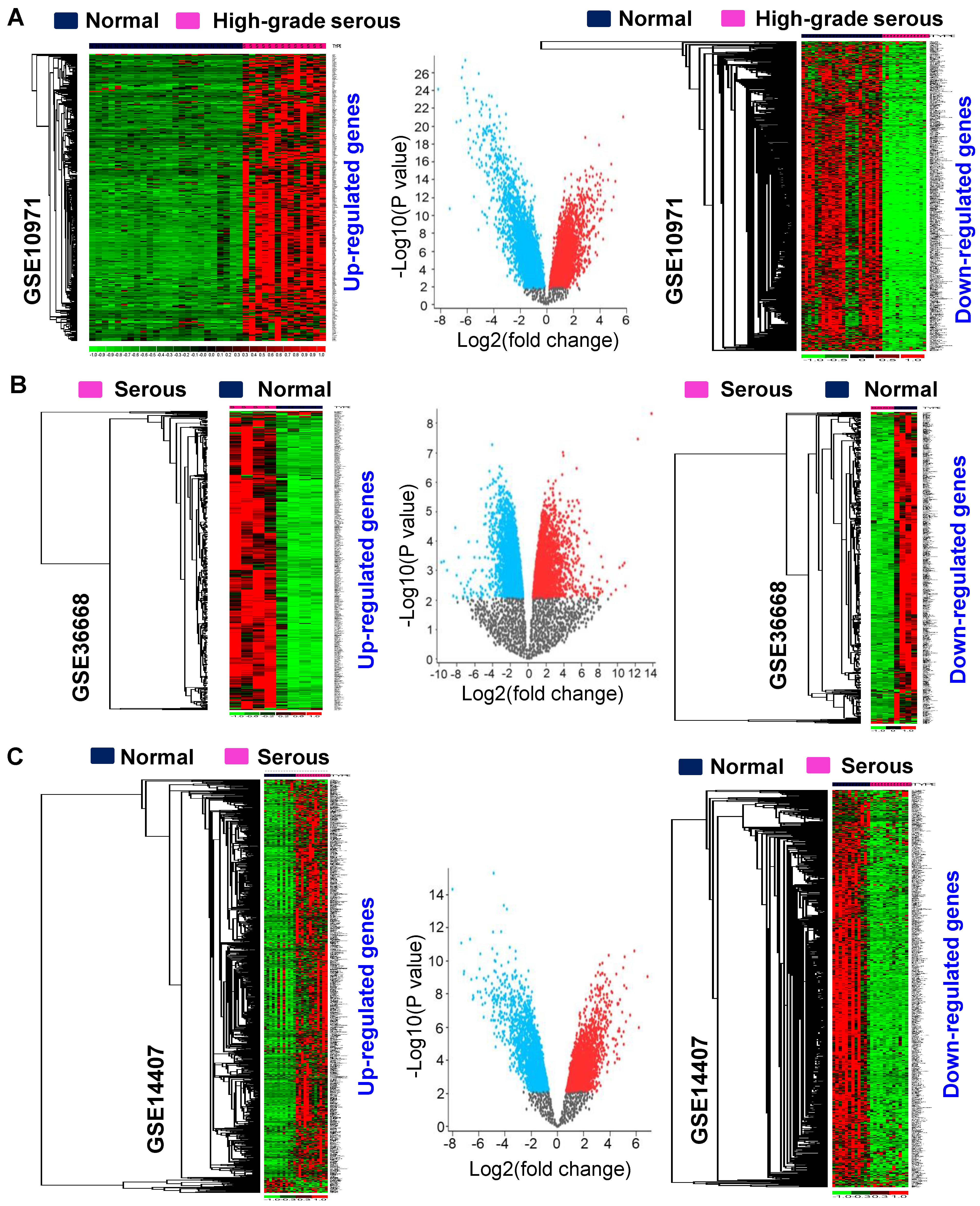
3.1. Identification of Differentially Expressed Genes in Serous-Type Ovarian Cancer
3.2. Identification of Consistently Upregulated Genes in Serous-Type Ovarian Tumors
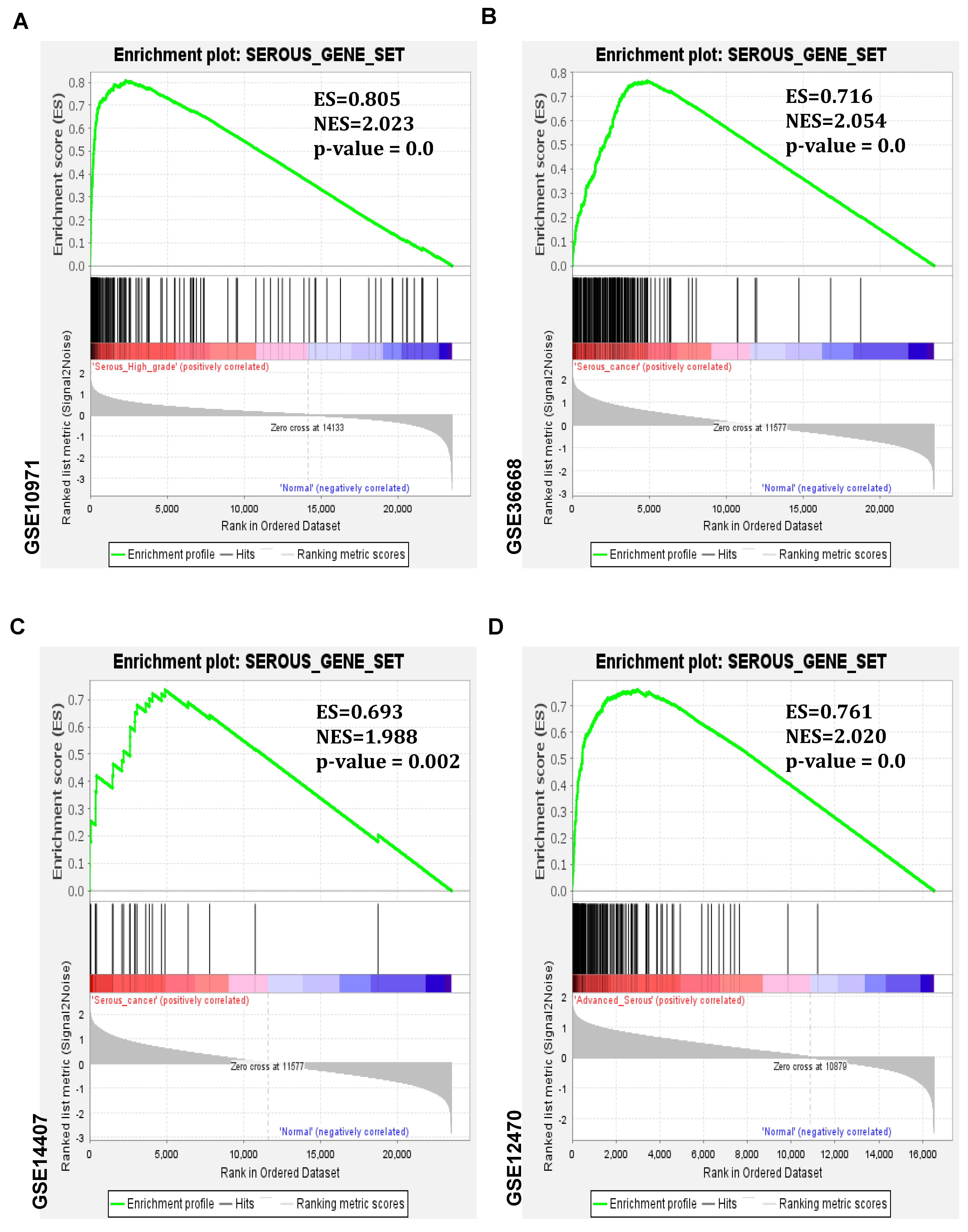
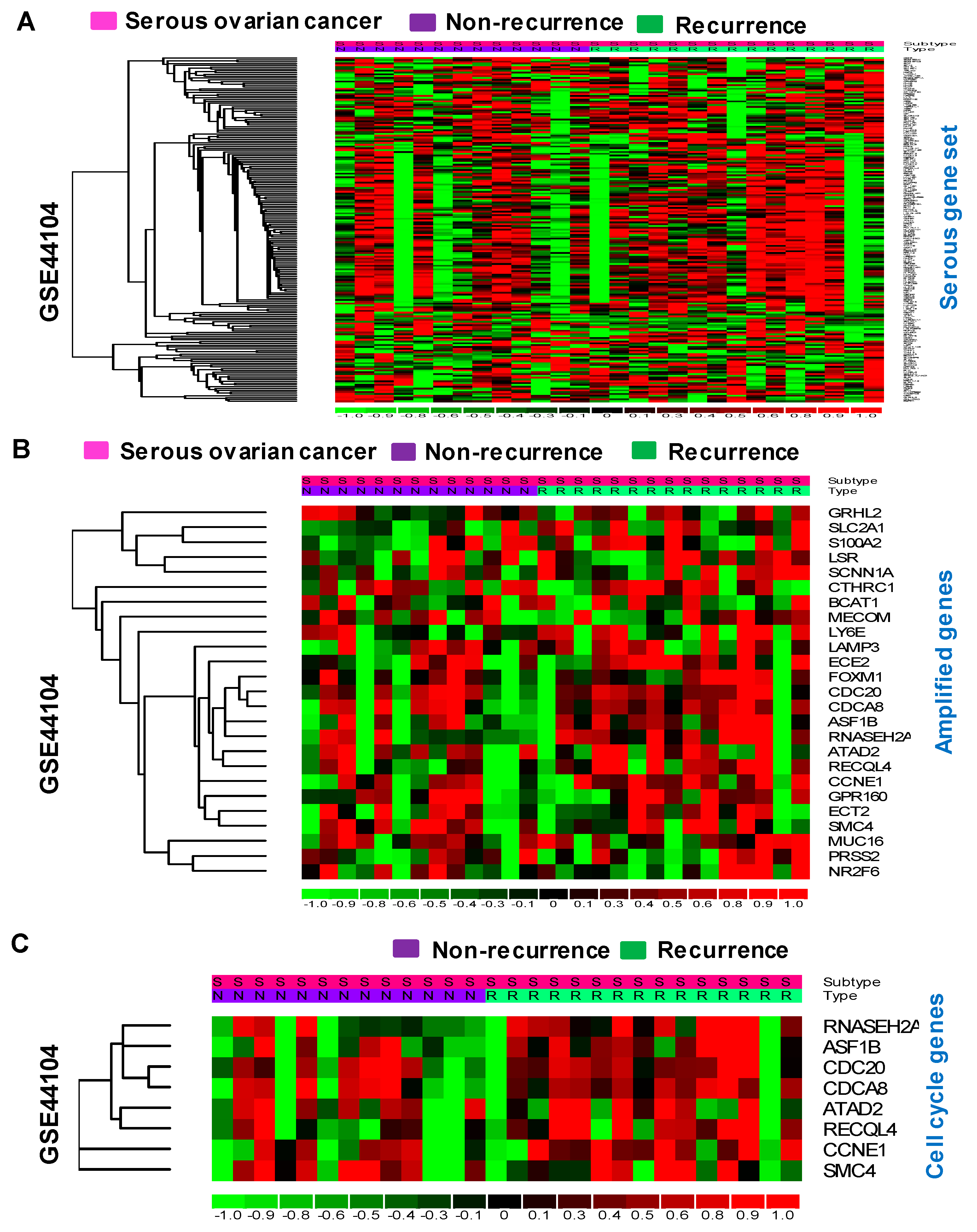
3.3. Serous Gene Set Significantly Amplified in HGSO Adenocarcinoma
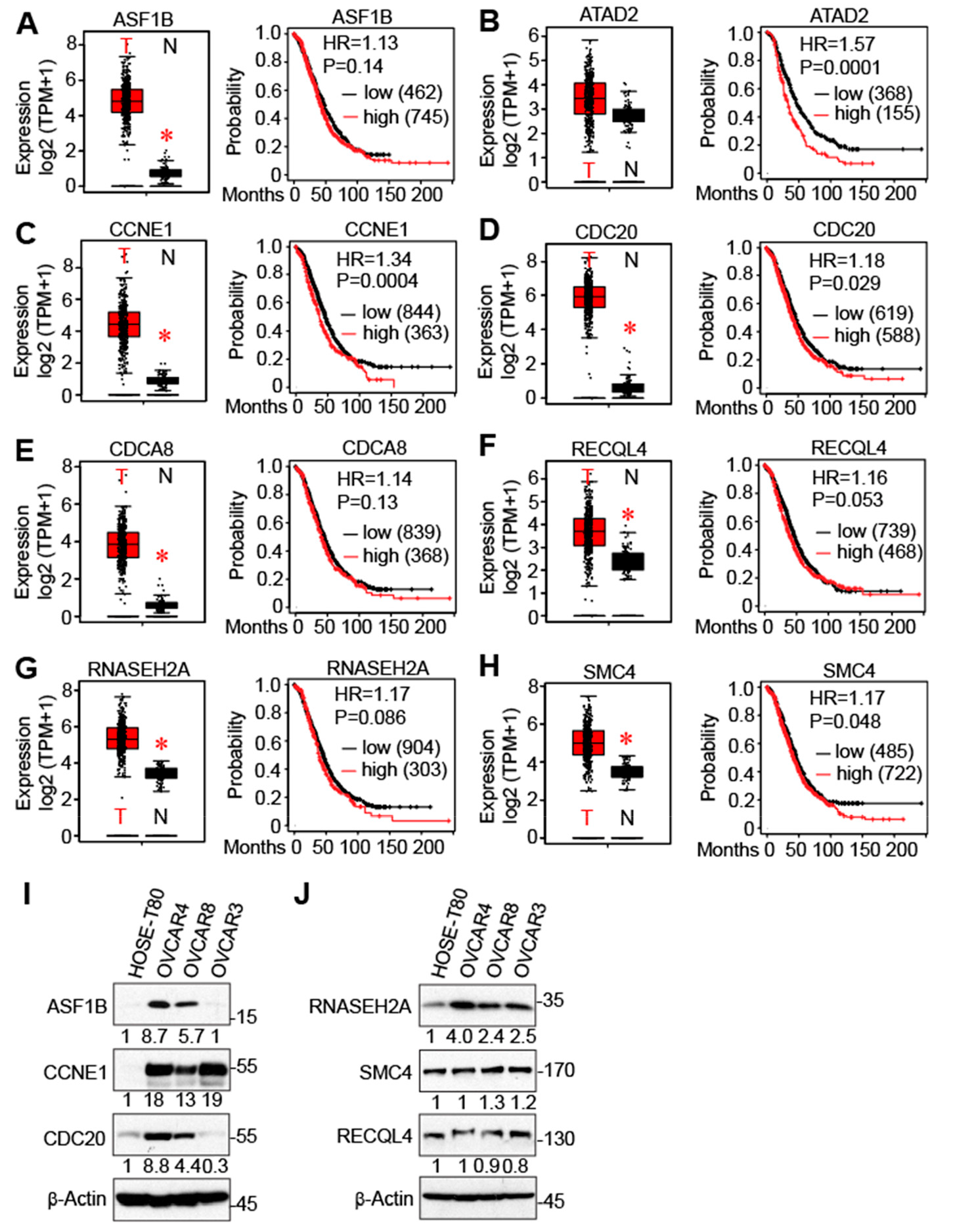
3.4. Identification of Cell Cycle Genes as Promising Prognostic Markers for Serous-Type Ovarian Cancer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Duval, A.J.; Adli, M.; Matei, D. Biology-Driven Therapy Advances in High-Grade Serous Ovarian Cancer. J. Clin. Invest. 2024, 134, e174013. [Google Scholar] [CrossRef] [PubMed]
- Spriggs, D.R.; Zivanovic, O. Ovarian Cancer Treatment-Are We Getting Warmer? N. Engl. J. Med. 2018, 378, 293–294. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Kotnik, E.N.; Mullen, M.M.; Spies, N.C.; Li, T.; Inkman, M.; Zhang, J.; Martins-Rodrigues, F.; Hagemann, I.S.; McCourt, C.K.; Thaker, P.H.; et al. Genetic Characterization of Primary and Metastatic High-Grade Serous Ovarian Cancer Tumors Reveals Distinct Features Associated with Survival. Commun. Biol. 2023, 6, 688. [Google Scholar] [CrossRef] [PubMed]
- Sowamber, R.; Lukey, A.; Huntsman, D.; Hanley, G. Ovarian Cancer: From Precursor Lesion Identification to Population-Based Prevention Programs. Curr. Oncol. 2023, 30, 10179–10194. [Google Scholar] [CrossRef] [PubMed]
- Bergsten, T.M.; Burdette, J.E.; Dean, M. Fallopian Tube Initiation of High Grade Serous Ovarian Cancer and Ovarian Metastasis: Mechanisms and Therapeutic Implications. Cancer Lett. 2020, 476, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Bowtell, D.D.; Böhm, S.; Ahmed, A.A.; Aspuria, P.-J.; Bast, R.C.; Beral, V.; Berek, J.S.; Birrer, M.J.; Blagden, S.; Bookman, M.A.; et al. Rethinking Ovarian Cancer II: Reducing Mortality from High-Grade Serous Ovarian Cancer. Nat. Rev. Cancer 2015, 15, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, T.G.; Dick, F.A. Principles of Dormancy Evident in High-Grade Serous Ovarian Cancer. Cell Div. 2022, 17, 2. [Google Scholar] [CrossRef] [PubMed]
- Karnezis, A.N.; Cho, K.R.; Gilks, C.B.; Pearce, C.L.; Huntsman, D.G. The Disparate Origins of Ovarian Cancers: Pathogenesis and Prevention Strategies. Nat. Rev. Cancer 2017, 17, 65–74. [Google Scholar] [CrossRef]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for Functional Genomics Data Sets--Update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef]
- Wilson, C.L.; Miller, C.J. Simpleaffy: A BioConductor Package for Affymetrix Quality Control and Data Analysis. Bioinformatics 2005, 21, 3683–3685. [Google Scholar] [CrossRef] [PubMed]
- Smyth, G.K. Linear Models and Empirical Bayes Methods for Assessing Differential Expression in Microarray Experiments. Stat. Appl. Genet. Mol. Biol. 2004, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.; Meltzer, P.S. GEOquery: A Bridge between the Gene Expression Omnibus (GEO) and BioConductor. Bioinformatics 2007, 23, 1846–1847. [Google Scholar] [CrossRef] [PubMed]
- Tarca, A.L.; Romero, R.; Draghici, S. Analysis of Microarray Experiments of Gene Expression Profiling. Am. J. Obstet. Gynecol. 2006, 195, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wong, W.H. DNA-Chip Analyzer (dChip). In The Analysis of Gene Expression Data: Methods and Software; Parmigiani, G., Garrett, E.S., Irizarry, R.A., Zeger, S.L., Eds.; Springer: New York, NY, USA, 2003; pp. 120–141. [Google Scholar] [CrossRef]
- Balakrishnan, K. Salt-Driven Chromatin Remodeling Associated with Senescence Dysregulation Plays a Crucial Role in the Carcinogenesis of Gastric Cancer Subtype. Comput. Toxicol. 2023, 25, 100262. [Google Scholar] [CrossRef]
- Balakrishnan, K. The Hepatocellular Carcinoma (HCC) Stage Carcinogenesis Is Associated with Genomic Instability Features. Hum. Gene 2023, 38, 201228. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Gotea, V.; Ovcharenko, I. DiRE: Identifying distant regulatory elements of co-expressed genes. Nucleic Acids Res. 2008, 36 (Suppl. S2), W133–W139. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An Enhanced Web Server for Large-Scale Expression Profiling and Interactive Analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Balakrishnan, K. Hepatocellular Carcinoma Stage: An Almost Loss of Fatty Acid Metabolism and Gain of Glucose Metabolic Pathways Dysregulation. Med. Oncol. 2022, 39, 247. [Google Scholar] [CrossRef] [PubMed]
- Menyhárt, O.; Nagy, Á.; Győrffy, B. Determining Consistent Prognostic Biomarkers of Overall Survival and Vascular Invasion in Hepatocellular Carcinoma. R. Soc. Open Sci. 2018, 5, 181006. [Google Scholar] [CrossRef] [PubMed]
- Nagy, Á.; Lánczky, A.; Menyhárt, O.; Győrffy, B. Validation of miRNA Prognostic Power in Hepatocellular Carcinoma Using Expression Data of Independent Datasets. Sci. Rep. 2018, 8, 9227. [Google Scholar] [CrossRef]
- Balakrishnan, K. Lactate Dehydrogenase Isoform Expressions Differing Impacts on Gastrointestinal Carcinogenesis. Human. Gene 2024, 39, 201243. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. The Logrank Test. BMJ 2004, 328, 1073. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Chen, Y.; Ji, M.; Volle, D.J.; Lewis, R.E.; Tsai, M.Y.; Dong, J. KIBRA protein phosphorylation is regulated by mitotic kinase aurora and protein phosphatase 1. J. Biol. Chem. 2011, 286, 36304–36315. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhou, L.; Cheng, H.; Wang, S.; Luan, W.; Cai, E.; Ye, X.; Zhu, H.; Cui, H.; Li, Y.; et al. Characterization of Candidate Factors Associated with the Metastasis and Progression of High-Grade Serous Ovarian Cancer. Chin. Med. J. 2023, 136, 2974–2982. [Google Scholar] [CrossRef]
- Kostova, I.; Mandal, R.; Becker, S.; Strebhardt, K. The Role of Caspase-8 in the Tumor Microenvironment of Ovarian Cancer. Cancer Metastasis Rev. 2021, 40, 303–318. [Google Scholar] [CrossRef]
- Hollis, R.L. Molecular Characteristics and Clinical Behaviour of Epithelial Ovarian Cancers. Cancer Lett. 2023, 555, 216057. [Google Scholar] [CrossRef] [PubMed]
- Patch, A.-M.; Christie, E.L.; Etemadmoghadam, D.; Garsed, D.W.; George, J.; Fereday, S.; Nones, K.; Cowin, P.; Alsop, K.; Bailey, P.J.; et al. Whole-Genome Characterization of Chemoresistant Ovarian Cancer. Nature 2015, 521, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Adamson, A.W.; Ding, Y.C.; Steele, L.; Leong, L.A.; Morgan, R.; Wakabayashi, M.T.; Han, E.S.; Dellinger, T.H.; Lin, P.S.; Hakim, A.A.; et al. Genomic Analyses of Germline and Somatic Variation in High-Grade Serous Ovarian Cancer. J. Ovarian Res. 2023, 16, 141. [Google Scholar] [CrossRef] [PubMed]
- Ciriello, G.; Miller, M.L.; Aksoy, B.A.; Senbabaoglu, Y.; Schultz, N.; Sander, C. Emerging Landscape of Oncogenic Signatures across Human Cancers. Nat. Genet. 2013, 45, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Hollstein, M.; Xu, Y. P53 Gain-of-Function Cancer Mutants Induce Genetic Instability by Inactivating ATM. Nat. Cell Biol. 2007, 9, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, K.; Chen, Y.; Dong, J. Amplification of Hippo Signaling Pathway Genes Is Governed and Implicated in the Serous Subtype-Specific Ovarian Carcino-Genesis. Cancers 2024, 16, 1781. [Google Scholar] [CrossRef] [PubMed]
- Hollis, R.L.; Meynert, A.M.; Michie, C.O.; Rye, T.; Churchman, M.; Hallas-Potts, A.; Croy, I.; McCluggage, W.G.; Williams, A.R.W.; Bartos, C.; et al. Multiomic Characterization of High-Grade Serous Ovarian Carcinoma Enables High-Resolution Patient Stratification. Clin. Cancer Res. 2022, 28, 3546–3556. [Google Scholar] [CrossRef]
- Etemadmoghadam, D.; deFazio, A.; Beroukhim, R.; Mermel, C.; George, J.; Getz, G.; Tothill, R.; Okamoto, A.; Raeder, M.B.; Harnett, P.; et al. Integrated Genome-Wide DNA Copy Number and Expression Analysis Identifies Distinct Mechanisms of Primary Chemoresistance in Ovarian Carcinomas. Clin. Cancer Res. 2009, 15, 1417–1427. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balakrishnan, K.; Chen, Y.; Dong, J. Amplified Cell Cycle Genes Identified in High-Grade Serous Ovarian Cancer. Cancers 2024, 16, 2783. https://doi.org/10.3390/cancers16162783
Balakrishnan K, Chen Y, Dong J. Amplified Cell Cycle Genes Identified in High-Grade Serous Ovarian Cancer. Cancers. 2024; 16(16):2783. https://doi.org/10.3390/cancers16162783
Chicago/Turabian StyleBalakrishnan, Karthik, Yuanhong Chen, and Jixin Dong. 2024. "Amplified Cell Cycle Genes Identified in High-Grade Serous Ovarian Cancer" Cancers 16, no. 16: 2783. https://doi.org/10.3390/cancers16162783
APA StyleBalakrishnan, K., Chen, Y., & Dong, J. (2024). Amplified Cell Cycle Genes Identified in High-Grade Serous Ovarian Cancer. Cancers, 16(16), 2783. https://doi.org/10.3390/cancers16162783




