Enhanced Recovery after Surgery (ERAS) Program for Patients with Peritoneal Surface Malignancies Undergoing Cytoreductive Surgery with or without HIPEC: A Systematic Review and a Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
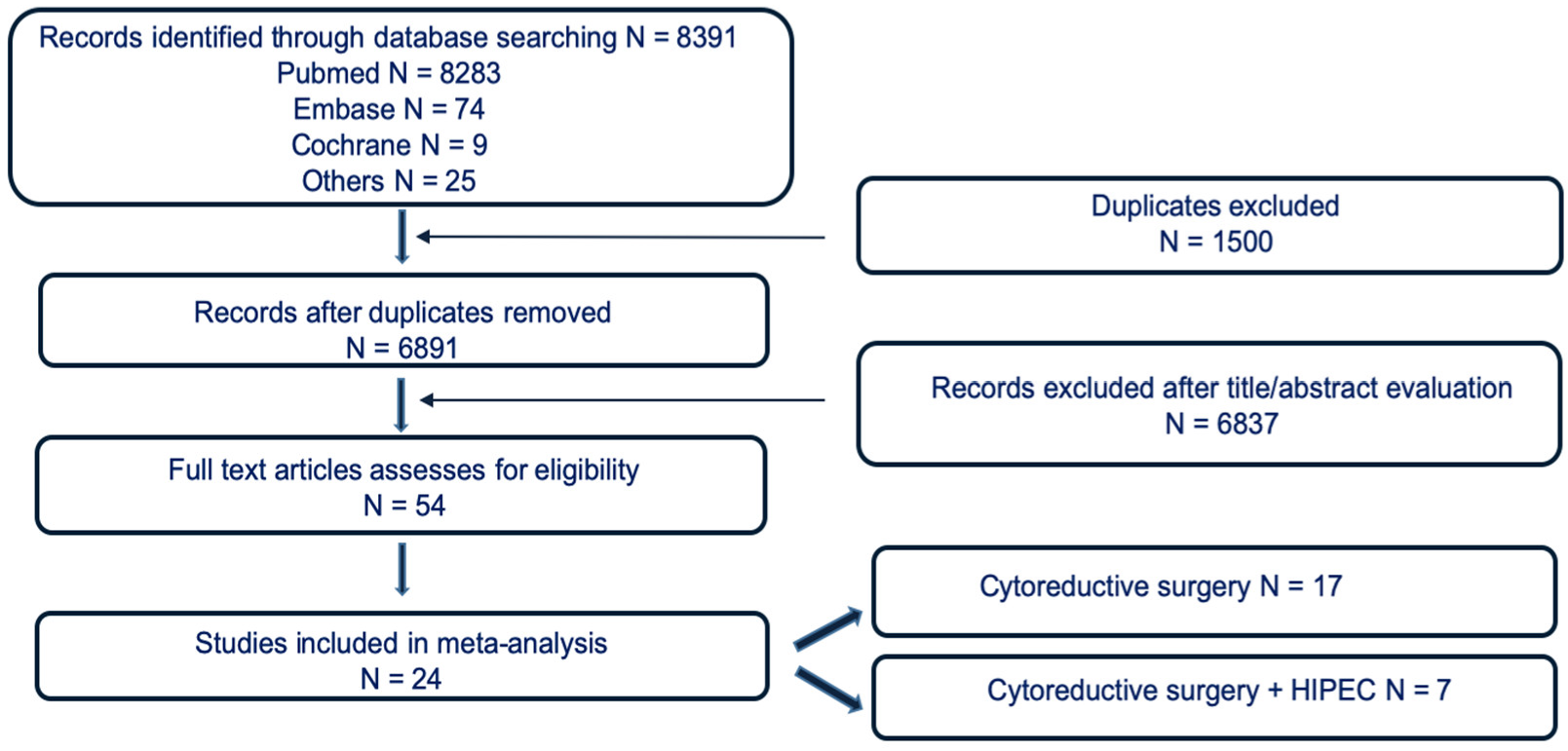
2.1. Eligibility Criteria and Study Selection
2.2. Data Source and Extraction
2.3. Statistical Analysis
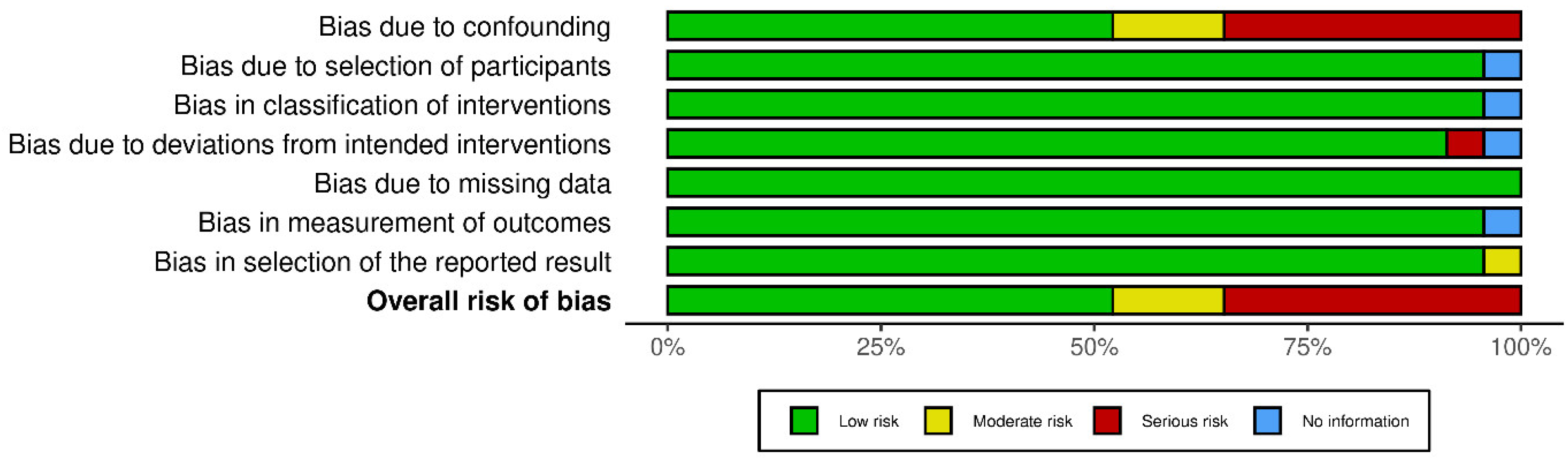
3. Results
3.1. Study Characteristics
3.2. Postoperative Length of Stay
3.3. Postoperative Morbidity and Mortality Rate
3.4. Readmission Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rout, S.; Renehan, A.; Parkinson, M.F.; Saunders, M.P.; Fulford, P.E.; Wilson, M.S.; O’Dwyer, S.T. Treatments and Outcomes of Peritoneal Surface Tumors Through a Centralized National Service (United Kingdom). Dis. Colon Rectum 2009, 52, 1705–1714. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, U.; Dahlke, M.; Klempnauer, J.; Schlitt, H.; Piso, P. Perioperative morbidity and quality of life in long-term survivors following cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur. J. Surg. Oncol. (EJSO) 2005, 31, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Newton, A.D.; Bartlett, E.K.; Karakousis, G.C. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: A review of factors contributing to morbidity and mortality. J. Gastrointest. Oncol. 2016, 7, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Levine, E.A.; Stewart, J.H.; Shen, P.; Russell, G.B.; Loggie, B.L.; Votanopoulos, K.I. Intraperitoneal Chemotherapy for Peritoneal Surface Malignancy: Experience with 1000 Patients. J. Am. Coll. Surg. 2013, 218, 573–585. [Google Scholar] [CrossRef] [Green Version]
- Spiliotis, J.; Halkia, E.; de Bree, E. Treatment of Peritoneal Surface Malignancies with Hyperthermic Intraperitoneal Chemotherapy—Current Perspectives. Curr. Oncol. 2016, 23, 266–275. [Google Scholar] [CrossRef] [Green Version]
- Mortensen, K.; Nilsson, M.; Slim, K.; Schäfer, M.; Mariette, C.; Braga, M.; Carli, F.; Demartines, N.; Griffin, S.M.; Lassen, K.; et al. Consensus guidelines for enhanced recovery after gastrectomy. Br. J. Surg. 2014, 101, 1209–1229. [Google Scholar] [CrossRef]
- The ERAS Compliance Group. The Impact of Enhanced Recovery Protocol Compliance on Elective Colorectal Cancer Resection: Results from an International Registry. Ann. Surg. 2015, 261, 1153–1159. [Google Scholar] [CrossRef]
- Wijk, L.; Udumyan, R.; Pache, B.; Altman, A.D.; Williams, L.L.; Elias, K.M.; McGee, J.; Wells, T.; Gramlich, L.; Holcomb, K.; et al. International validation of Enhanced Recovery After Surgery Society guidelines on enhanced recovery for gynecologic surgery. Am. J. Obstet. Gynecol. 2019, 221, 237.e1–237.e11. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef]
- Hong, H.; Chu, H.; Zhang, J.; Carlin, B.P. A Bayesian missing data framework for generalized multiple outcome mixed treatment comparisons. Res. Synth. Methods 2015, 7, 6–22. [Google Scholar] [CrossRef]
- Hoffman, M.D. The No-U-Turn Sampler: Adaptively Setting Path Lengths in Hamiltonian Monte Carlo. J. Mach. Learn. Res. 2014, 15, 1593–1623. [Google Scholar]
- Makowski, D.; Ben-Shachar, M.S.; Chen, S.H.A.; Lüdecke, D. Indices of Effect Existence and Significance in the Bayesian Framework. Front. Psychol. 2019, 10, 2767. [Google Scholar] [CrossRef] [Green Version]
- Makowski, D.; Ben-Shachar, M.; Lüdecke, D. bayestestR: Describing Effects and their Uncertainty, Existence and Significance within the Bayesian Framework. J. Open Source Softw. 2019, 4. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 23 June 2022).
- Lin, L.; Zhang, J.; Hodges, J.S.; Chu, H. Performing Arm-Based Network Meta-Analysis in R with the pcnetmeta Package. J. Stat. Softw. 2017, 80, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Phillippo, D.M. multinma: Network Meta-Analysis of Individual and Aggregate Data in Stan, R Package Version 0.4.1; GitHub: San Francisco, CA, USA, 2022. [CrossRef]
- Kalogera, E.; Bakkum-Gamez, J.N.; Jankowski, C.J.; Trabuco, E.; Lovely, J.K.; Dhanorker, S.; Grubbs, P.L.; Weaver, A.L.; Haas, L.R.; Borah, B.J.; et al. Enhanced Recovery in Gynecologic Surgery. Obstet. Gynecol. 2013, 122, 319–328. [Google Scholar] [CrossRef]
- Kay, A.H.; Venn, M.; Urban, R.; Gray, H.J.; Goff, B. Postoperative narcotic use in patients with ovarian cancer on an Enhanced Recovery After Surgery (ERAS) pathway. Gynecol. Oncol. 2020, 156, 624–628. [Google Scholar] [CrossRef]
- Ferrari, F.; Forte, S.; Sbalzer, N.; Zizioli, V.; Mauri, M.; Maggi, C.; Sartori, E.; Odicino, F. Validation of an enhanced recovery after surgery protocol in gynecologic surgery: An Italian randomized study. Am. J. Obstet. Gynecol. 2020, 223, 543.e1–543.e14. [Google Scholar] [CrossRef]
- Azhar, H.; Hafeez, M.H.; Ahmed, S.Z. Enhanced Recovery after Surgery (ERAS) versus Traditional Care in Patients Hospitalized for Colorectal Surgery: A Meta-Analysis. Arch. Surg. Res. 2022, 2. [Google Scholar] [CrossRef]
- Reuter, S.; Woelber, L.; Trepte, C.C.; Perez, D.; Zapf, A.; Cevirme, S.; Mueller, V.; Schmalfeldt, B.; Jaeger, A. The impact of Enhanced Recovery after Surgery (ERAS) pathways with regard to perioperative outcome in patients with ovarian cancer. Arch. Gynecol. Obstet. 2021, 306, 199–207. [Google Scholar] [CrossRef]
- Webb, C.; Day, R.; Velazco, C.S.; Pockaj, B.A.; Gray, R.J.; Stucky, C.-C.; Young-Fadok, T.; Wasif, N. Implementation of an Enhanced Recovery After Surgery (ERAS) Program is Associated with Improved Outcomes in Patients Undergoing Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Ann. Surg. Oncol. 2019, 27, 303–312. [Google Scholar] [CrossRef]
- Mendivil, A.A.; Busch, J.R.; Richards, D.C.; Vittori, H.; Goldstein, B.H. The Impact of an Enhanced Recovery After Surgery Program on Patients Treated for Gynecologic Cancer in the Community Hospital Setting. Int. J. Gynecol. Cancer 2018, 28, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Iglesias, J.L.; Carbonell-Socias, M.; Pérez-Benavente, M.A.; Clua, S.M.; Manrique-Muñoz, S.; Gorriz, M.G.; Burgos-Peláez, R.; Gurrutxaga, H.S.; Serrano, M.P.; Gutiérrez-Barceló, M.D.P.; et al. PROFAST: A randomised trial implementing enhanced recovery after surgery for highcomplexity advanced ovarian cancer surgery. Eur. J. Cancer 2020, 136, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.W.; Fields, A.C.; Shabat, G.; Bleday, R.; Goldberg, J.E.; Irani, J.; Stopfkuchen-Evans, M.; Melnitchouk, N. Cytoreductive Surgery and HIPEC in an Enhanced Recovery After Surgery Program: A Feasibility Study. J. Surg. Res. 2019, 247, 59–65. [Google Scholar] [CrossRef] [PubMed]
- White, B.; Dahdaleh, F.; Naffouje, S.A.; Kothari, N.; Berg, J.; Wiemann, W.; Salti, G.I. Impact of Enhanced Recovery after Surgery on Postoperative Outcomes for Patients Undergoing Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Ann. Surg. Oncol. 2021, 28, 5265–5272. [Google Scholar] [CrossRef]
- Cascales-Campos, P.; Sánchez-Fuentes, P.; Gil, J.; Gil, E.; López-López, V.; Gomez-Hidalgo, N.R.; Fuentes, D.; Parrilla, P. Effectiveness and failures of a fast track protocol after cytoreduction and hyperthermic intraoperative intraperitoneal chemotherapy in patients with peritoneal surface malignancies. Surg. Oncol. 2016, 25, 349–354. [Google Scholar] [CrossRef]
- Siddharthan, R.; Dewey, E.; Billingsley, K.; Gilbert, E.; Tsikitis, V.L. Feasibility and benefits of an enhanced recovery after surgery protocol for patients undergoing cytoreductive surgery and heated intraperitoneal chemotharpy: A single institution experience. Am. J. Surg. 2019, 219, 1073–1075. [Google Scholar] [CrossRef]
- Tankou, J.I.; Foley, O.; Falzone, M.; Kalyanaraman, R.; Elias, K.M. Enhanced recovery after surgery protocols improve time to return to intended oncology treatment following interval cytoreductive surgery for advanced gynecologic cancers. Int. J. Gynecol. Cancer 2021, 31, 1145–1153. [Google Scholar] [CrossRef]
- Marx, C.; Rasmussen, T.; Jakobsen, D.H.; Ottosen, C.; Lundvall, L.; Ottesen, B.; Callesen, T.; Kehlet, H. The effect of accelerated rehabilitation on recovery after surgery for ovarian malignancy. Acta Obstet. Gynecol. Scand. 2006, 85, 488–492. [Google Scholar] [CrossRef]
- Gerardi, M.A.; Santillan, A.; Meisner, B.; Zahurak, M.L.; Montes, T.P.D.; Giuntoli, R.L.; Bristow, R.E. A clinical pathway for patients undergoing primary cytoreductive surgery with rectosigmoid colectomy for advanced ovarian and primary peritoneal cancers. Gynecol. Oncol. 2008, 108, 282–286. [Google Scholar] [CrossRef]
- Myriokefalitaki, E.; Smith, M.; Ahmed, A.S. Implementation of enhanced recovery after surgery (ERAS) in gynaecological oncology. Arch. Gynecol. Obstet. 2015, 294, 137–143. [Google Scholar] [CrossRef]
- Modesitt, S.C.; Sarosiek, B.M.; Trowbridge, E.R.; Redick, D.L.; Shah, P.M.; Thiele, R.; Tiouririne, M.; Hedrick, T.L. Enhanced Recovery Implementation in Major Gynecologic Surgeries. Obstet. Gynecol. 2016, 128, 457–466. [Google Scholar] [CrossRef] [Green Version]
- Pache, B.; Jurt, J.; Grass, F.; Hübner, M.; Demartines, N.; Mathevet, P.; Achtari, C. Compliance with enhanced recovery after surgery program in gynecology: Are all items of equal importance? Int. J. Gynecol. Cancer 2019, 29, 810–815. [Google Scholar] [CrossRef]
- Bisch, S.; Wells, T.; Gramlich, L.; Faris, P.; Wang, X.; Tran, D.; Thanh, N.; Glaze, S.; Chu, P.; Ghatage, P.; et al. Enhanced Recovery after Surgery (ERAS) in gynecologic oncology: System-wide implementation and audit leads to improved value and patient outcomes. Gynecol. Oncol. 2018, 151, 117–123. [Google Scholar] [CrossRef]
- Boitano, T.K.; Smith, H.J.; Rushton, T.; Johnston, M.C.; Lawson, P.; Leath, C.A.; Xhaja, A.; Guthrie, M.P.; Straughn, J.M. Impact of enhanced recovery after surgery (ERAS) protocol on gastrointestinal function in gynecologic oncology patients undergoing laparotomy. Gynecol. Oncol. 2018, 151, 282–286. [Google Scholar] [CrossRef]
- Agarwal, R.; Rajanbabu, A.; V, N.P.V.; Goel, G.; Madhusudanan, L.; G, U.U. A prospective study evaluating the impact of implementing the ERAS protocol on patients undergoing surgery for advanced ovarian cancer. Int. J. Gynecol. Cancer 2019, 29, 605–612. [Google Scholar] [CrossRef]
- Bergstrom, J.E.; Scott, M.E.; Alimi, Y.; Yen, T.-T.; Hobson, D.; Machado, K.K.; Tanner, E.J.; Fader, A.N.; Temkin, S.M.; Wethington, S.; et al. Narcotics reduction, quality and safety in gynecologic oncology surgery in the first year of enhanced recovery after surgery protocol implementation. Gynecol. Oncol. 2018, 149, 554–559. [Google Scholar] [CrossRef]
- Meyer, L.A.; Lasala, J.; Iniesta, M.D.; Nick, A.M.; Munsell, M.F.; Shi, Q.; Wang, X.S.; Cain, K.E.; Lu, K.H.; Ramirez, P.T. Effect of an Enhanced Recovery after Surgery Program on Opioid Use and Patient-Reported Outcomes. Obstet. Gynecol. 2018, 132, 281–290. [Google Scholar] [CrossRef]
- Bernard, L.; McGinnis, J.M.; Su, J.; Alyafi, M.; Palmer, D.; Potts, L.; Nancekivell, K.; Thomas, H.; Kokus, H.; Eiriksson, L.R.; et al. Thirty-day outcomes after gynecologic oncology surgery: A single-center experience of enhanced recovery after surgery pathways. Acta Obstet. Gynecol. Scand. 2020, 100, 353–361. [Google Scholar] [CrossRef]
- Duzgun, O. Evaluation of Enhanced Recovery after Following a Surgical Protocol for Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Carcinomatosis. Med. Arch. 2019, 73, 331–337. [Google Scholar] [CrossRef]
- Martin, R.C.; Marshall, B.M.; Philips, P.; Egger, M.; McMasters, K.M.; Scoggins, C.R. Enhanced recovery after surgery is safe for cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Am. J. Surg. 2020, 220, 1428–1432. [Google Scholar] [CrossRef]
- Bisch, S.; Jago, C.; Kalogera, E.; Ganshorn, H.; Meyer, L.; Ramirez, P.; Dowdy, S.; Nelson, G. Outcomes of enhanced recovery after surgery (ERAS) in gynecologic oncology—A systematic review and meta-analysis. Gynecol. Oncol. 2020, 161, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Hübner, M.; Kusamura, S.; Villeneuve, L.; Al-Niaimi, A.; Alyami, M.; Balonov, K.; Bell, J.; Bristow, R.; Guiral, D.C.; Fagotti, A.; et al. Guidelines for Perioperative Care in Cytoreductive Surgery (CRS) with or without hyperthermic IntraPEritoneal chemotherapy (HIPEC): Enhanced recovery after surgery (ERAS®) Society Recommendations—Part I: Preoperative and intraoperative management. Eur. J. Surg. Oncol. (EJSO) 2020, 46, 2292–2310. [Google Scholar] [CrossRef] [PubMed]
- Hübner, M.; Kusamura, S.; Villeneuve, L.; Al-Niaimi, A.; Alyami, M.; Balonov, K.; Bell, J.; Bristow, R.; Guiral, D.C.; Fagotti, A.; et al. Guidelines for Perioperative Care in Cytoreductive Surgery (CRS) with or without hyperthermic IntraPEritoneal chemotherapy (HIPEC): Enhanced Recovery After Surgery (ERAS®) Society Recommendations—Part II: Postoperative management and special considerations. Eur. J. Surg. Oncol. (EJSO) 2020, 46, 2311–2323. [Google Scholar] [CrossRef] [PubMed]
- Mao, F.; Huang, Z. Enhanced Recovery After Surgery for Patients Undergoing Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: A Systematic Review and Meta-Analysis. Front. Surg. 2021, 8. [Google Scholar] [CrossRef]
- Małczak, P.; Pisarska-Adamczyk, M.; Piotr, M.; Wysocki, M.; Budzyński, A.; Pędziwiatr, M. Enhanced Recovery after Bariatric Surgery: Systematic Review and Meta-Analysis. Obes. Surg. 2016, 27, 226–235. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, C.-L.; Ye, X.-Z.; Zhang, X.-D.; Chen, B.-C.; Yu, Z. Enhanced Recovery After Surgery Programs Versus Traditional Care for Colorectal Surgery. Dis. Colon Rectum 2013, 56, 667–678. [Google Scholar] [CrossRef] [Green Version]
- Maessen, J.; Dejong, C.H.C.; Hausel, J.; Nygren, J.; Lassen, K.; Andersen, J.; Kessels, A.G.H.; Revhaug, A.; Kehlet, H.; Ljungqvist, O.; et al. A protocol is not enough to implement an enhanced recovery programme for colorectal resection. Br. J. Surg. 2007, 94, 224–231. [Google Scholar] [CrossRef]
- Noblett, S.E.; Snowden, C.P.; Shenton, B.K.; Horgan, A.F. Randomized clinical trial assessing the effect of Doppler-optimized fluid management on outcome after elective colorectal resection. Br. J. Surg. 2006, 93, 1069–1076. [Google Scholar] [CrossRef]
- Wakeling, H.G.; McFall, M.R.; Jenkins, C.S.; Woods, W.G.A.; Miles, W.F.A.; Barclay, G.R.; Fleming, S.C. Intraoperative oesophageal Doppler guided fluid management shortens postoperative hospital stay after major bowel surgery. Br. J. Anaesth. 2005, 95, 634–642. [Google Scholar] [CrossRef] [Green Version]
- Conway, D.H.; Mayall, R.; Abdul-Latif, M.S.; Gilligan, S.; Tackaberry, C. Randomised controlled trial investigating the influence of intravenous fluid titration using oesophageal Doppler monitoring during bowel surgery*. Anaesthesia 2002, 57, 845–849. [Google Scholar] [CrossRef]
- Garijo, M.E.C.; Blanco, A.S.; Gonzalez, J.T.; Casado, A.M.; de Moya, J.I.M.; Vidal, G.Y.; Rodriguez, A.F.; García, C.M.; Muoz-Casares, F.C.; Ruiz, J.P. Fluid administration in cytoreductive surgery with hyperthermic intraperitoneal chemotherapy: Neither too much nor too little. Braz. J. Anesthesiol. 2021, 72, 695–701. [Google Scholar] [CrossRef]
- Hendrix, R.J.; Damle, A.; Williams, C.; Harris, A.; Spanakis, S.; Lambert, D.H.; Lambert, L.A. Restrictive Intraoperative Fluid Therapy is Associated with Decreased Morbidity and Length of Stay Following Hyperthermic Intraperitoneal Chemoperfusion. Ann. Surg. Oncol. 2018, 26, 490–496. [Google Scholar] [CrossRef]
- Varadhan, K.K.; Lobo, D.N. A meta-analysis of randomised controlled trials of intravenous fluid therapy in major elective open abdominal surgery: Getting the balance right. Proc. Nutr. Soc. 2010, 69, 488–498. [Google Scholar] [CrossRef] [Green Version]
- Wijk, L.; Franzen, K.; Ljungqvist, O.; Nilsson, K. Implementing a structured Enhanced Recovery After Surgery (ERAS) protocol reduces length of stay after abdominal hysterectomy. Acta Obstet. Gynecol. Scand. 2014, 93, 749–756. [Google Scholar] [CrossRef]
- Jurt, J.; Slieker, J.; Frauche, P.; Addor, V.; Sola, J.; Demartines, N.; Hubner, M. Enhanced Recovery After Surgery: Can We Rely on the Key Factors or Do We Need the Bel Ensemble? World J. Surg. 2017, 41, 2464–2470. [Google Scholar] [CrossRef]
- Cakir, H.; van Stijn, M.F.M.; Cardozo, A.M.F.L.; Langenhorst, B.L.A.M.; Schreurs, W.H.; van der Ploeg, T.J.; Bemelman, W.A.; Houdijk, A.P.J. Adherence to Enhanced Recovery after Surgery and length of stay after colonic resection. Color. Dis. 2013, 15, 1019–1025. [Google Scholar] [CrossRef]
- Gustafsson, U.O.; Hausel, J.; Thorell, A.; Ljungqvist, O.; Soop, M.; Nygren, J. Adherence to the Enhanced Recovery after Surgery Protocol and Outcomes after Colorectal Cancer Surgery. Arch. Surg. 2011, 146, 571–577. [Google Scholar] [CrossRef] [Green Version]
- Francis, N.K.; Mason, J.; Salib, E.; Allanby, L.; Messenger, D.; Allison, A.S.; Smart, N.; Ockrim, J.B. Factors predicting 30-day readmission after laparoscopic colorectal cancer surgery within an enhanced recovery programme. Color. Dis. 2015, 17, O148–O154. [Google Scholar] [CrossRef]
| Study | Sample Size | Mean Age (Years) | Female (%) | PCI | Primary Tumor | HIPEC Drug | Mean Operative Time (min) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ERAS | Control | ERAS | Control | ERAS | Control | ERAS | Control | ERAS | Control | ERAS | Control | ERAS | Control | |
| Duzgun 2019 [18] | 62 | 40 | 57.3 | 56.1 | 58.1 | 57.5 | 12.8 | 12.4 | CRC 26 OC 11 STS 8 GC 5 Other 12 | CRC 20 OC 8 STS 6 GC 5 Other 11 | NR | NR | 472 | 395 |
| Martin 2020 [19] | 20 | 105 | 51.7 * | 58.7 * | 12 | 43 | 3 * | 5.5 * | PMP 9 CRC 8 GC 1 PP 1 Other 1 | PMP 7 CRC 4 GC 3 PP 7 Other 10 | CDDP 1 IRI 2 MMC 11 OXA 6 | CDDP/DXR 3 CDDP 2 IRI 1 MMC 55 OXA/IRI 11 OXA 27 Other 6 | 270 * | 300 * |
| Siddhartan 2020 [20] | 15 | 16 | 60 * | 57 * | NR | NR | 3 * | 6 * | NR | NR | MMC | MMC | 418 * | 452 * |
| Webb 2020 [21] | 81 | 49 | 54.4 | 56.0 | 39 | 43 | 12 | 11.5 | PMP 47 CRC 18 MPM 7 OC 2 GC 5 Other 2 | PMP 26 CRC 14 MPM 4 OC 3 GC 0 Other 2 | CDDP MMC | CDDP MMC | 390 | 390 |
| Lu 2020 [22] | 20 | 11 | 50 | 47 | 12 | 43 | 13.5 | 10 | PMP 12 CRC 4 Other 4 | PMP 7 CRC 4 | MMC | MMC | 347 | 391 |
| White 2021 [23] | 80 | 88 | 56.5 | 56.7 | 62.5% | 63.6% | 13.2 | 13.6 | NR | NR | CDDP 23.8% MMC 76.2% | CDDP 26.1% MMC 73.9% | 370 | 360 |
| Cascales Campos 2016 [24] | 156 | 57 * | 148 (94.9%) | 8 (0–32) | OC 113 CRC18 PMP 13 STS 5 Other 5 | OC: Paclitaxel/CDDP PMP and CRC: MMC Sarcomas: CDDP + DXR | 300 * | |||||||
| Veneto Institute of Oncology | 33 | 62.3 | 22 (66.6%) | 15.4 | OC 12 CRC 7 MPM 3 PMP 5 STS 2 Other 4 | CDDP 10 MMC 1 CDDP + MMC 15 CDDP + DXR 7 | 558.2 | |||||||
| Candiolo Cancer Institute | 28 | 58.57 | 18 (64.28%) | 11,2 | OC 4 CRC 9 MPM 1 PMP 13 PP 1 | CDDP 7 MMC 3 CDDP + MMC 18 | 341.4 | |||||||
| Study | Sample Size | Mean Age (Years) | Female (%) | Aletti Score | Primary Tumor | Operative Time (min) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ERAS | Control | ERAS | Control | ERAS | Control | ERAS | Control | ERAS | Control | ERAS | Control | |
| Marx, 2006 [25] | 69 | 72 | 61 * | 62 * | 69 (100%) | 72 (100%) | NR | NR | OC 61 | OC 62 | 134 * | 122 * |
| Gerardi, 2008 [26] | 19 | 45 | 62 | 58 | 19 (100%) | 45 (100%) | NR | NR | OC 19 | OC 45 | NR | NR |
| Kalogera, 2013 [17] | 81 | 78 | 64.3 | 65.1 | 81 (100%) | 78 (100%) | NR | NR | Gyn 100% | Gyn 100% | 227 | 278 |
| Myriokefalitaki, 2016 [27] | 99 | 99 | 61.6 | 61.0 | 99 (100%) | 99 (100%) | High 46 | High 58 | Gyn 100% | Gyn 100% | NR | NR |
| Modesitt, 2016 [28] | 136 | 211 | 51.8 | 51.1 | 136 (100%) | 211 (100%) | NR | NR | Gyn 100% | Gyn 100% | 228 | 222 |
| Bisch, 2018 [29] | 367 | 152 | 57 * | 61 * | 367 (100%) | 152 (100%) | Low 253 Med/High 114 | Low 94 Med/High 58 | OC EC Benign | OC EC Benign | NR | NR |
| Agarwal, 2018 [30] | 45 | 45 | 53 * | 58 * | 45 (100%) | 45 (100%) | NR | NR | OC 45 | OC 45 | 229 | 219 |
| Boitano, 2018 [31] | 179 | 197 | 55.9 | 57.8 | 179 (100%) | 197 (100%) | Low 124 Mod 46 High 9 | Low 144 Mod 47 High 6 | OC 62 Uterine 20 Cervix 4 Others 93 | OC 59 Uterine 40 Cervix 6 Others 92 | NR | NR |
| Meyer, 2018 [32] | 533 | 74 | 58 | 58 | 533 (100%) | 74 (100%) | NR | NR | Adv. OC 288 | Adv. OC 48 | 216 * | 236 * |
| Bergstrom, 2018 [33] | 109 | 158 | 55.2 | 51.7 | 109 (100%) | 158 (100%) | NR | NR | Adv. OC 54 | Adv. OC 41 | 285 | 238 |
| Bernard, 2020 [34] | 187 | 441 | 58.6 | 60.3 | 187 (100%) | 441 (100%) | NR | NR | OC 129 Uterine 36 Cervix 22 | OC 335 Uterine 101 Cervix 5 | 145 * | 121 * |
| Sanchez-Iglesias, 2020 [35] | 50 | 49 | 57.8 | 57.2 | 50 (100%) | 49 (100%) | Low 11 Med 16 High 23 | Low 6 Med 17 High 26 | OC 48 Others 2 | OC 47 Others 2 | 288 | 287 |
| Tankou, 2021 [36] | 128 | 150 | NR | NR | 128 (100%) | 150 (100%) | Low 90 Med28 High 10 | Low 114 Med 33 High 3 | OC/PP 120 Uterine 8 | OC/PP 150 Uterine 0 | NR | NR |
| Ferrari, 2020 [37] | 83 | 85 | 56.5 | 54.9 | 83 (100%) | 85 (100%) | NR | NR | Adv. OC 24 | Adv. OC 25 | 139 | 160 |
| Mendivil 2018 [38] | 86 | 91 | 63.87 | 56.01 | 86 (100%) | 91 (100%) | NR | NR | Gyn 100% | Gyn 100% | NR | NR |
| Kay, 2020 [39] | 94 | 42 | 63.1 | 60.1 | 94 (100%) | 42 (100%) | NR | NR | OC 100% | OC 100% | NR | NR |
| Reuter, 2021 [40] | 47 | 87 | 65 * | 60 * | 47 (100%) | 87 (100%) | NR | NR | OC 100% | OC 100% | 303 ± 91 | 306 ± 103 |
| Veneto Institute of Oncology | 33 | 66.87 | 30 (90.9%) | PCI 12.24 | OC 24 CRC 2 PMP 1 Other 6 | 363.63 | ||||||
| Candiolo Cancer Institute | 33 | 61 | 26 (78.7%) | PCI 12.5 | OC 17 CRC10 PMP 6 | 244 | ||||||
| Study | Arms | Sample Size N | LOS Mean (Days) | LOS (SD) | Readmission N | Reoperation N | Complications N | Major complic. N | Death N | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | ERAS | Control | ERAS | Control | ERAS | Control | ERAS | Control | ERAS | Control | ERAS | Control | ERAS | Control | ERAS | |||
| CRS + HIPEC | P.A Cascales Campos 2016 [24] | 1 | 156 | 14 | 48 | 21 | ||||||||||||
| Duzgun 2019 [18] | 2 | 40 | 62 | 10 | 7 | 4.50 | 1,10 | 2 | 2 | 14 | 9 | 4 | 1 | 3 | 3 | |||
| Webb 2020 [21] | 2 | 49 | 81 | 10.30 | 6.90 | 8.90 | 5 | 7 | 13 | 6 | 5 | 12 | 12 | |||||
| Siddharthan 2020 [20] | 2 | 16 | 15 | 11 | 7 | 2.38 | 16.30 | 4 | 3 | |||||||||
| Lu 2020 [22] | 2 | 11 | 20 | 9 | 6 | 2.96 | 1.85 | 3 | 1 | 1 | 1 | |||||||
| Martin 2020 [19] | 2 | 105 | 20 | 11 | 9 | 28.96 | 16.30 | 19 | 6 | 7 | 1 | |||||||
| White 2021 [23] | 2 | 88 | 80 | 10 | 7.90 | 4.50 | 6.40 | 12 | 13 | 34 | 19 | 19 | 1 | |||||
| CRS | Marx 2006 [25] | 2 | 72 | 69 | 6 | 5 | 45 | 21.50 | 7 | 2 | 9 | 3 | 23 | 17 | 18 | 4 | 2 | 0 |
| Gerardi 2008 [26] | 2 | 45 | 19 | 11.40 | 8.70 | 18 | 17 | 15 | 4 | 26 | 12 | |||||||
| Kalogera, 2013 [17] | 2 | 78 | 81 | 10.70 | 6.50 | 11.40 | 3.50 | 14 | 21 | 56 | 51 | 16 | 17 | 1 | 1 | |||
| Myriokefalitaki, 2016 [27] | 2 | 99 | 99 | 7.20 | 4.30 | 5.68 | 2.78 | 6 | 5 | 27 | 30 | 4 | 2 | 1 | 0 | |||
| Modesitt, 2016 [28] | 2 | 211 | 136 | 3 | 2 | 0.74 | 0.74 | 13 | 7 | 1 | 2 | 85 | 29 | 2 | 0 | |||
| Bisch 2018 [29] | 2 | 152 | 367 | 6.40 | 4.50 | 7.50 | 5.90 | 10 | 22 | 81 | 133 | 0 | 2 | |||||
| Boitano 2018 [31] | 2 | 197 | 179 | 4 | 2.90 | 2.40 | 1.90 | 21 | 18 | |||||||||
| Bergstrom 2018 [33] | 2 | 158 | 109 | 5 | 5.50 | 2.96 | 2.96 | 15 | 13 | 32 | 15 | |||||||
| Meyer 2018 [32] | 2 | 74 | 533 | 4 | 3 | 6.75 | 14 | 10 | 70 | 4 | 12 | 0 | 1 | |||||
| Mendivil 2018 [38] | 2 | 91 | 86 | 8.04 | 4.88 | 7.19 | 4.23 | 4 | 2 | |||||||||
| Agarwal 2019 [30] | 2 | 45 | 45 | 6 | 4 | 10.40 | 4.44 | 5 | 1 | 17 | 11 | |||||||
| Sanchez-Iglesias 2020 [35] | 2 | 49 | 50 | 9 | 7 | 3.70 | 2.96 | 10 | 3 | 5 | 4 | 35 | 30 | 8 | 9 | 2 | 2 | |
| Ferrari 2020 [37] | 2 | 85 | 83 | 7 | 4 | 5.18 | 2.22 | 5 | 4 | 28 | 15 | 8 | 1 | |||||
| Bernard 2020 [34] | 2 | 441 | 187 | 4.70 | 3.80 | 3.80 | 3.20 | 35 | 9 | 7 | 5 | 107 | 30 | 2 | 0 | |||
| Kay, 2020 [39] | 2 | 42 | 94 | 6.7 | 4.2 | 3 | 9 | |||||||||||
| Tankou, 2021 [36] | 2 | 150 | 128 | 4 | 3 | 1.48 | 0.74 | 13 | 14 | 2 | 0 | 1 | 0 | |||||
| Reuter, 2021 [40] | 2 | 87 | 47 | 13 | 11 | 3.7 | 2.22 | 18 | 7 | 46 | 14 | 9 | 2 | |||||
| Outcome | Treatment | BMA AD 2 Arms + AD 1 Arms Median (95% CrI) | pd | IBMA AD 2 Arms + IPD Median (95% CrI) | pd | No AD Studies with 2 Arms | No AD Studies with 1 Arm | No IPD Studies 2 Arms |
|---|---|---|---|---|---|---|---|---|
| Hospital stay (days) | HIPEC + ERAS vs. HIPEC | −3.17 (−4.68, −1.69) | 0.99 | −3.00 (−7.84, 1.55) | 0.90 | 6 | 0 | 2 |
| CRS + ERAS vs. CRS | −1.65 (−2.32, −1.06) | 0.99 | −1.28 (−3.01, 0.39) | 0.93 | 17 | 0 | 2 | |
| Major complications | HIPEC + ERAS vs. HIPEC | 0.53 (0.18, 1.59) | 0.88 | 0.48 (0.22, 0.98) | 0.98 | 5 | 1 | 2 |
| CRS + ERAS vs. CRS | 0.70 (0.33, 1.52) | 0.83 | 0.58 (0.32, 0.94) | 0.98 | 8 | 0 | 2 | |
| Reoperation | HIPEC + ERAS vs. HIPEC | 0.63 (0.09, 4.48) | 0.69 | 0.58 (0.15, 2.07) | 0.82 | 3 | 0 | 2 |
| CRS + ERAS vs. CRS | 0.65 (0.17, 2.54) | 0.74 | 0.67 (0.27, 1.64) | 0.83 | 6 | 0 | 2 | |
| Readmission | HIPEC + ERAS vs. HIPEC | 0.84 (0.23, 2.84) | 0.62 | 1.16 (0.63, 2.08) | 0.69 | 4 | 1 | 2 |
| CRS + ERAS vs. CRS | 0.79 (0.48, 1.28) | 0.84 | 0.84 (0.64, 1.09) | 0.92 | 17 | 0 | 2 | |
| Complications | HIPEC + ERAS vs. HIPEC | 0.57 (0.05, 7.31) | 0.70 | 0.31 (0.10, 0.89) | 0.99 | 1 | 1 | 2 |
| CRS + ERAS vs. CRS | 0.61 (0.37, 1.01) | 0.95 | 0.56 (0.44, 0.71) | 0.99 | 10 | 0 | 2 | |
| Death | HIPEC + ERAS vs. HIPEC | 0.23 (0.02, 3.40) | 0.87 | 0.15 (0.01, 1.76) | 0.94 | 2 | 0 | 2 |
| CRS + ERAS vs. CRS | 0.49 (0.08, 3.25) | 0.79 | 0.43 (0.05, 1.96) | 0.88 | 10 | 0 | 2 |
| Study | Preoperative Information/ Counseling | Nutritional Supplement | No Bowel Preparation | Carbohydrate Loading | Multimodal Analgesia | PONV Management | Goal Directed Fluid Therapy | Avoidance Abdominal Drains | Avoidance NGT | Early NGT Removal (<24 h) | Early UC Removal (<24 h) | Time to Fluid Intake (<24 h) | Early Solid Intake (<48 h) | Early Mobilization/ Deambulation | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRS + HIPEC | Cascales Campos, 2016 [24] | x | x | x | x | x | x | ||||||||
| Duzgun, 2019 [18] | x | x | x | x | x | x | x | x | x | x | x | ||||
| Webb, 2020 [21] | x | x | x | x | x | x | |||||||||
| Siddhartan, 2020 [20] | x | x | x | x | x | x | x | x | x | x | x | ||||
| Lu, 2020 [22] | x | x | x | x | x | x | x | x | x | ||||||
| Martin, 2020 [19] | x | x | x | x | x | x | x | x | x | x | |||||
| White, 2021 [23] | x | x | x | x | x | x | x | x | x | x | x | x | |||
| Candiolo Cancer Institute | x | x | x | x | x | x | x | x | x | x | x | ||||
| Veneto Institute of Oncology | x | x | x | x | x | x | x | x | x | ||||||
| CRS | Marx, 2006 [25] | x | x | x | x | x | x | x | x | ||||||
| Gerardi, 2008 [26] | x | x | x | ||||||||||||
| Kalogera, 2013 [17] | x | x | x | x | x | x | x | x | x | ||||||
| Myriokefalitak, 2016 [27] | x | x | x | x | x | x | x | x | x | x | x | x | x | ||
| Modesitt, 2016 [28] | x | x | x | x | x | x | |||||||||
| Bisch, 2018 [29] | x | x | x | x | x | x | x | x | x | x | x | ||||
| Boitano, 2018 [31] | x | x | x | x | x | x | x | x | x | x | x | ||||
| Bergstrom, 2018 [33] | x | x | x | x | x | x | x | x | x | x | x | x | |||
| Meyer 2018 [32] | x | x | x | x | x | x | x | x | x | x | x | ||||
| Mendivil, 2018 [38] | x | x | x | x | x | x | x | x | x | ||||||
| Agarwal, 2019 [30] | x | x | x | x | x | x | x | x | x | x | x | x | x | ||
| Sanchez- Iglesias, 2020 [35] | x | x | x | x | x | x | x | x | x | x | x | x | |||
| Ferrari, 2020 [37] | x | x | x | x | x | x | x | x | x | x | x | x | x | ||
| Bernard, 2020 [34] | x | x | x | x | x | x | x | x | x | x | x | x | |||
| Kay, 2020 [39] | x | x | x | x | x | x | x | x | x | ||||||
| Tankou, 2021 [36] | x | x | x | x | x | x | x | x | |||||||
| Reuter, 2021 [40] | x | x | x | x | x | x | x | ||||||||
| Candiolo Cancer Institute | x | x | x | x | x | x | x | x | x | x | x | ||||
| Veneto Institute of Oncology | x | x | x | x | x | x | x | x | x |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robella, M.; Tonello, M.; Berchialla, P.; Sciannameo, V.; Ilari Civit, A.M.; Sommariva, A.; Sassaroli, C.; Di Giorgio, A.; Gelmini, R.; Ghirardi, V.; et al. Enhanced Recovery after Surgery (ERAS) Program for Patients with Peritoneal Surface Malignancies Undergoing Cytoreductive Surgery with or without HIPEC: A Systematic Review and a Meta-Analysis. Cancers 2023, 15, 570. https://doi.org/10.3390/cancers15030570
Robella M, Tonello M, Berchialla P, Sciannameo V, Ilari Civit AM, Sommariva A, Sassaroli C, Di Giorgio A, Gelmini R, Ghirardi V, et al. Enhanced Recovery after Surgery (ERAS) Program for Patients with Peritoneal Surface Malignancies Undergoing Cytoreductive Surgery with or without HIPEC: A Systematic Review and a Meta-Analysis. Cancers. 2023; 15(3):570. https://doi.org/10.3390/cancers15030570
Chicago/Turabian StyleRobella, Manuela, Marco Tonello, Paola Berchialla, Veronica Sciannameo, Alba Maria Ilari Civit, Antonio Sommariva, Cinzia Sassaroli, Andrea Di Giorgio, Roberta Gelmini, Valentina Ghirardi, and et al. 2023. "Enhanced Recovery after Surgery (ERAS) Program for Patients with Peritoneal Surface Malignancies Undergoing Cytoreductive Surgery with or without HIPEC: A Systematic Review and a Meta-Analysis" Cancers 15, no. 3: 570. https://doi.org/10.3390/cancers15030570






