Strategy for Pediatric Patients with Relapsed or Refractory Anaplastic Lymphoma Kinase-Positive Anaplastic Large Cell Lymphoma: A Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Clinical Features
3. Oncogenic Mechanism
4. Risk Factors with Poor Prognosis
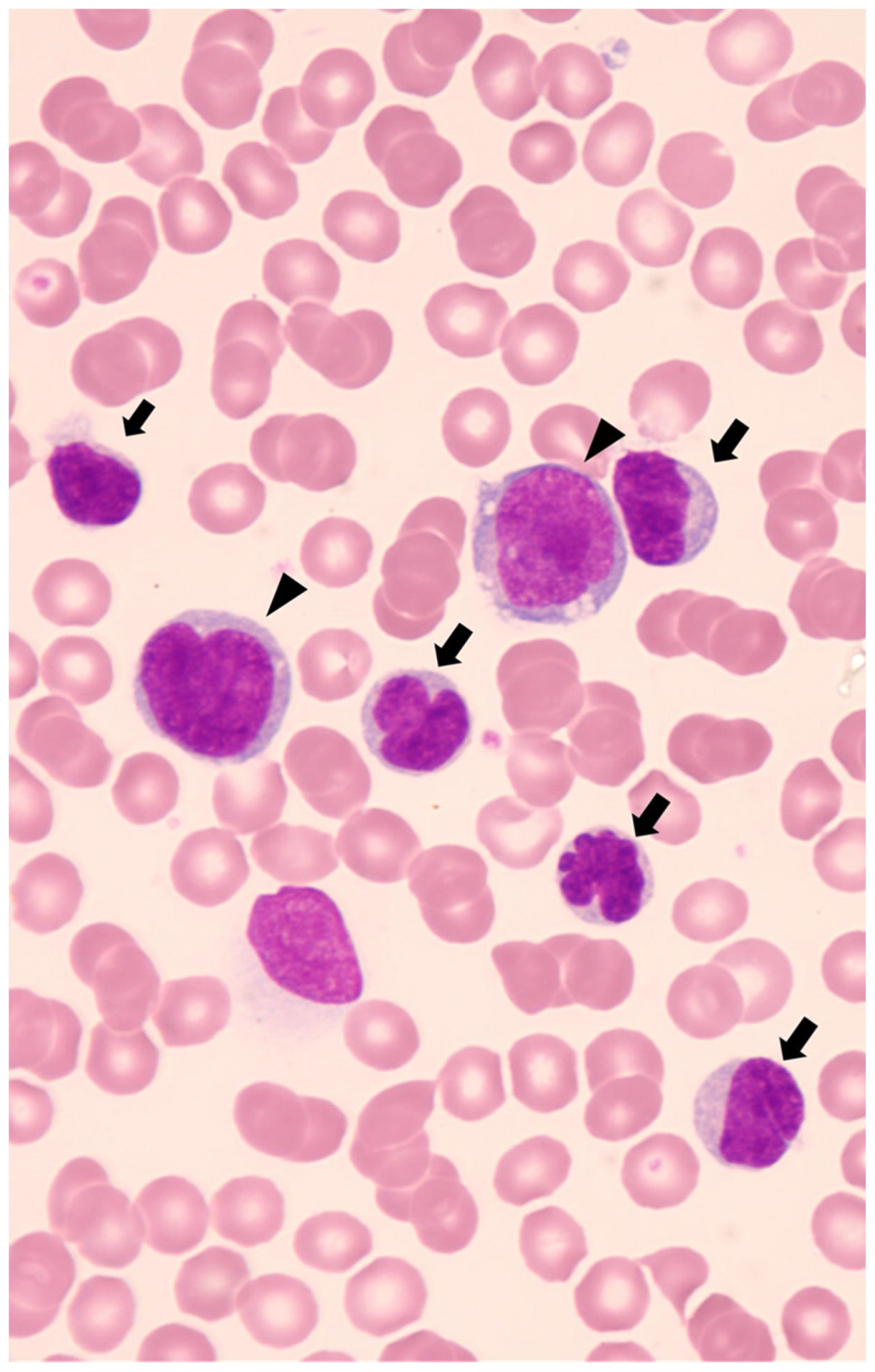
4.1. Morphological Pattern
4.2. Leukemic Presentation
4.3. Minimal Disseminated Disease
4.4. CNS Involvement
5. Treatment
5.1. Treatment for Newly Diagnosed ALK-Positive ALCL
5.2. Treatment for Relapsed or Refractory ALK-Positive ALCL
5.2.1. Weekly Vinblastine
5.2.2. Brentuximab Vedotin
5.2.3. ALK Inhibitors
Crizotinib
Alectinib
Ceritinib
5.2.4. Nivolumab
5.2.5. Hematopoietic Stem Cell Transplantation
Autologous HSCT
Allogeneic HSCT
5.2.6. Treatment Strategy for Pediatric Patients with Relapsed or Refractory ALK-Positive ALCL
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stein, H.; Foss, H.-D. CD30+ Anaplastic Large Cell Lymphoma: A Review of Its Histopathologic, Genetic, and Clinical Features. Blood 2000, 96, 3681–3695. [Google Scholar] [CrossRef] [PubMed]
- Perkins, S.L.; Pickering, D.; Lowe, E.J.; Zwick, D.; Abromowitch, M.; Davenport, G.; Cairo, M.S.; Sanger, W.G. Childhood Anaplastic Large Cell Lymphoma Has a High Incidence of ALK Gene Rearrangement as Determined by Immunohistochemical Staining and Fluorescent in Situ Hybridisation: A Genetic and Pathological Correlation. Br. J. Haematol. 2005, 131, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J. (Eds.) WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; IARC: Lyon, France, 2017; ISBN 978-92-832-4494-3. [Google Scholar]
- Falini, B.; Pileri, S.; Zinzani, P.L.; Carbone, A.; Zagonel, V.; Wolf-Peeters, C.; Verhoef, G.; Menestrina, F.; Todeschini, G.; Paulli, M.; et al. ALK+ Lymphoma: Clinico-Pathological Findings and Outcome. Blood 1999, 93, 2697–2706. [Google Scholar] [PubMed]
- Shiota, M.; Nakamura, S.; Ichinohasama, R.; Abe, M.; Akagi, T.; Takeshita, M.; Mori, N.; Fujimoto, J.; Miyauchi, J.; Mikata, A.; et al. Anaplastic Large Cell Lymphomas Expressing the Novel Chimeric Protein P80NPM/ALK. A Distinct Clinicopathologic Entity. Blood 1995, 86, 1954–1960. [Google Scholar] [CrossRef] [PubMed]
- Tsuyama, N.; Sakamoto, K.; Sakata, S.; Dobashi, A.; Takeuchi, K. Anaplastic Large Cell Lymphoma: Pathology, Genetics, and Clinical Aspects. J. Clin. Exp. Hematop. 2017, 57, 120–142. [Google Scholar] [CrossRef] [PubMed]
- Bohling, S.D.; Jenson, S.D.; Crockett, D.K.; Schumacher, J.A.; Elenitoba-Johnson, K.S.J.; Lim, M.S. Analysis of Gene Expression Profile of TPM3-ALK Positive Anaplastic Large Cell Lymphoma Reveals Overlapping and Unique Patterns with That of NPM-ALK Positive Anaplastic Large Cell Lymphoma. Leuk. Res. 2008, 32, 383–393. [Google Scholar] [CrossRef]
- Mathew, P.; Sanger, W.G.; Weisenburger, D.D.; Valentine, M.; Valentine, V.; Pickering, D.; Higgins, C.; Hess, M.; Cui, X.; Srivastava, D.K.; et al. Detection of the t(2;5)(p23;q35) and NPM-ALK Fusion in Non-Hodgkin’s Lymphoma by Two-Color Fluorescence In Situ Hybridization. Blood 1997, 89, 1678–1685. [Google Scholar] [CrossRef] [PubMed]
- Brugières, L.; Pacquement, H.; Le Deley, M.-C.; Leverger, G.; Lutz, P.; Paillard, C.; Baruchel, A.; Frappaz, D.; Nelken, B.; Lamant, L.; et al. Single-Drug Vinblastine As Salvage Treatment for Refractory or Relapsed Anaplastic Large-Cell Lymphoma: A Report From the French Society of Pediatric Oncology. J. Clin. Oncol. 2009, 27, 5056–5061. [Google Scholar] [CrossRef]
- Lamant, L.; McCarthy, K.; d’Amore, E.; Klapper, W.; Nakagawa, A.; Fraga, M.; Maldyk, J.; Simonitsch-Klupp, I.; Oschlies, I.; Delsol, G.; et al. Prognostic Impact of Morphologic and Phenotypic Features of Childhood ALK-Positive Anaplastic Large-Cell Lymphoma: Results of the ALCL99 Study. J. Clin. Oncol. 2011, 29, 4669–4676. [Google Scholar] [CrossRef]
- Locatelli, F.; Mauz-Koerholz, C.; Neville, K.; Llort, A.; Beishuizen, A.; Daw, S.; Pillon, M.; Aladjidi, N.; Klingebiel, T.; Landman-Parker, J.; et al. Brentuximab Vedotin for Paediatric Relapsed or Refractory Hodgkin’s Lymphoma and Anaplastic Large-Cell Lymphoma: A Multicentre, Open-Label, Phase 1/2 Study. Lancet Haematol. 2018, 5, e450–e461. [Google Scholar] [CrossRef]
- Pro, B.; Advani, R.; Brice, P.; Bartlett, N.L.; Rosenblatt, J.D.; Illidge, T.; Matous, J.; Ramchandren, R.; Fanale, M.; Connors, J.M.; et al. Brentuximab Vedotin (SGN-35) in Patients With Relapsed or Refractory Systemic Anaplastic Large-Cell Lymphoma: Results of a Phase II Study. J. Clin. Oncol. 2012, 30, 2190–2196. [Google Scholar] [CrossRef] [PubMed]
- Pro, B.; Advani, R.; Brice, P.; Bartlett, N.L.; Rosenblatt, J.D.; Illidge, T.; Matous, J.; Ramchandren, R.; Fanale, M.; Connors, J.M.; et al. Five-Year Results of Brentuximab Vedotin in Patients with Relapsed or Refractory Systemic Anaplastic Large Cell Lymphoma. Blood 2017, 130, 2709–2717. [Google Scholar] [CrossRef] [PubMed]
- Woessmann, W.; Zimmermann, M.; Lenhard, M.; Burkhardt, B.; Rossig, C.; Kremens, B.; Lang, P.; Attarbaschi, A.; Mann, G.; Oschlies, I.; et al. Relapsed or Refractory Anaplastic Large-Cell Lymphoma in Children and Adolescents After Berlin-Frankfurt-Muenster (BFM)–Type First-Line Therapy: A BFM-Group Study. J. Clin. Oncol. 2011, 29, 3065–3071. [Google Scholar] [CrossRef] [PubMed]
- Strullu, M.; Thomas, C.; Le Deley, M.-C.; Chevance, A.; Kanold, J.; Bertrand, Y.; Jubert, C.; Dalle, J.-H.; Paillard, C.; Baruchel, A.; et al. Hematopoietic Stem Cell Transplantation in Relapsed ALK+ Anaplastic Large Cell Lymphoma in Children and Adolescents: A Study on Behalf of the SFCE and SFGM-TC. Bone Marrow Transplant. 2015, 50, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Woessmann, W.; Peters, C.; Lenhard, M.; Burkhardt, B.; Sykora, K.-W.; Dilloo, D.; Kremens, B.; Lang, P.; Fuhrer, M.; Kuhne, T.; et al. Allogeneic Haematopoietic Stem Cell Transplantation in Relapsed or Refractory Anaplastic Large Cell Lymphoma of Children and Adolescents—A Berlin-Frankfurt-Munster Group Report. Br. J. Haematol. 2006, 133, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Bischof, D.; Pulford, K.; Mason, D.Y.; Morris, S.W. Role of the Nucleophosmin (NPM) Portion of the Non-Hodgkin’s Lymphoma-Associated NPM-Anaplastic Lymphoma Kinase Fusion Protein in Oncogenesis. Mol. Cell. Biol. 1997, 17, 2312–2325. [Google Scholar] [CrossRef] [PubMed]
- Mussolin, L.; Le Deley, M.-C.; Carraro, E.; Damm-Welk, C.; Attarbaschi, A.; Williams, D.; Burke, A.; Horibe, K.; Nakazawa, A.; Wrobel, G.; et al. Prognostic Factors in Childhood Anaplastic Large Cell Lymphoma: Long Term Results of the International ALCL99 Trial. Cancers 2020, 12, 2747. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.; Mori, T.; Reiter, A.; Woessman, W.; Rosolen, A.; Wrobel, G.; Zsiros, J.; Uyttebroeck, A.; Marky, I.; Le Deley, M.-C.; et al. Central Nervous System Involvement in Anaplastic Large Cell Lymphoma in Childhood: Results from a Multicentre European and Japanese Study: CNS Involvement in Anaplastic Large Cell Lymphoma. Pediatr. Blood Cancer 2013, 60, E118–E121. [Google Scholar] [CrossRef]
- Imamura, R.; Mouri, F.; Nomura, K.; Nakamura, T.; Oku, E.; Morishige, S.; Takata, Y.; Seki, R.; Osaki, K.; Hashiguchi, M.; et al. Successful Treatment of Small Cell Variant Anaplastic Large Cell Lymphoma with Allogeneic Peripheral Blood Stem Cell Transplantation, and Review of the Literature. Int. J. Hematol. 2013, 97, 139–143. [Google Scholar] [CrossRef]
- Noguchi, K.; Ikawa, Y.; Takenaka, M.; Sakai, Y.; Fujiki, T.; Kuroda, R.; Wada, T. Characterisation of Two Tumour Cell Populations in the Small Cell Variant of Anaplastic Lymphoma Kinase-positive Anaplastic Large Cell Lymphoma. Br. J. Haematol. 2022, 196, 241–243. [Google Scholar] [CrossRef]
- Onciu, M.; Behm, F.G.; Raimondi, S.C.; Moore, S.; Harwood, E.L.; Pui, C.-H.; Sandlund, J.T. ALK-Positive Anaplastic Large Cell Lymphoma With Leukemic Peripheral Blood Involvement Is a Clinicopathologic Entity With an Unfavorable Prognosis: Report of Three Cases and Review of the Literature. Am. J. Clin. Pathol. 2003, 120, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, A.; Paillard, C.; Ducassou, S.; Perel, Y.; Plantaz, D.; Strullu, M.; Eischen, A.; Lutz, P.; Lamant, L.; Le Deley, M.-C.; et al. Paediatric Anaplastic Large Cell Lymphoma with Leukaemic Presentation in Children: A Report of Nine French Cases. Br. J. Haematol. 2014, 165, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Damm-Welk, C.; Busch, K.; Burkhardt, B.; Schieferstein, J.; Viehmann, S.; Oschlies, I.; Klapper, W.; Zimmermann, M.; Harbott, J.; Reiter, A.; et al. Prognostic Significance of Circulating Tumor Cells in Bone Marrow or Peripheral Blood as Detected by Qualitative and Quantitative PCR in Pediatric NPM-ALK–Positive Anaplastic Large-Cell Lymphoma. Blood 2007, 110, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Mussolin, L.; Pillon, M.; d’Amore, E.S.; Santoro, N.; Lombardi, A.; Fagioli, F.; Zanesco, L.; Rosolen, A. Prevalence and Clinical Implications of Bone Marrow Involvement in Pediatric Anaplastic Large Cell Lymphoma. Leukemia 2005, 19, 1643–1647. [Google Scholar] [CrossRef] [PubMed]
- Iijima-Yamashita, Y.; Mori, T.; Nakazawa, A.; Fukano, R.; Takimoto, T.; Tsurusawa, M.; Kobayashi, R.; Horibe, K. Prognostic Impact of Minimal Disseminated Disease and Immune Response to NPM-ALK in Japanese Children with ALK-Positive Anaplastic Large Cell Lymphoma. Int. J. Hematol. 2018, 107, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Damm-Welk, C.; Lovisa, F.; Contarini, G.; Lüdersen, J.; Carraro, E.; Knörr, F.; Förster, J.; Zimmermann, M.; Sala, A.; Vinti, L.; et al. Quantification of Minimal Disease by Digital PCR in ALK-Positive Anaplastic Large Cell Lymphoma: A Step towards Risk Stratification in International Trials? Cancers 2022, 14, 1703. [Google Scholar] [CrossRef] [PubMed]
- Damm-Welk, C.; Kutscher, N.; Zimmermann, M.; Attarbaschi, A.; Schieferstein, J.; Knörr, F.; Oschlies, I.; Klapper, W.; Woessmann, W. Quantification of Minimal Disseminated Disease by Quantitative Polymerase Chain Reaction and Digital Polymerase Chain Reaction for NPM-ALK as a Prognostic Factor in Children with Anaplastic Large Cell Lymphoma. Haematologica 2020, 105, 2141–2149. [Google Scholar] [CrossRef] [PubMed]
- Brugières, L.; Deley, M.C.L.; Pacquement, H.; Meguerian-Bedoyan, Z.; Terrier-Lacombe, M.J.; Robert, A.; Pondarré, C.; Leverger, G.; Devalck, C.; Rodary, C.; et al. CD30+ Anaplastic Large-Cell Lymphoma in Children: Analysis of 82 Patients Enrolled in Two Consecutive Studies of the French Society of Pediatric Oncology. Blood 1998, 92, 3591–3598. [Google Scholar]
- Seidemann, K. Short-Pulse B-Non-Hodgkin Lymphoma-Type Chemotherapy Is Efficacious Treatment for Pediatric Anaplastic Large Cell Lymphoma: A Report of the Berlin-Frankfurt-Munster Group Trial NHL-BFM 90. Blood 2001, 97, 3699–3706. [Google Scholar] [CrossRef]
- Williams, D.M.; Hobson, R.; Imeson, J.; Gerrard, M.; McCarthy, K.; Pinkerton, C.R.; The United Kingdom Children’s Cancer Study Group. Anaplastic Large Cell Lymphoma in Childhood: Analysis of 72 Patients Treated on The United Kingdom Children’s Cancer Study Group Chemotherapy Regimens. Br. J. Haematol. 2002, 117, 812–820. [Google Scholar] [CrossRef]
- Rosolen, A.; Pillon, M.; Garaventa, A.; Burnelli, R.; d’Amore, E.S.; Giuliano, M.; Comis, M.; Cesaro, S.; Tettoni, K.; Luisa Moleti, M.; et al. Anaplastic Large Cell Lymphoma Treated with a Leukemia-like Therapy: Report of the Italian Association of Pediatric Hematology and Oncology (AIEOP) LNH-92 Protocol. Cancer 2005, 104, 2133–2140. [Google Scholar] [CrossRef] [PubMed]
- Laver, J.H.; Kraveka, J.M.; Hutchison, R.E.; Chang, M.; Kepner, J.; Schwenn, M.; Tarbell, N.; Desai, S.; Weitzman, S.; Weinstein, H.J.; et al. Advanced-Stage Large-Cell Lymphoma in Children and Adolescents: Results of a Randomized Trial Incorporating Intermediate-Dose Methotrexate and High-Dose Cytarabine in the Maintenance Phase of the APO Regimen: A Pediatric Oncology Group Phase III Trial. J. Clin. Oncol. 2005, 23, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Lowe, E.J.; Sposto, R.; Perkins, S.L.; Gross, T.G.; Finlay, J.; Zwick, D.; Abromowitch, M. Intensive Chemotherapy for Systemic Anaplastic Large Cell Lymphoma in Children and Adolescents: Final Results of Children’s Cancer Group Study 5941. Pediatr. Blood Cancer 2009, 52, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.; Kraveka, J.M.; Weitzman, S.; Lowe, E.; Smith, L.; Lynch, J.C.; Chang, M.; Kinney, M.C.; Perkins, S.L.; Laver, J.; et al. Advanced Stage Anaplastic Large Cell Lymphoma in Children and Adolescents: Results of ANHL0131, a Randomized Phase III Trial of APO versus a Modified Regimen with Vinblastine: A Report from the Children’s Oncology Group. Pediatr. Blood Cancer 2014, 61, 2236–2242. [Google Scholar] [CrossRef] [PubMed]
- Lowe, E.J.; Reilly, A.F.; Lim, M.S.; Gross, T.G.; Saguilig, L.; Barkauskas, D.A.; Wu, R.; Alexander, S.; Bollard, C.M. Brentuximab Vedotin in Combination with Chemotherapy for Pediatric Patients with ALK+ ALCL: Results of COG Trial ANHL12P1. Blood 2021, 137, 3595–3603. [Google Scholar] [CrossRef] [PubMed]
- Lowe, E.J.; Reilly, A.F.; Lim, M.S.; Gross, T.G.; Saguilig, L.; Barkauskas, D.A.; Wu, R.; Alexander, S.; Bollard, C.M. Crizotinib in Combination With Chemotherapy for Pediatric Patients With ALK+ Anaplastic Large-Cell Lymphoma: The Results of Children’s Oncology Group Trial ANHL12P1. J. Clin. Oncol. 2023, 41, 2043–2053. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, S.; O’Connor, O.A.; Pro, B.; Illidge, T.; Fanale, M.; Advani, R.; Bartlett, N.L.; Christensen, J.H.; Morschhauser, F.; Domingo-Domenech, E.; et al. Brentuximab Vedotin with Chemotherapy for CD30-Positive Peripheral T-Cell Lymphoma (ECHELON-2): A Global, Double-Blind, Randomised, Phase 3 Trial. Lancet 2019, 393, 229–240. [Google Scholar] [CrossRef]
- Gadner, H.; Grois, N.; Arico, M.; Broadbent, V.; Ceci, A.; Jakobson, A.; Komp, D.; Michaelis, J.; Nicholson, S.; Pötschger, U.; et al. A Randomized Trial of Treatment for Multisystem Langerhans’ Cell Histiocytosis. J. Pediatr. 2001, 138, 728–734. [Google Scholar] [CrossRef]
- Gadgeel, S.M.; Gandhi, L.; Riely, G.J.; Chiappori, A.A.; West, H.L.; Azada, M.C.; Morcos, P.N.; Lee, R.-M.; Garcia, L.; Yu, L.; et al. Safety and Activity of Alectinib against Systemic Disease and Brain Metastases in Patients with Crizotinib-Resistant ALK-Rearranged Non-Small-Cell Lung Cancer (AF-002JG): Results from the Dose-Finding Portion of a Phase 1/2 Study. Lancet Oncol. 2014, 15, 1119–1128. [Google Scholar] [CrossRef]
- Saito, S.; Tashiro, H.; Sumiyoshi, R.; Matsuo, T.; Yamamoto, T.; Matsumoto, K.; Ooi, J.; Shirafuji, N. Second Allogeneic Transplantation Using Umbilical Cord Blood for a Patient with Relapsed ALK+ Anaplastic Large Cell Lymphoma after Allogeneic Bone Marrow Transplantation in the Era of ALK Inhibitors: A Case Report. Medicine 2021, 100, e25576. [Google Scholar] [CrossRef]
- Subbiah, V.; Kuravi, S.; Ganguly, S.; Welch, D.R.; Vivian, C.J.; Mushtaq, M.U.; Hegde, A.; Iyer, S.; Behrang, A.; Ali, S.M.; et al. Precision Therapy with Anaplastic Lymphoma Kinase Inhibitor Ceritinib in ALK-Rearranged Anaplastic Large Cell Lymphoma. ESMO Open 2021, 6, 100172. [Google Scholar] [CrossRef] [PubMed]
- Mossé, Y.P.; Voss, S.D.; Lim, M.S.; Rolland, D.; Minard, C.G.; Fox, E.; Adamson, P.; Wilner, K.; Blaney, S.M.; Weigel, B.J. Targeting ALK With Crizotinib in Pediatric Anaplastic Large Cell Lymphoma and Inflammatory Myofibroblastic Tumor: A Children’s Oncology Group Study. J. Clin. Oncol. 2017, 35, 3215–3221. [Google Scholar] [CrossRef] [PubMed]
- Hebart, H.; Lang, P.; Woessmann, W. Nivolumab for Refractory Anaplastic Large Cell Lymphoma: A Case Report. Ann. Intern. Med. 2016, 165, 607–608. [Google Scholar] [CrossRef] [PubMed]
- Rigaud, C.; Abbou, S.; Minard-Colin, V.; Geoerger, B.; Scoazec, J.Y.; Vassal, G.; Jaff, N.; Heuberger, L.; Valteau-Couanet, D.; Brugieres, L. Efficacy of Nivolumab in a Patient with Systemic Refractory ALK+ Anaplastic Large Cell Lymphoma. Pediatr. Blood Cancer 2018, 65, e26902. [Google Scholar] [CrossRef] [PubMed]
- Ruf, S.; Hebart, H.; Hjalgrim, L.L.; Kabickova, E.; Lang, P.; Steinbach, D.; Schwabe, G.C.; Woessmann, W. CNS Progression during Vinblastine or Targeted Therapies for High-risk Relapsed ALK-positive Anaplastic Large Cell Lymphoma: A Case Series. Pediatr. Blood Cancer 2018, 65, e27003. [Google Scholar] [CrossRef] [PubMed]
- Van Der Weyden, C.A.; Pileri, S.A.; Feldman, A.L.; Whisstock, J.; Prince, H.M. Understanding CD30 Biology and Therapeutic Targeting: A Historical Perspective Providing Insight into Future Directions. Blood Cancer J. 2017, 7, e603. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, M.D.; Evens, A.M.; Fenske, T.S.; Hamlin, P.; Coiffier, B.; Engert, A.; Moskowitz, A.J.; Ghosh, N.; Petrich, A.M.; Lomasney, J.; et al. Pancreatitis in Patients Treated with Brentuximab Vedotin: A Previously Unrecognized Serious Adverse Event. Blood 2014, 123, 2895–2897. [Google Scholar] [CrossRef] [PubMed]
- Carson, K.R.; Newsome, S.D.; Kim, E.J.; Wagner-Johnston, N.D.; Von Geldern, G.; Moskowitz, C.H.; Moskowitz, A.J.; Rook, A.H.; Jalan, P.; Loren, A.W.; et al. Progressive Multifocal Leukoencephalopathy Associated with Brentuximab Vedotin Therapy: A Report of 5 Cases from the Southern Network on Adverse Reactions (SONAR) Project: Brentuximab-Associated PML. Cancer 2014, 120, 2464–2471. [Google Scholar] [CrossRef]
- Chen, R.; Hou, J.; Newman, E.; Kim, Y.; Donohue, C.; Liu, X.; Thomas, S.H.; Forman, S.J.; Kane, S.E. CD30 Downregulation, MMAE Resistance, and MDR1 Upregulation Are All Associated with Resistance to Brentuximab Vedotin. Mol. Cancer Ther. 2015, 14, 1376–1384. [Google Scholar] [CrossRef]
- Al-Rohil, R.N.; Torres-Cabala, C.A.; Patel, A.; Tetzlaff, M.T.; Ivan, D.; Nagarajan, P.; Curry, J.L.; Miranda, R.N.; Duvic, M.; Prieto, V.G.; et al. Loss of CD30 Expression after Treatment with Brentuximab Vedotin in a Patient with Anaplastic Large Cell Lymphoma: A Novel Finding. J. Cutan. Pathol. 2016, 43, 1161–1166. [Google Scholar] [CrossRef]
- Jagadeesh, D.; Horwitz, S.; Bartlett, N.L.; Kim, Y.; Jacobsen, E.; Duvic, M.; Little, M.; Trepicchio, W.; Fenton, K.; Onsum, M.; et al. Response to Brentuximab Vedotin by CD30 Expression in Non-Hodgkin Lymphoma. Oncology 2022, 27, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Kwak, E.L.; Shaw, A.T.; Ou, S.-H.I.; Varella-Garcia, M.; Stubbs, H.; Gandhi, L.; Ratain, M.J.; Wilner, K.; Iafrate, A.J. Anaplastic Lymphoma Kinase Inhibition in Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2010, 363, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Richly, H.; Kim, T.M.; Schuler, M.; Kim, D.-W.; Harrison, S.J.; Shaw, A.T.; Boral, A.L.; Yovine, A.; Solomon, B. Ceritinib in Patients with Advanced Anaplastic Lymphoma Kinase–Rearranged Anaplastic Large-Cell Lymphoma. Blood 2015, 126, 1257–1258. [Google Scholar] [CrossRef]
- Iseki, M.; Kaburaki, T.; Aihara, M.; Sawamura, H. Late-Onset Ocular Toxicity Presenting as Uveitis Caused by Crizotinib. Neuro-Ophthalmol. 2022, 46, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Gambacorti-Passerini, C.; Mussolin, L.; Brugieres, L. Abrupt Relapse of ALK-Positive Lymphoma after Discontinuation of Crizotinib. N. Engl. J. Med. 2016, 374, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Cleary, J.M.; Rodig, S.; Barr, P.M.; Shinagare, A.B.; Clark, J.W.; Shapiro, G.I.; Armand, P. Crizotinib as Salvage and Maintenance With Allogeneic Stem Cell Transplantation for Refractory Anaplastic Large Cell Lymphoma. J. Natl. Compr. Cancer Netw. 2014, 12, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Gambacorti Passerini, C.; Farina, F.; Stasia, A.; Redaelli, S.; Ceccon, M.; Mologni, L.; Messa, C.; Guerra, L.; Giudici, G.; Sala, E.; et al. Crizotinib in Advanced, Chemoresistant Anaplastic Lymphoma Kinase–Positive Lymphoma Patients. JNCI J. Natl. Cancer Inst. 2014, 106, djt378. [Google Scholar] [CrossRef]
- Kay, M.; Dehghanian, F. Exploring the Crizotinib Resistance Mechanism of NSCLC with the L1196M Mutation Using Molecular Dynamics Simulation. J. Mol. Model. 2017, 23, 323. [Google Scholar] [CrossRef]
- Sakamoto, H.; Tsukaguchi, T.; Hiroshima, S.; Kodama, T.; Kobayashi, T.; Fukami, T.A.; Oikawa, N.; Tsukuda, T.; Ishii, N.; Aoki, Y. CH5424802, a Selective ALK Inhibitor Capable of Blocking the Resistant Gatekeeper Mutant. Cancer Cell 2011, 19, 679–690. [Google Scholar] [CrossRef]
- Fukano, R.; Mori, T.; Sekimizu, M.; Choi, I.; Kada, A.; Saito, A.M.; Asada, R.; Takeuchi, K.; Terauchi, T.; Tateishi, U.; et al. Alectinib for Relapsed or Refractory Anaplastic Lymphoma Kinase-positive Anaplastic Large Cell Lymphoma: An Open-label Phase II Trial. Cancer Sci. 2020, 111, 4540–4547. [Google Scholar] [CrossRef]
- Gainor, J.F.; Dardaei, L.; Yoda, S.; Friboulet, L.; Leshchiner, I.; Katayama, R.; Dagogo-Jack, I.; Gadgeel, S.; Schultz, K.; Singh, M.; et al. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov. 2016, 6, 1118–1133. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, K.; Ikawa, Y.; Takenaka, M.; Sakai, Y.; Fujiki, T.; Kuroda, R.; Ikeda, H.; Abe, T.; Sakai, S.; Wada, T. Acquired L1196M ALK Mutation in Anaplastic Lymphoma Kinase-positive Anaplastic Large Cell Lymphoma during Alectinib Administration. eJHaem 2023, 4, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, J.; Gu, W.-Y.; Jin, L.; Duan, Y.-L.; Huang, S.; Zhang, M.; Wang, X.-S.; Liu, Y.; Zhou, C.-J.; et al. Central Nervous System Relapse in a Pediatric Anaplastic Large Cell Lymphoma Patient with CLTC/ALK Translocation Treated with Alectinib: A Case Report. World J. Clin. Cases 2020, 8, 1685–1692. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Kim, D.-W.; Mehra, R.; Tan, D.S.W.; Felip, E.; Chow, L.Q.M.; Camidge, D.R.; Vansteenkiste, J.; Sharma, S.; De Pas, T.; et al. Ceritinib in ALK -Rearranged Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2014, 370, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Brugières, L.; Quartier, P.; Le Deley, M.C.; Pacquement, H.; Perel, Y.; Bergeron, C.; Schmitt, C.; Landmann, J.; Patte, C.; Terrier-Lacombe, M.J.; et al. Relapses of Childhood Anaplastic Large-Cell Lymphoma: Treatment Results in a Series of 41 Children—A Report from the French Society of Pediatric Oncology. Ann. Oncol. 2000, 11, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Fukano, R.; Mori, T.; Kobayashi, R.; Mitsui, T.; Fujita, N.; Iwasaki, F.; Suzumiya, J.; Chin, M.; Goto, H.; Takahashi, Y.; et al. Haematopoietic Stem Cell Transplantation for Relapsed or Refractory Anaplastic Large Cell Lymphoma: A Study of Children and Adolescents in Japan. Br. J. Haematol. 2015, 168, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Gross, T.G.; Hale, G.A.; He, W.; Camitta, B.M.; Sanders, J.E.; Cairo, M.S.; Hayashi, R.J.; Termuhlen, A.M.; Zhang, M.-J.; Davies, S.M.; et al. Hematopoietic Stem Cell Transplantation for Refractory or Recurrent Non-Hodgkin Lymphoma in Children and Adolescents. Biol. Blood Marrow Transplant. 2010, 16, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Knörr, F.; Brugières, L.; Pillon, M.; Zimmermann, M.; Ruf, S.; Attarbaschi, A.; Mellgren, K.; Burke, G.A.A.; Uyttebroeck, A.; Wróbel, G.; et al. Stem Cell Transplantation and Vinblastine Monotherapy for Relapsed Pediatric Anaplastic Large Cell Lymphoma: Results of the International, Prospective ALCL-Relapse Trial. J. Clin. Oncol. 2020, 38, 3999–4009. [Google Scholar] [CrossRef]
- Fossard, G.; Broussais, F.; Coelho, I.; Bailly, S.; Nicolas-Virelizier, E.; Toussaint, E.; Lancesseur, C.; Le Bras, F.; Willems, E.; Tchernonog, E.; et al. Role of Up-Front Autologous Stem-Cell Transplantation in Peripheral T-Cell Lymphoma for Patients in Response after Induction: An Analysis of Patients from LYSA Centers. Ann. Oncol. 2018, 29, 715–723. [Google Scholar] [CrossRef]
- Fukano, R.; Mori, T.; Fujita, N.; Kobayashi, R.; Mitsui, T.; Kato, K.; Suzuki, R.; Suzumiya, J.; Fukuda, T.; Shindo, M.; et al. Successful Outcome with Reduced-Intensity Condition Regimen Followed by Allogeneic Hematopoietic Stem Cell Transplantation for Relapsed or Refractory Anaplastic Large-Cell Lymphoma. Int. J. Hematol. 2019, 110, 723–728. [Google Scholar] [CrossRef]
- Bayle, C.; Charpentier, A.; Duchayne, E.; Manel, A.-M.; Pages, M.-P.; Robert, A.; Lamant, L.; Dastugue, N.; Bertrand, Y.; Dijoud, F.; et al. Leukaemic Presentation of Small Cell Variant Anaplastic Large Cell Lymphoma: Report of Four Cases: Leukaemic Form of Anaplastic Large Cell Lymphoma. Br. J. Haematol. 1999, 104, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Villamor, N.; Rozman, M.; Esteve, J.; Aymerich, M.; Colomer, D.; Aguilar, J.L.; Campo, E.; Montserrat, E. Anaplastic Large-Cell Lymphoma with Rapid Evolution to Leukemic Phase. Ann. Hematol. 1999, 78, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Meech, S.J.; McGavran, L.; Odom, L.F.; Liang, X.; Meltesen, L.; Gump, J.; Wei, Q.; Carlsen, S.; Hunger, S.P. Unusual Childhood Extramedullary Hematologic Malignancy with Natural Killer Cell Properties That Contains Tropomyosin 4–Anaplastic Lymphoma Kinase Gene Fusion. Blood 2001, 98, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Awaya, N.; Mori, S.; Takeuchi, H.; Mori, S.; Sugano, Y.; Kamata, T.; Takeuchi, T.; Abe, T. CD30 and the NPM-ALK Fusion Protein (P80) Are Differentially Expressed between Peripheral Blood and Bone Marrow in Primary Small Cell Variant of Anaplastic Large Cell Lymphoma. Am. J. Hematol. 2002, 69, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.-Y.; Cho, H.-J.; Suk, J.-H.; Tak, E.-Y.; Ko, Y.H.; Kim, K.; Kim, S.-H. A Novel Complex t(2;5;13)(P23;Q35;Q14) in Small Cell Variant Type Anaplastic Large Cell Lymphoma with Peripheral Involvement. Cancer Genet. Cytogenet. 2004, 154, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Grewal, J.S.; Smith, L.B.; Winegarden, J.D.; Krauss, J.C.; Tworek, J.A.; Schnitzer, B. Highly Aggressive ALK-Positive Anaplastic Large Cell Lymphoma with a Leukemic Phase and Multi-Organ Involvement: A Report of Three Cases and a Review of the Literature. Ann. Hematol. 2007, 86, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, D.; Nagatoshi, Y.; Nagayama, J.; Inagaki, J.; Itonoaga, N.; Takeshita, M.; Okamura, J. Anaplastic Large Cell Lymphoma in Leukemic Presentation: A Case Report and a Review of the Literature. J. Pediatr. Hematol./Oncol. 2008, 30, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.T.; Condron, M.R.; Nguyen, N.D.; De, J.; Medeiros, L.J.; Padula, A. Anaplastic Large Cell Lymphoma in Leukemic Phase: Extraordinarily High White Blood Cell Count. Pathol. Int. 2009, 59, 345–353. [Google Scholar] [CrossRef]
- Nakai, R.; Fukuhara, S.; Maeshima, A.M.; Kim, S.; Ito, Y.; Hatta, S.; Suzuki, T.; Yuda, S.; Makita, S.; Munakata, W.; et al. Alectinib, an Anaplastic Lymphoma Kinase (ALK) Inhibitor, as a Bridge to Allogeneic Stem Cell Transplantation in a Patient with ALK-positive Anaplastic Large-cell Lymphoma Refractory to Chemotherapy and Brentuximab Vedotin. Clin. Case Rep. 2019, 7, 2500–2504. [Google Scholar] [CrossRef]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). In Non-Hodgkin’s Lymphomas; NCCN: Plymouth Meeting, PA, USA, 2015.
- Tanaka, M.; Miura, H.; Ishimaru, S.; Furukawa, G.; Kawamura, Y.; Kozawa, K.; Yamada, S.; Ito, F.; Kudo, K.; Yoshikawa, T. Future Perspective for ALK-Positive Anaplastic Large Cell Lymphoma with Initial Central Nervous System (CNS) Involvement: Could Next-Generation ALK Inhibitors Replace Brain Radiotherapy for the Prevention of Further CNS Relapse? Pediatr. Rep. 2023, 15, 333–340. [Google Scholar] [CrossRef]
| Protocol | Study Group | Study Period | Treatment Strategy | Number of Patients | EFS (Year) | OS (Year) | Reference |
|---|---|---|---|---|---|---|---|
| HM89/91 | SFOP | 1988–1997 | COPADM (CY, DXR, PSL, MTX, VCR) × 2 with maintenance treatment | 82 | 66% (3) | 83% (3) | Blood. 1998 [29] |
| NHL-BFM90 | BFM | 1990–1995 | K1/2 arm: 3/6 courses (MTX, DEX, oxazaphorins, ETP, AraC, DXR, IT) K3 arm: 6 intensified courses including HD-MTX/HD-AraC/HD-ETP | 89 | 76% (5) | ND | Blood. 2001 [30] |
| NHL 9000/9602 | UKCCSG | 1990–1998 | NHL 9000 including VCR, DXR, PSL, MTX, AraC, 6-TG NHL 9602 including COPADM × 3 with CYM (CY, MTX) × 2 intensified courses including HD-MTX/HD-AraC for CNS-positive disease | 72 | 59% (5) | 65% (5) | Br. J. Haematol. 2002 [31] |
| LNH92 | AIEOP | 1993–1997 | Induction therapy (CY, VCR, DEX, DNR, IT), consolidation therapy (6-TG, AraC, ASP, HD-MTX, IT) with maintenance treatment | 34 | 65% (5) | 85% (5) | Cancer. 2005 [32] |
| POG9315 | POG | 1994–2000 | APO (DXR, VCR, PSL, 6MP, MTX) with randomization of ID-MTX and HD-AraC | 86 | 72% (4) | 88% (4) | J. Clin. Oncol. 2005 [33] |
| CCG5941 | CCG | 1996–2001 | Induction therapy (VCR, PSL, CY, DNR, ASP, IT, G-CSF), consolidation therapy (VCR, PSL, ETP, 6-TG, AraC, ASP, MTX, IT, G-CSF) with maintenance treatment | 86 | 68% (5) | 80% (5) | Pediatr. Blood Cancer. 2009 [34] |
| ALCL99 | EICNHL | 1999–2006 | DEX, CY, IT, IFO, AraC, ETP with randomization of VBL | 352 | 72% (10) | 92% (10) | Cancers (Basel). 2020 [18] |
| ANHL0131 | COG | 2004–2008 | APO (DXR, VCR, PSL, 6MP, MTX) with randomization of VBL | 125 | Non-VBL arm: 74% (3) VBL arm: 79% (3) | Non-VBL arm: 84% (3) VBL arm: 86% (3) | Pediatr. Blood Cancer. 2014 [35] |
| Number of Patients | CR Rate | EFS (Year) | OS (Year) | CNS Penetrability | Adverse Effect | Reference | ||
|---|---|---|---|---|---|---|---|---|
| Weekly vinblastine | 31 | 83% | 65% (5) | 30% (5) | Low | Mild leukopenia (34%) | J. Pediatr. 2001 [39], J. Clin. Oncol. 2009 [9] | |
| Brentuximab vedotin | 17 | 41% (ORR: 53%) | ND | ND | Low | Fever (44%), nausea (36%), peripheral neuropathy (33%) | Lancet Haematol. 2018 [11] | |
| ALK inhibitor | Crizotinib | 26 | 81% (ORR: 88%) | ND | ND | Low | Visual disturbance (40–60%), gastrointestinal symptoms (29–54%) | J. Clin. Oncol. 2017 [40] |
| Alectinib | 10 | 60% (ORR: 80%) | 70% (1) | 70% (1) | High | Gastrointestinal symptoms (<15%) | Cancer Sci. 2020 [41] | |
| Ceritinib | 3 | 2 out of 3 cases: CR 1 out of 3 cases: PR | ND | ND | ND | Gastrointestinal symptoms (32–82%) | Blood. 2015 [42], ESMO Open. 2021 [43] | |
| Nivolumab | 2 case reports | 2 out of 2 cases: CR | ND | ND | ND | ND | Ann. Intern. Med. 2016 [44], Pediatr. Blood Cancer. 2018 [45] | |
| Case | Age (Year)/Sex | CNS Lesion | Extranodal Lesion | 1st Line | 2nd Line | 3rd Line | HSCT | Status at HSCT | Conditioning Regimen | Donor Source | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1/M | ND | Li, Sp, PE | CsA, mPSL, CY, AraC, DXR, VP, MTX → CR | - | - | Blood. 2001 [74] | ||||
| 2 | 12/F | - | Li, Sp | ALCL99+VBL → CR | - | - | Br. J. Haematol. 2014 [23] | ||||
| 3 | 10/F | - | Li, Sp, Sk, Lu | ALCL99 → PR | DEX, VDS, AraC, VP → CR | + | 1st CR | ND | ND | Br. J. Haematol. 2014 [23] | |
| 4 | 11/M | - | Li, Sp, Sk, Lu, A | ALCL99 → CR → relapse | VBL+glucocorticoid → 2nd CR | + | 2nd CR | ND | ND | Br. J. Haematol. 2014 [23] | |
| 5 | 18/F | - | Li, Sp, Sk, Lu | CHOP → relapse | chemotherapy | + | 2nd CR | ND | Allo | Br. J. Haematol. 1999 [72] | |
| 6 | 6/F | - | K, Lu | DXR, PSL, VCR → PR | MTX, IFO, VP, DEX → PR | AraC, CCNU, VBL, BLM → PR | + | PR | TBI/TEPA/VP/CY/alemtuzumab | Allo | Am. J. Clin. Pathol. 2003 [22] |
| 7 | 40/M | + | Li, Sp | chemotherapy for ALL → PR, CNS+ | IT, MTX, AraC → CNS- | + | PR | TBI/VP/CY | Allo | Int. Hematol. 2013 [20] | |
| 8 | 10/M | - | Li, Sp, Lu, Sk, PE | ALCL99 → PR, CNS+ | alectinib → CR | + | 1st CR | TBI/VP/CY | Allo | Br. J. Haematol. 2022 [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noguchi, K.; Ikawa, Y. Strategy for Pediatric Patients with Relapsed or Refractory Anaplastic Lymphoma Kinase-Positive Anaplastic Large Cell Lymphoma: A Review. Cancers 2023, 15, 5733. https://doi.org/10.3390/cancers15245733
Noguchi K, Ikawa Y. Strategy for Pediatric Patients with Relapsed or Refractory Anaplastic Lymphoma Kinase-Positive Anaplastic Large Cell Lymphoma: A Review. Cancers. 2023; 15(24):5733. https://doi.org/10.3390/cancers15245733
Chicago/Turabian StyleNoguchi, Kazuhiro, and Yasuhiro Ikawa. 2023. "Strategy for Pediatric Patients with Relapsed or Refractory Anaplastic Lymphoma Kinase-Positive Anaplastic Large Cell Lymphoma: A Review" Cancers 15, no. 24: 5733. https://doi.org/10.3390/cancers15245733





