Impact of Low Skeletal Muscle Mass on Long-Term Outcomes in Hepatocellular Carcinoma Treated with Trans-Arterial Radioembolization: A Retrospective Multi-Center Study
Abstract
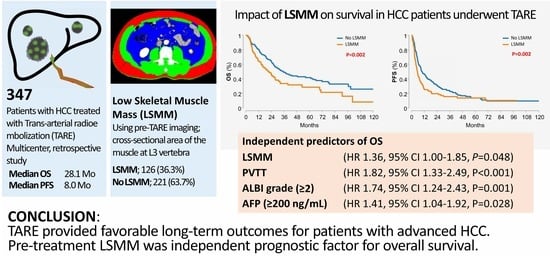
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Endpoints
2.3. Data Collection
2.4. TARE Procedure
2.5. Outcomes and Assessments
2.6. Measurement of Skeletal Muscle Mass
2.7. Definition of LSMM
2.8. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Response to TARE
3.3. Factors Associated with Overall Survival
3.4. Factors Associated with Progression-Free Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019, 5, 1749–1768. [Google Scholar] [PubMed]
- Petrick, J.L.; Kelly, S.P.; Altekruse, S.F.; McGlynn, K.A.; Rosenberg, P.S. Future of Hepatocellular Carcinoma Incidence in the United States Forecast Through 2030. J. Clin. Oncol. 2016, 34, 1787–1794. [Google Scholar] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [PubMed]
- Omata, M.; Cheng, A.L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.H.; Chawla, Y.K.; Shiina, S.; et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar]
- Choi, J.W.; Kim, H.C. Radioembolization for hepatocellular carcinoma: What clinicians need to know. J. Liver Cancer 2022, 22, 4–13. [Google Scholar]
- Martin, L.; Birdsell, L.; Macdonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar]
- Fujiwara, N.; Nakagawa, H.; Kudo, Y.; Tateishi, R.; Taguri, M.; Watadani, T.; Nakagomi, R.; Kondo, M.; Nakatsuka, T.; Minami, T.; et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J. Hepatol. 2015, 63, 131–140. [Google Scholar]
- Oura, K.; Morishita, A.; Manabe, T.; Takuma, K.; Nakahara, M.; Tadokoro, T.; Fujita, K.; Mimura, S.; Tani, J.; Ono, M.; et al. Relationship between Accurate Diagnosis of Sarcopenia and Prognosis in Patients with Hepatocellular Carcinoma Treated with Atezolizumab plus Bevacizumab Combination Therapy. Cancers 2023, 15, 3243. [Google Scholar]
- Imai, K.; Takai, K.; Unome, S.; Miwa, T.; Hanai, T.; Suetsugu, A.; Shimizu, M. Lenvatinib or Sorafenib Treatment Causing a Decrease in Skeletal Muscle Mass, an Independent Prognostic Factor in Hepatocellular Carcinoma: A Survival Analysis Using Time-Varying Covariates. Cancers 2023, 15, 4223. [Google Scholar]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [PubMed]
- March, C.; Omari, J.; Thormann, M.; Pech, M.; Wienke, A.; Surov, A. Prevalence and role of low skeletal muscle mass (LSMM) in hepatocellular carcinoma. A systematic review and meta-analysis. Clin. Nutr. ESPEN 2022, 49, 103–113. [Google Scholar] [PubMed]
- Jiang, C.; Wang, Y.; Fu, W.; Zhang, G.; Feng, X.; Wang, X.; Wang, F.; Zhang, L.; Deng, Y. Association between sarcopenia and prognosis of hepatocellular carcinoma: A systematic review and meta-analysis. Front. Nutr. 2022, 9, 978110. [Google Scholar] [PubMed]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [PubMed]
- Lee, J.S.; Kim, B.K.; Kim, S.U.; Park, J.Y.; Ahn, S.H.; Seong, J.S.; Han, K.H.; Kim, D.Y. A survey on transarterial chemoembolization refractoriness and a real-world treatment pattern for hepatocellular carcinoma in Korea. Clin. Mol. Hepatol. 2020, 26, 24–32. [Google Scholar]
- Salem, R.; Gabr, A.; Riaz, A.; Mora, R.; Ali, R.; Abecassis, M.; Hickey, R.; Kulik, L.; Ganger, D.; Flamm, S.; et al. Institutional decision to adopt Y90 as primary treatment for hepatocellular carcinoma informed by a 1000-patient 15-year experience. Hepatology 2018, 68, 1429–1440. [Google Scholar]
- Kim, J.; Kim, J.Y.; Lee, J.H.; Sinn, D.H.; Hur, M.H.; Hong, J.H.; Park, M.K.; Cho, H.J.; Choi, N.R.; Lee, Y.B.; et al. Long-Term Outcomes of Transarterial Radioembolization for Large Single Hepatocellular Carcinoma: A Comparison to Resection. J. Nucl. Med. 2022, 63, 1215–1222. [Google Scholar]
- Salem, R.; Gilbertsen, M.; Butt, Z.; Memon, K.; Vouche, M.; Hickey, R.; Baker, T.; Abecassis, M.M.; Atassi, R.; Riaz, A.; et al. Increased quality of life among hepatocellular carcinoma patients treated with radioembolization, compared with chemoembolization. Clin. Gastroenterol. Hepatol. 2013, 11, 1358–1365. [Google Scholar]
- Biederman, D.M.; Titano, J.J.; Tabori, N.E.; Pierobon, E.S.; Alshebeeb, K.; Schwartz, M.; Facciuto, M.E.; Gunasekaran, G.; Florman, S.; Fischman, A.M.; et al. Outcomes of Radioembolization in the Treatment of Hepatocellular Carcinoma with Portal Vein Invasion: Resin versus Glass Microspheres. J. Vasc. Interv. Radiol. 2016, 27, 812–821. [Google Scholar]
- Guo, Y.; Ren, Y.; Zhu, L.; Yang, L.; Zheng, C. Association between sarcopenia and clinical outcomes in patients with hepatocellular carcinoma: An updated meta-analysis. Sci. Rep. 2023, 13, 934. [Google Scholar]
- Lee, J.; Cho, Y.; Park, S.; Kim, J.W.; Lee, I.J. Skeletal Muscle Depletion Predicts the Prognosis of Patients With Hepatocellular Carcinoma Treated With Radiotherapy. Front. Oncol. 2019, 9, 1075. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Takahashi, A.; Hayashi, M.; Okai, K.; Abe, K.; Ohira, H. Skeletal muscle volume loss during transarterial chemoembolization predicts poor prognosis in patients with hepatocellular carcinoma. Hepatol. Res. 2019, 49, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Jeong, W.K.; Baik, S.K.; Cha, S.H.; Kim, M.Y. Impact of sarcopenia on prognostic value of cirrhosis: Going beyond the hepatic venous pressure gradient and MELD score. J. Cachexia Sarcopenia Muscle 2018, 9, 860–870. [Google Scholar] [CrossRef]
- Goh, M.J.; Kang, W.; Jeong, W.K.; Sinn, D.H.; Gwak, G.Y.; Paik, Y.H.; Choi, M.S.; Lee, J.H.; Koh, K.C.; Paik, S.W. Prognostic significance of cachexia index in patients with advanced hepatocellular carcinoma treated with systemic chemotherapy. Sci. Rep. 2022, 12, 7647. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, J.H.; Cho, E.S.; Lee, H.S.; Shin, S.J.; Park, E.J.; Baik, S.H.; Lee, K.Y.; Kang, J. Albumin-myosteatosis gauge as a novel prognostic risk factor in patients with non-metastatic colorectal cancer. J. Cachexia Sarcopenia Muscle 2023, 14, 860–868. [Google Scholar] [CrossRef]
- Chew, V.; Lee, Y.H.; Pan, L.; Nasir, N.J.M.; Lim, C.J.; Chua, C.; Lai, L.; Hazirah, S.N.; Lim, T.K.H.; Goh, B.K.P.; et al. Immune activation underlies a sustained clinical response to Yttrium-90 radioembolisation in hepatocellular carcinoma. Gut 2019, 68, 335–346. [Google Scholar] [CrossRef]
- Chhetri, J.K.; de Souto Barreto, P.; Fougere, B.; Rolland, Y.; Vellas, B.; Cesari, M. Chronic inflammation and sarcopenia: A regenerative cell therapy perspective. Exp. Gerontol. 2018, 103, 115–123. [Google Scholar] [CrossRef]
- Salem, R.; Gordon, A.C.; Mouli, S.; Hickey, R.; Kallini, J.; Gabr, A.; Mulcahy, M.F.; Baker, T.; Abecassis, M.; Miller, F.H.; et al. Y90 Radioembolization Significantly Prolongs Time to Progression Compared with Chemoembolization in Patients with Hepatocellular Carcinoma. Gastroenterology 2016, 151, 1155–1163. [Google Scholar] [CrossRef]
- Martelletti, C.; Ricotti, A.; Gesualdo, M.; Carucci, P.; Gaia, S.; Rolle, E.; Burlone, M.E.; Okolicsanyi, S.; Mattalia, A.; Pirisi, M.; et al. Radioembolization vs sorafenib in locally advanced hepatocellular carcinoma with portal vein tumor thrombosis: A propensity score and Bayesian analysis. J. Dig. Dis. 2021, 22, 496–502. [Google Scholar] [CrossRef]
- Vilgrain, V.; Pereira, H.; Assenat, E.; Guiu, B.; Ilonca, A.D.; Pageaux, G.P.; Sibert, A.; Bouattour, M.; Lebtahi, R.; Allaham, W.; et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): An open-label randomised controlled phase 3 trial. Lancet Oncol. 2017, 18, 1624–1636. [Google Scholar] [CrossRef]
- Chow, P.K.H.; Gandhi, M.; Tan, S.B.; Khin, M.W.; Khasbazar, A.; Ong, J.; Choo, S.P.; Cheow, P.C.; Chotipanich, C.; Lim, K.; et al. SIRveNIB: Selective Internal Radiation Therapy Versus Sorafenib in Asia-Pacific Patients with Hepatocellular Carcinoma. J. Clin. Oncol. 2018, 36, 1913–1921. [Google Scholar] [CrossRef]
- Garin, E.; Tzelikas, L.; Guiu, B.; Chalaye, J.; Edeline, J.; De Baere, T.; Tacher, V.; Robert, C.; Assenat, E.; Terroir-Cassou-Mounat, M.; et al. Major impact of personalized dosimetry using 90Y loaded glass microspheres SIRT in HCC: Final overall survival analysis of a multicenter randomized phase II study (DOSISPHERE-01). J. Clin. Oncol. 2020, 38, 516. [Google Scholar] [CrossRef]
- Spreafico, C.; Sposito, C.; Vaiani, M.; Cascella, T.; Bhoori, S.; Morosi, C.; Lanocita, R.; Romito, R.; Chiesa, C.; Maccauro, M.; et al. Development of a prognostic score to predict response to Yttrium-90 radioembolization for hepatocellular carcinoma with portal vein invasion. J. Hepatol. 2018, 68, 724–732. [Google Scholar] [CrossRef]
- Lewandowski, R.J.; Gabr, A.; Abouchaleh, N.; Ali, R.; Al Asadi, A.; Mora, R.A.; Kulik, L.; Ganger, D.; Desai, K.; Thornburg, B.; et al. Radiation Segmentectomy: Potential Curative Therapy for Early Hepatocellular Carcinoma. Radiology 2018, 287, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Guiu, B.; Garin, E.; Allimant, C.; Edeline, J.; Salem, R. TARE in Hepatocellular Carcinoma: From the Right to the Left of BCLC. Cardiovasc. Intervent Radiol. 2022, 45, 1599–1607. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Kim, J.H.; Jeong, W.K.; Lee, J.; Kim, Y.K.; Choi, D.; Lee, W.J. Semiautomatic software for measurement of abdominal muscle and adipose areas using computed tomography: A STROBE-compliant article. Medicine 2019, 98, e15867. [Google Scholar] [CrossRef] [PubMed]




| Variables | Total (n = 347) | No LSMM (n = 221) | LSMM (n = 126) | p Value |
|---|---|---|---|---|
| Median age, years | 65 (55–75) | 62 (54–72) | 71 (59–78) | <0.001 |
| Male sex | 284 (81.8%) | 195 (88.2%) | 89 (70.6%) | <0.001 |
| Mean BMI (Kg/m2) | 23.9 (3.4) | 24.5 (3.2) | 22.9 (3.4) | <0.001 |
| Etiology of disease * | 0.010 | |||
| Hepatitis B virus | 199 (57.3%) | 141 (63.8%) | 58 (46.0%) | |
| Hepatitis C virus | 35 (10.1%) | 17 (7.7%) | 18 (14.3%) | |
| Alcohol | 59 (17.0%) | 29 (13.1%) | 30 (23.8%) | |
| Non-alcoholic steatohepatitis | 35 (10.1%) | 22 (10.0%) | 13 (10.3%) | |
| Other | 19 (5.5%) | 12 (5.4%) | 7 (5.6%) | |
| ECOG PS | 0.560 | |||
| 0 | 289 (83.3%) | 186 (84.2%) | 103 (81.7%) | |
| 1 | 58 (16.7%) | 35 (15.8%) | 23 (18.3%) | |
| Mean total bilirubin (mg/dL) | 0.8 (0.6) | 0.8 (0.6) | 0.7 (0.6) | 0.063 |
| Mean albumin (g/dL) | 3.7 (0.5) | 3.8 (0.5) | 3.6 (0.5) | 0.002 |
| Median INR | 1.1 (1.0–1.1) | 1.1 (1.0–1.1) | 1.0 (1.0–1.2) | 0.200 |
| Child–Pugh class | 0.130 | |||
| A | 328 (94.5%) | 212 (95.9%) | 116 (92.1%) | |
| B | 19 (5.5%) | 9 (4.1%) | 10 (7.9%) | |
| ALBI grade | 0.023 | |||
| 1 | 149 (42.9%) | 107 (48.4%) | 42 (33.3%) | |
| 2 | 190 (54.8%) | 109 (49.3%) | 81 (64.3%) | |
| 3 | 8 (2.3%) | 5 (2.3%) | 3 (2.4%) | |
| Median tumor diameter (cm) | 8.4 (6.2–10.6) | 8.0 (6.1–10.0) | 9.0 (6.5–11.6) | 0.059 |
| Tumor number (≥2) | 186 (53.6%) | 119 (53.8%) | 67 (53.2%) | 0.900 |
| PVTT | 108 (31.1%) | 73 (33.0%) | 35 (27.8%) | 0.310 |
| BCLC stage | 0.860 | |||
| A | 65 (18.7%) | 40 (18.1%) | 25 (19.8%) | |
| B | 144 (41.5%) | 94 (42.5%) | 50 (39.7%) | |
| C | 138 (39.8%) | 87 (39.4%) | 51 (40.5%) | |
| Median AFP (ng/mL) | 103.0 (10.7–2235.3) | 58.1 (11.7–1958.5) | 198.1 (9.6–2922.0) | 0.240 |
| Median PIVKA-II (mAU/mL) | 961.0 (108.0–7032.0) | 862.0 (96.0–5592.0) | 1740.0 (132.5–13607.0) | 0.120 |
| Variables | Total (n = 333) | no LSMM (n = 212) | LSMM (n = 121) | p Value |
|---|---|---|---|---|
| Overall response | 0.007 | |||
| Complete response | 26 (7.8%) | 18 (8.5%) | 8 (6.6%) | |
| Partial response | 121 (36.3%) | 85 (40.1%) | 36 (29.8%) | |
| Stable disease | 106 (31.8%) | 71 (33.5%) | 35 (28.9%) | |
| Progressive disease | 80 (24.0%) | 38 (17.9%) | 42 (34.7%) | |
| Objective response | 147 (44.1%) | 103 (48.6%) | 44 (36.4%) | 0.031 |
| Disease control rate | 253 (76.0%) | 174 (82.1%) | 79 (65.3%) | <0.001 |
| Characteristics | Overall Survival | Progression-Free Survival | ||||
|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||
| p Value | HR (95% CI) | p Value | p Value | HR (95% CI) | p Value | |
| Age (≥65) | 0.555 | 0.208 | ||||
| Sex (male) | 0.168 | 0.518 | ||||
| Etiology (viral hepatitis) | 0.931 | 0.250 | ||||
| LSMM (yes) | 0.002 | 1.363 (1.002–1.854) | 0.048 | 0.002 | 1.535 (1.192–1.978) | 0.001 |
| PVTT (yes) | <0.001 | 1.822 (1.333–2.491) | <0.001 | <0.001 | 2.075 (1.585–2.716) | <0.001 |
| AFP (≥200 ng/mL) | <0.001 | 1.411 (1.037–1.920) | 0.028 | <0.001 | 1.376 (1.070–1.770) | 0.013 |
| ALBI (grade 2–3) | <0.001 | 1.737 (1.244–2.425) | 0.001 | <0.001 | 1.492 (1.152–1.934) | 0.002 |
| Tumor number (≥2) | 0.001 | 1.304 (0.946–1.798) | 0.105 | <0.001 | 1.553 (1.198–2.014) | 0.001 |
| Largest tumor diameter (>8 cm) | 0.001 | 1.348 (0.986–1.843) | 0.061 | 0.012 | 1.056 (0.819–1.362) | 0.674 |
| BMI (>25 kg/m2) | 0.316 | 0.248 | ||||
| Previous TACE (yes) | 0.068 | 0.140 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, H.; Yang, H.; Chun, H.S.; Lee, H.A.; Nam, J.Y.; Jang, J.W.; Seo, Y.S.; Kim, D.Y.; Kim, Y.J.; Bae, S.H. Impact of Low Skeletal Muscle Mass on Long-Term Outcomes in Hepatocellular Carcinoma Treated with Trans-Arterial Radioembolization: A Retrospective Multi-Center Study. Cancers 2023, 15, 5195. https://doi.org/10.3390/cancers15215195
Nam H, Yang H, Chun HS, Lee HA, Nam JY, Jang JW, Seo YS, Kim DY, Kim YJ, Bae SH. Impact of Low Skeletal Muscle Mass on Long-Term Outcomes in Hepatocellular Carcinoma Treated with Trans-Arterial Radioembolization: A Retrospective Multi-Center Study. Cancers. 2023; 15(21):5195. https://doi.org/10.3390/cancers15215195
Chicago/Turabian StyleNam, Heechul, Hyun Yang, Ho Soo Chun, Han Ah Lee, Joon Yeul Nam, Jeong Won Jang, Yeon Seok Seo, Do Young Kim, Yoon Jun Kim, and Si Hyun Bae. 2023. "Impact of Low Skeletal Muscle Mass on Long-Term Outcomes in Hepatocellular Carcinoma Treated with Trans-Arterial Radioembolization: A Retrospective Multi-Center Study" Cancers 15, no. 21: 5195. https://doi.org/10.3390/cancers15215195