Hashimoto’s Thyroiditis and the Risk of Papillary Thyroid Cancer in Children
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Review of Papillary Thyroid Carcinoma Pathology
2.4. Diagnosis of Hashimoto’s Thyroiditis
2.5. Additional Clinical Data
2.6. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Association of HT and PTC
3.3. Association of HT and Patient Cancer Outcomes
3.4. Association of HT and PTC Patient Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vergamini, L.B.; Frazier, A.L.; Abrantes, F.L.; Ribeiro, K.B.; Rodriguez-Galindo, C. Increase in the Incidence of Differentiated Thyroid Carcinoma in Children, Adolescents, and Young Adults: A Population-Based Study. J. Pediatr. 2014, 164, 1481–1485. [Google Scholar] [CrossRef] [PubMed]
- Vaccarella, S.; Lortet-Tieulent, J.; Colombet, M.; Davies, L.; Stiller, C.A.; Schüz, J.; Togawa, K.; Bray, F.; Franceschi, S.; Maso, L.D.; et al. Global Patterns and Trends in Incidence and Mortality of Thyroid Cancer in Children and Adolescents: A Population-Based Study. Lancet Diabetes Endocrinol. 2021, 9, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.A.; King, J.; Tai, E.; Buchanan, N.; Ajani, U.A.; Li, J. Cancer Incidence Rates and Trends Among Children and Adolescents in the United States, 2001–2009. Pediatrics 2014, 134, e945–e955. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.A.; Richardson, L.C.; Henley, S.J.; Wilson, R.J.; Dowling, N.F.; Weir, H.K.; Tai, E.W.; Lunsford, N.B. Pediatric Cancer Mortality and Survival in the United States, 2001–2016. Cancer 2020, 126, 4379–4389. [Google Scholar] [CrossRef]
- Reiners, C.; Wegscheider, K.; Schicha, H.; Theissen, P.; Vaupel, R.; Wrbitzky, R.; Schumm-Draeger, P.-M. Prevalence of Thyroid Disorders in the Working Population of Germany: Ultrasonography Screening in 96,278 Unselected Employees. Thyroid 2004, 14, 926–932. [Google Scholar] [CrossRef]
- Guille, J.T.; Opoku-Boateng, A.; Thibeault, S.L.; Chen, H. Evaluation and Management of the Pediatric Thyroid Nodule. Oncologist 2015, 20, 19–27. [Google Scholar] [CrossRef]
- Gupta, A.; Ly, S.; Castroneves, L.A.; Frates, M.C.; Benson, C.B.; Feldman, H.A.; Wassner, A.J.; Smith, J.R.; Marqusee, E.; Alexander, E.K.; et al. A Standardized Assessment of Thyroid Nodules in Children Confirms Higher Cancer Prevalence Than in Adults. J. Clin. Endocrinol. Metab. 2013, 98, 3238–3245. [Google Scholar] [CrossRef]
- Wang, H.; Mehrad, M.; Ely, K.A.; Liang, J.; Solórzano, C.C.; Neblett, W.W.; Coogan, A.C.; Weiss, V.L. Incidence and Malignancy Rates of Indeterminate Pediatric Thyroid Nodules. Cancer Cytopathol. 2019, 127, 231–239. [Google Scholar] [CrossRef]
- Francis, G.L.; Waguespack, S.G.; Bauer, A.J.; Angelos, P.; Benvenga, S.; Cerutti, J.M.; Dinauer, C.A.; Hamilton, J.; Hay, I.D.; Luster, M.; et al. Management Guidelines for Children with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2015, 25, 716–759. [Google Scholar] [CrossRef]
- Kandil, E.; Noureldine, S.I.; Abbas, A.; Tufano, R.P. The Impact of Surgical Volume on Patient Outcomes Following Thyroid Surgery. Surgery 2013, 154, 1346–1353. [Google Scholar] [CrossRef]
- Drews, J.D.; Cooper, J.N.; Onwuka, E.A.; Minneci, P.C.; Aldrink, J.H. The Relationships of Surgeon Volume and Specialty with Outcomes Following Pediatric Thyroidectomy. J. Pediatr. Surg. 2019, 54, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Gil, L.A.; Cooper, J.N.; Patterson, K.N.; Aldrink, J.H.; Diesen, D.L. Practice Patterns in the Operative Management of Pediatric Thyroid Disease Across Surgical Specializations. J. Pediatr. Surg. 2023. [Google Scholar] [CrossRef] [PubMed]
- Gallant, J.-N.; Chen, S.-C.; Ortega, C.A.; Rohde, S.L.; Belcher, R.H.; Netterville, J.L.; Baregamian, N.; Wang, H.; Liang, J.; Ye, F.; et al. Evaluation of the Molecular Landscape of Pediatric Thyroid Nodules and Use of a Multigene Genomic Classifier in Children. JAMA Oncol. 2022, 8, 1323–1327. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, M.L.; Schmidt, A.; Ghuzlan, A.A.; Lacroix, L.; de Vathaire, F.; Chevillard, S.; Schlumberger, M. Radiation Exposure and Thyroid Cancer: A Review. Arch. Endocrinol. Metab. 2017, 61, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Sigurdson, A.J.; Ronckers, C.M.; Mertens, A.C.; Stovall, M.; Smith, S.A.; Liu, Y.; Berkow, R.L.; Hammond, S.; Neglia, J.P.; Meadows, A.T.; et al. Primary Thyroid Cancer after a First Tumour in Childhood (the Childhood Cancer Survivor Study): A Nested Case-Control Study. Lancet 2005, 365, 2014–2023. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Dailey, M.E.; Lindsay, S.; Shaken, R. Relation of Thyroid Neoplasms to Hashimoto Disease of the Thyroid Gland. AMA Arch. Surg. 1955, 70, 291–297. [Google Scholar] [CrossRef]
- De Paiva, C.R.; Grønhøj, C.; Feldt-Rasmussen, U.; Buchwald, C. von Association between Hashimoto’s Thyroiditis and Thyroid Cancer in 64,628 Patients. Front. Oncol. 2017, 7, 53. [Google Scholar] [CrossRef]
- Lai, X.; Xia, Y.; Zhang, B.; Li, J.; Jiang, Y. A Meta-Analysis of Hashimoto’s Thyroiditis and Papillary Thyroid Carcinoma Risk. Oncotarget 2017, 8, 62414–62424. [Google Scholar] [CrossRef]
- Sur, M.L.; Gaga, R.; Lazăr, C.; Lazea, C.; Aldea, C.; Sur, D. Papillary Thyroid Carcinoma in Children with Hashimoto’s Thyroiditis—A Review of the Literature between 2000 and 2020. J. Pediatr. Endocrinol. Metab. 2020, 33, 1511–1517. [Google Scholar] [CrossRef] [PubMed]
- Yeker, R.M.; Shaffer, A.D.; Viswanathan, P.; Witchel, S.F.; Mollen, K.; Yip, L.; Monaco, S.E.; Duvvuri, U.; Simons, J.P. Chronic Lymphocytic Thyroiditis and Aggressiveness of Pediatric Differentiated Thyroid Cancer. Laryngoscope 2022, 132, 1668–1674. [Google Scholar] [CrossRef] [PubMed]
- Keefe, G.; Culbreath, K.; Cherella, C.E.; Smith, J.R.; Zendejas, B.; Shamberger, R.C.; Richman, D.M.; Hollowell, M.L.; Modi, B.P.; Wassner, A.J. Autoimmune Thyroiditis and Risk of Malignancy in Children with Thyroid Nodules. Thyroid 2022, 32, 1109–1117. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. BMJ 2007, 335, 806. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Schiavi, F.; Cascon, A.; Qin, Y.; Inglada-Pérez, L.; King, E.E.; Toledo, R.A.; Ercolino, T.; Rapizzi, E.; Ricketts, C.J.; et al. Spectrum and Prevalence of FP/TMEM127 Gene Mutations in Pheochromocytomas and Paragangliomas. JAMA 2010, 304, 2611–2619. [Google Scholar] [CrossRef] [PubMed]
- Baloch, Z.W.; Asa, S.L.; Barletta, J.A.; Ghossein, R.A.; Juhlin, C.C.; Jung, C.K.; LiVolsi, V.A.; Papotti, M.G.; Sobrinho-Simões, M.; Tallini, G.; et al. Overview of the 2022 WHO Classification of Thyroid Neoplasms. Endocr. Pathol. 2022, 33, 27–63. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. (Eds.) AJCC Cancer Staging Manual, 8th ed.; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-40617-6. [Google Scholar]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Kamma, H.; Fujii, K.; Ogata, T. Lymphocytic Infiltration in Juvenile Thyroid Carcinoma. Cancer 1988, 62, 1988–1993. [Google Scholar] [CrossRef]
- Corrias, A.; Cassio, A.; Weber, G.; Mussa, A.; Wasniewska, M.; Rapa, A.; Gastaldi, R.; Einaudi, S.; Baronio, F.; Vigone, M.C.; et al. Thyroid Nodules and Cancer in Children and Adolescents Affected by Autoimmune Thyroiditis. Arch. Pediatr. Adolesc. Med. 2008, 162, 526–531. [Google Scholar] [CrossRef]
- Keskin, M.; Savas-Erdeve, S.; Aycan, Z. Co-Existence of Thyroid Nodule and Thyroid Cancer in Children and Adolescents with Hashimoto Thyroiditis: A Single-Center Study. Horm. Res. Paediatr. 2016, 85, 181–187. [Google Scholar] [CrossRef]
- Radetti, G.; Loche, S.; D’Antonio, V.; Salerno, M.; Guzzetti, C.; Aversa, T.; Cassio, A.; Cappa, M.; Gastaldi, R.; Deluca, F.; et al. Influence of Hashimoto’s Thyroiditis on the Development of Thyroid Nodules and Cancer in Children and Adolescents. J. Endocr. Soc. 2019, 3, 607–616. [Google Scholar] [CrossRef]
- Iliadou, P.K.; Effraimidis, G.; Konstantinos, M.; Grigorios, P.; Mitsakis, P.; Patakiouta, F.; Pazaitou-Panayiotou, K. Chronic Lymphocytic Thyroiditis Is Associated with Invasive Characteristics of Differentiated Thyroid Carcinoma in Children and Adolescents. Eur. J. Endocrinol. 2015, 173, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Cho, Y.J.; Heo, Y.J.; Chung, E.; Choi, Y.H.; Kim, J.; Park, Y.J.; Shin, C.H.; Lee, Y.A. Thyroid Nodules in Childhood-onset Hashimoto’s Thyroiditis: Frequency, Risk Factors, Follow-up Course and Genetic Alterations of Thyroid Cancer. Clin. Endocrinol. 2021, 95, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, H.; Ji, Q.; Zhu, Y.; Wang, Z.; Wang, Y.; Huang, C.; Shen, Q.; Li, D.; Wu, Y. The Clinical Features of Papillary Thyroid Cancer in Hashimoto’s Thyroiditis Patients from an Area with a High Prevalence of Hashimoto’s Disease. BMC Cancer 2012, 12, 610. [Google Scholar] [CrossRef]
- Ren, P.-Y.; Liu, J.; Xue, S.; Chen, G. Pediatric Differentiated Thyroid Carcinoma: The Clinicopathological Features and the Coexistence of Hashimoto’s Thyroiditis. Asian J. Surg. 2019, 42, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Grani, G.; Carbotta, G.; Nesca, A.; D’Alessandri, M.; Vitale, M.; Sordo, M.D.; Fumarola, A. A Comprehensive Score to Diagnose Hashimoto’s Thyroiditis: A Proposal. Endocrine 2015, 49, 361–365. [Google Scholar] [CrossRef]
- Caturegli, P.; Remigis, A.D.; Rose, N.R. Hashimoto Thyroiditis: Clinical and Diagnostic Criteria. Autoimmun. Rev. 2014, 13, 391–397. [Google Scholar] [CrossRef]
- Guan, H.; de Morais, N.S.; Stuart, J.; Ahmadi, S.; Marqusee, E.; Kim, M.I.; Alexander, E.K. Discordance of Serological and Sonographic Markers for Hashimoto’s Thyroiditis with Gold Standard Histopathology. Eur. J. Endocrinol. 2019, 181, 539–544. [Google Scholar] [CrossRef]
- Vuong, H.G.; Kondo, T.; Pham, T.Q.; Oishi, N.; Mochizuki, K.; Nakazawa, T.; Hassell, L.; Katoh, R. Prognostic Significance of Diffuse Sclerosing Variant Papillary Thyroid Carcinoma: A Systematic Review and Meta-Analysis. Eur. J. Endocrinol. 2017, 176, 433–441. [Google Scholar] [CrossRef]
- Pillai, S.; Gopalan, V.; Smith, R.A.; Lam, A.K.-Y. Diffuse Sclerosing Variant of Papillary Thyroid Carcinoma—An Update of Its Clinicopathological Features and Molecular Biology. Crit. Rev. Oncol. Hematol. 2015, 94, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Castagna, M.G.; Belardini, V.; Memmo, S.; Maino, F.; Santo, A.D.; Toti, P.; Carli, A.F.; Caruso, G.; Pacini, F. Nodules in Autoimmune Thyroiditis Are Associated with Increased Risk of Thyroid Cancer in Surgical Series but Not in Cytological Series: Evidence for Selection Bias. J. Clin. Endocrinol. Metab. 2014, 99, 3193–3198. [Google Scholar] [CrossRef] [PubMed]
- De Morais, N.S.; Stuart, J.; Guan, H.; Wang, Z.; Cibas, E.S.; Frates, M.C.; Benson, C.B.; Cho, N.L.; Nehs, M.A.; Alexander, C.A.; et al. The Impact of Hashimoto’s Thyroiditis on Thyroid Nodule Cytology & Risk of Thyroid Cancer. J. Endocr. Soc. 2019, 3, 791–800. [Google Scholar] [CrossRef]
| Variable | All Patients (n = 153) | Without HT (n = 118) | With HT (n = 35) | Statistic |
|---|---|---|---|---|
| Age at surgery, years, median (Q1, Q3) | 16.5 (14.2, 18.3) | 16.8 (14.2, 18.6) | 16.1 (13.9, 17.2) | p = 0.22 a |
| Sex, n (%) | p = 0.37 b | |||
| Female | 123 (80.4) | 93 (78.8) | 30 (85.7) | |
| Male | 30 (19.6) | 25 (21.2) | 5 (14.3) | |
| Race/ethnicity, n (%) | p = 0.40 c | |||
| White | 128 (83.7) | 99 (83.9) | 29 (82.9) | |
| Black | 11 (7.2) | 10 (8.5) | 1 (2.9) | |
| Hispanic | 9 (5.9) | 6 (5.1) | 3 (8.6) | |
| Arab | 5 (3.3) | 3 (2.5) | 2 (5.7) | |
| HT features, n (%) | ||||
| LT | 43 (28.1) | 8 (6.8) | 35 (100.0) | p < 0.001 b |
| TSH | 52 (34.0) | 31 (26.2) | 21 (60.0) | p < 0.001 b |
| Anti-Tg | 28 (18.3) | 9 (7.6) | 19 (54.2) | p < 0.001 b |
| Anti-TPO | 40 (26.1) | 15 (12.7) | 25 (71.4) | p < 0.001 b |
| Positive clinical history | 32 (20.9) | 8 (6.8) | 24 (68.6) | p < 0.001 b |
| Follow-up, months, median (Q1, Q3) | 58.6 (20.7, 105.4) | 58.6 (18.0, 112.0) | 57.1 (24.7, 99.9) | p = 0.67 a |
| Variable | All Patients (n = 153) | Without HT (n = 118) | With HT (n = 35) | Statistic |
|---|---|---|---|---|
| Malignancy, n (%) a | 106 (69.3) | 77 (65.3) | 29 (82.9) | p = 0.05 b |
| T, n (%) | p = 0.63 b | |||
| T1 and T2 | 73 (68.9) | 52 (67.5) | 21 (72.4) | |
| T3 and T4 | 33 (31.1) | 25 (32.5) | 8 (27.6) | |
| N, n (%) | p = 0.20 c | |||
| NX and N0 | 43 (40.6) | 32 (41.6) | 11 (37.9) | |
| N1a | 18 (17.0) | 10 (13.0) | 8 (27.6) | |
| N1b | 45 (42.5) | 35 (45.5) | 10 (34.5) | |
| Histopathology, n (%) | p = 0.12 *,c | |||
| PTC | 92 (60.1) | 65 (55.1) | 27 (77.1) | p = 0.02 †,b |
| Non-PTC | 61 (39.9) | 53 (44.9) | 8 (22.9) | |
| FA | 47 (30.7) | 41 (34.7) | 6 (17.1) | |
| FTC | 8 (5.3) | 7 (5.9) | 1 (2.9) | |
| NIFTP | 6 (3.9) | 5 (4.2) | 1 (2.9) |
| Without HT (n = 65) | With HT (n = 27) | Statistic | |
|---|---|---|---|
| PTC subtype | p = 0.03 *,a | ||
| Classic, n (%) | 48 (73.8) | 20 (74.1) | p = 0.98 †,b |
| Non-classic, (%) | 17 (26.2) | 7 (25.9) | |
| Follicular variant, n (%) | 10 (15.4) | 0 (0) | |
| Diffuse-sclerosing, n (%) | 3 (4.6) | 5 (18.5) | |
| Cribiform-morular, n (%) | 1 (1.5) | 0 (0) | |
| Oncocytic, n (%) | 1 (1.5) | 0 (0) | |
| Solid, n (%) | 1 (1.5) | 1 (3.7) | |
| Tall cell, n (%) | 1 (1.5) | 0 (0) | |
| Warthin’s-like, n (%) | 0 (0) | 1 (3.7) |
| Variable | All Patients (n = 83) | Without HT (n = 57) | With HT (n = 26) | Statistic |
|---|---|---|---|---|
| Surgeries, n (%) | p = 0.93 a | |||
| 1 | 54 (65.1) | 36 (63.2) | 18 (69.2) | |
| 2 | 24 (28.9) | 17 (29.8) | 7 (26.9) | |
| 3 | 5 (6.0) | 4 (7.0) | 1 (3.8) | |
| RAI treatments, n (%) | p = 0.12 a | |||
| 0 | 29 (34.9) | 19 (33.3) | 10 (38.5) | |
| 1 | 43 (51.8) | 27 (47.4) | 16 (61.5) | |
| 2 | 7 (8.4) | 7 (12.3) | 0 (0.0) | |
| 3 | 4 (4.8) | 4 (7.0) | 0 (0.0) | |
| Outcomes, n (%) | 73 (68.9) | 52 (67.5) | 21 (72.4) | p = 0.08 a |
| Good | 58 (69.9) | 36 (63.2) | 22 (84.6) | |
| Indeterminate | 7 (8.4) | 7 (12.3) | 0 (0.0) | |
| Poor | 18 (21.7) | 14 (24.6) | 4 (15.4) | |
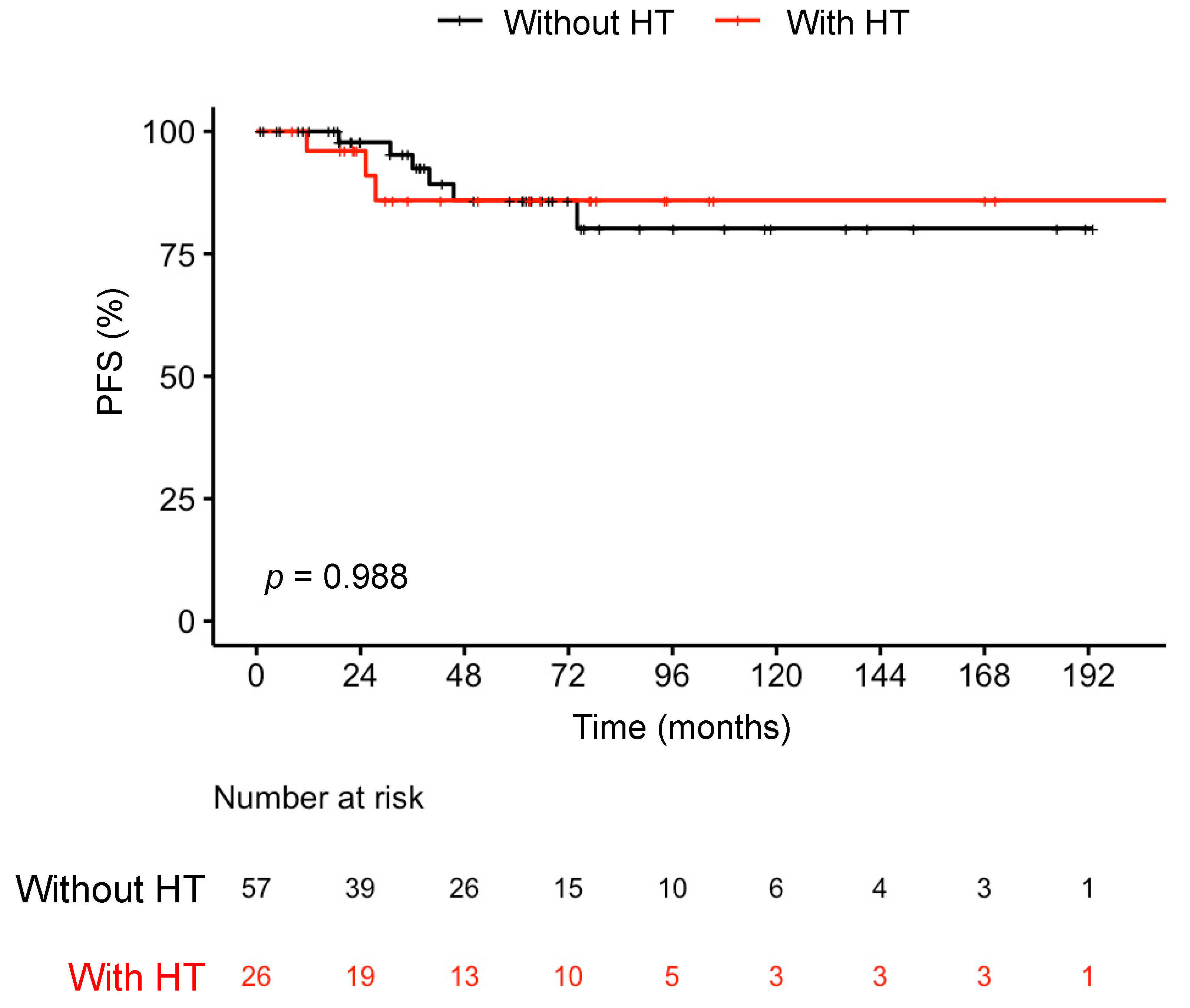
| PFS, survival rate, % (CI) | p = 0.99 b | |||
| 60 Months | 86 (77–96) | 86 (75–98) | 86% (72–100) | |
| 120 Months | 82 (72–94) | 80 (66–97) | 86% (72–100) | |
| 180 Months | 82 (72–94) | 80 (66–97) | 86% (72–100) | |
| 226.7 Months | 82 (72–94) | NA | 86% (72–100) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallant, J.-N.; Weiss, V.L.; Chen, S.-C.; Liang, J.; Belcher, R.H.; Ye, F.; Correa, H.; Wang, H. Hashimoto’s Thyroiditis and the Risk of Papillary Thyroid Cancer in Children. Cancers 2023, 15, 4902. https://doi.org/10.3390/cancers15194902
Gallant J-N, Weiss VL, Chen S-C, Liang J, Belcher RH, Ye F, Correa H, Wang H. Hashimoto’s Thyroiditis and the Risk of Papillary Thyroid Cancer in Children. Cancers. 2023; 15(19):4902. https://doi.org/10.3390/cancers15194902
Chicago/Turabian StyleGallant, Jean-Nicolas, Vivian L. Weiss, Sheau-Chiann Chen, Jiancong Liang, Ryan H. Belcher, Fei Ye, Hernan Correa, and Huiying Wang. 2023. "Hashimoto’s Thyroiditis and the Risk of Papillary Thyroid Cancer in Children" Cancers 15, no. 19: 4902. https://doi.org/10.3390/cancers15194902





