The Relative Dose Intensity Changes during Cycles of Standard Regimens in Patients with Diffuse Large B-Cell Lymphoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Clinical Information
2.2. Immunochemotherapy Regimens and Calculation of RDI
2.3. Outcome Measures
2.4. Sensitivity Analysis
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Multivariate Cox-RCS Models for ARDI Changes
3.3. Sensitivity Analysis for Survival Outcomes
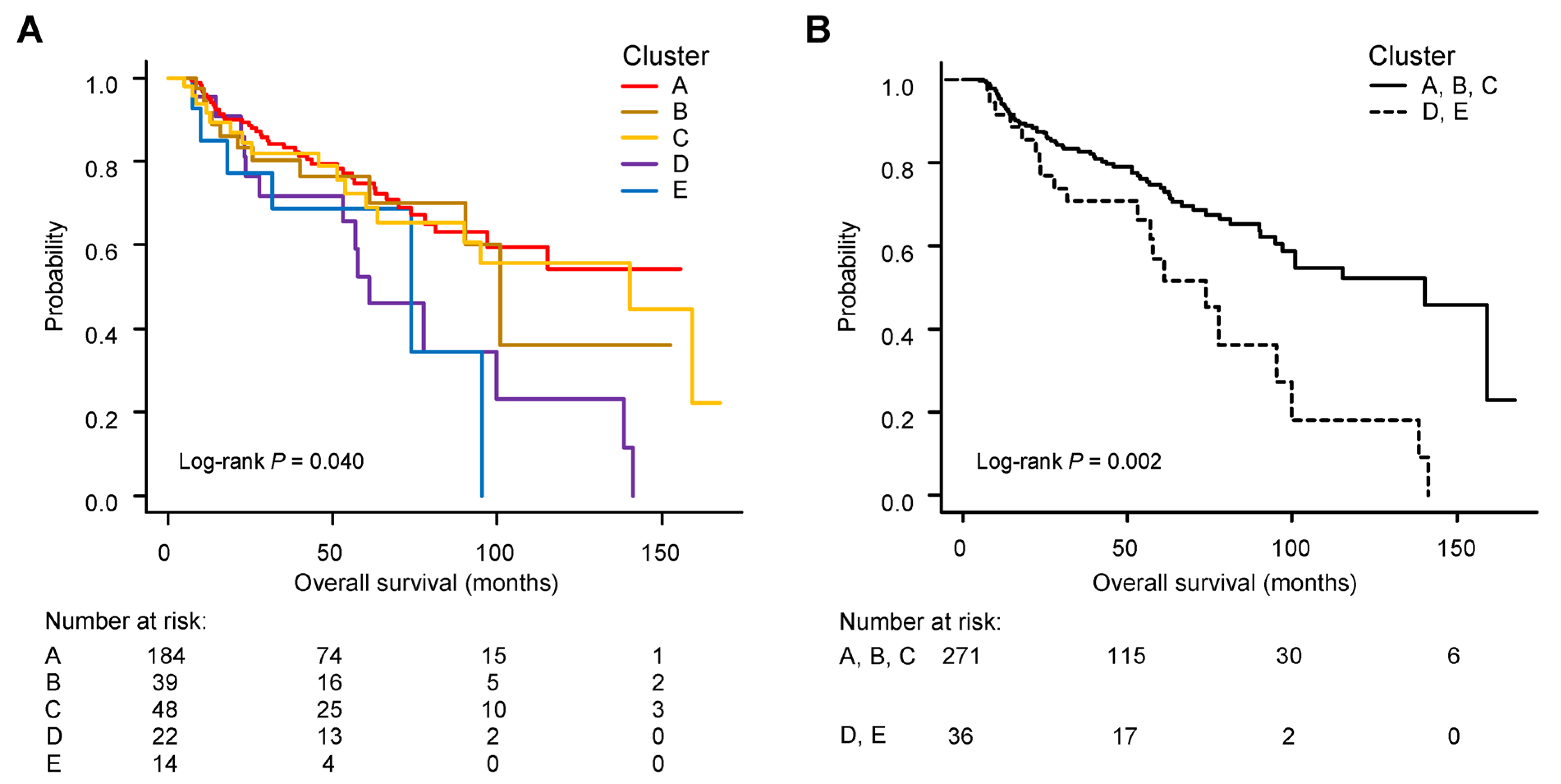
3.4. Group-Based Trajectory Modelling for a Pattern of ARDI Changes
3.5. Survival Curves and Clinical Factors in Each Patient Group According to ARDI Change
3.6. Clinical Factors Influencing ARDI Changes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morton, L.M.; Wang, S.S.; Devesa, S.S.; Hartge, P.; Weisenburger, D.D.; Linet, M.S. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood 2006, 107, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef]
- Pettengell, R.; Schwenkglenks, M.; Bosly, A. Association of reduced relative dose intensity and survival in lymphoma patients receiving CHOP-21 chemotherapy. Ann. Hematol. 2008, 87, 429–430. [Google Scholar] [CrossRef]
- Kwak, L.W.; Halpern, J.; Olshen, R.A.; Horning, S.J. Prognostic significance of actual dose intensity in diffuse large-cell lymphoma: Results of a tree-structured survival analysis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1990, 8, 963–977. [Google Scholar] [CrossRef] [PubMed]
- Długosz-Danecka, M.; Szmit, S.; Ogórka, T.; Skotnicki, A.B.; Jurczak, W. The average relative dose intensity of R-CHOP is an independent factor determining favorable overall survival in diffuse large B-cell lymphoma patients. Cancer Med. 2019, 8, 1103–1109. [Google Scholar] [CrossRef]
- Bataillard, E.J.; Cheah, C.Y.; Maurer, M.J.; Khurana, A.; Eyre, T.A.; El-Galaly, T.C. Impact of R-CHOP dose intensity on survival outcomes in diffuse large B-cell lymphoma: A systematic review. Blood Adv. 2021, 5, 2426–2437. [Google Scholar] [CrossRef]
- Bosly, A.; Bron, D.; Van Hoof, A.; De Bock, R.; Berneman, Z.; Ferrant, A.; Kaufman, L.; Dauwe, M.; Verhoef, G. Achievement of optimal average relative dose intensity and correlation with survival in diffuse large B-cell lymphoma patients treated with CHOP. Ann. Hematol. 2008, 87, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Kanemasa, Y.; Shimoyama, T.; Sasaki, Y.; Tamura, M.; Sawada, T.; Omuro, Y.; Hishima, T.; Maeda, Y. The impacts of initial and relative dose intensity of R-CHOP on outcomes of elderly patients with diffuse large B-cell lymphoma. Leuk. Lymphoma 2017, 58, 736–739. [Google Scholar] [CrossRef]
- Lee, S.; Fujita, K.; Negoro, E.; Morishita, T.; Oiwa, K.; Tsukasaki, H.; Kinoshita, K.; Kawai, Y.; Ueda, T.; Yamauchi, T. Impact of relative dose intensity of standard regimens on survival in elderly patients aged 80 years and older with diffuse large B-cell lymphoma. Haematologica 2020, 105, e415–e418. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J.; Vardiman, J.W. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed.; IARC Press: Lyon, France, 2008. [Google Scholar]
- Hara, T.; Yoshikawa, T.; Goto, H.; Sawada, M.; Yamada, T.; Fukuno, K.; Kasahara, S.; Shibata, Y.; Matsumoto, T.; Mabuchi, R.; et al. R-THP-COP versus R-CHOP in patients younger than 70 years with untreated diffuse large B cell lymphoma: A randomized, open-label, noninferiority phase 3 trial. Hematol. Oncol. 2018, 36, 638–644. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Konishi, J.; Miyake, T.; Makita, M.; Hojo, A.; Masaki, Y.; Uno, M.; Ozaki, J.; Yoshida, C.; Niiya, D.; et al. A Host-Dependent Prognostic Model for Elderly Patients with Diffuse Large B-Cell Lymphoma. Oncol. 2017, 22, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef]
- Kanemasa, Y.; Shimoyama, T.; Sasaki, Y.; Hishima, T.; Omuro, Y. Geriatric nutritional risk index as a prognostic factor in patients with diffuse large B cell lymphoma. Ann. Hematol. 2018, 97, 999–1007. [Google Scholar] [CrossRef]
- Lee, S.; Fujita, K.; Morishita, T.; Negoro, E.; Oiwa, K.; Tsukasaki, H.; Yamamura, O.; Ueda, T.; Yamauchi, T. Prognostic utility of a geriatric nutritional risk index in combination with a comorbidity index in elderly patients with diffuse large B cell lymphoma. Br. J. Haematol. 2021, 192, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Cheson, B.D.; Pfistner, B.; Juweid, M.E.; Gascoyne, R.D.; Specht, L.; Horning, S.J.; Coiffier, B.; Fisher, R.I.; Hagenbeek, A.; Zucca, E.; et al. Revised response criteria for malignant lymphoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Cheson, B.D.; Horning, S.J.; Coiffier, B.; Shipp, M.A.; Fisher, R.I.; Connors, J.M.; Lister, T.A.; Vose, J.; Grillo-Lopez, A.; Hagenbeek, A.; et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1999, 17, 1244. [Google Scholar] [CrossRef] [PubMed]
- Durrleman, S.; Simon, R. Flexible regression models with cubic splines. Stat. Med. 1989, 8, 551–561. [Google Scholar] [CrossRef]
- Saygin, C.; Jia, X.; Hill, B.; Dean, R.; Pohlman, B.; Smith, M.R.; Jagadeesh, D. Impact of comorbidities on outcomes of elderly patients with diffuse large B-cell lymphoma. Am. J. Hematol. 2017, 92, 989–996. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Pfreundschuh, M.; Schubert, J.; Ziepert, M.; Schmits, R.; Mohren, M.; Lengfelder, E.; Reiser, M.; Nickenig, C.; Clemens, M.; Peter, N.; et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: A randomised controlled trial (RICOVER-60). Lancet Oncol. 2008, 9, 105–116. [Google Scholar] [CrossRef]
- Oiwa, K.; Fujita, K.; Lee, S.; Morishita, T.; Tsukasaki, H.; Negoro, E.; Hara, T.; Tsurumi, H.; Ueda, T.; Yamauchi, T. Prognostic impact of six versus eight cycles of standard regimen in patients with diffuse large B-cell lymphoma: Propensity score-matching analysis. ESMO Open 2021, 6, 100210. [Google Scholar] [CrossRef] [PubMed]
- Wästerlid, T.; Biccler, J.L.; Brown, P.N.; Bøgsted, M.; Enblad, G.; Mészáros Jørgensen, J.; Christensen, J.H.; Wahlin, B.E.; Smedby, K.E.; El-Galaly, T.C.; et al. Six cycles of R-CHOP-21 are not inferior to eight cycles for treatment of diffuse large B-cell lymphoma: A Nordic Lymphoma Group Population-based Study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, 1882–1883. [Google Scholar] [CrossRef] [PubMed]
- Sehn, L.H.; Congiu, A.G.; Culligan, D.J.; Gironella, M.; Yoon, D.H.; Ogura, M.; Rosta, A.; Zhu, J.; Launonen, A.; Nielsen, T.; et al. No Added Benefit of Eight Versus Six Cycles of CHOP When Combined with Rituximab in Previously Untreated Diffuse Large B-Cell Lymphoma Patients: Results from the International Phase III GOYA Study. Blood 2018, 132, 783. [Google Scholar] [CrossRef]
- Yang, D.H.; Min, J.J.; Song, H.C.; Jeong, Y.Y.; Chung, W.K.; Bae, S.Y.; Ahn, J.S.; Kim, Y.K.; Bom, H.S.; Chung, I.J.; et al. Prognostic significance of interim ¹⁸F-FDG PET/CT after three or four cycles of R-CHOP chemotherapy in the treatment of diffuse large B-cell lymphoma. Eur. J. Cancer 2011, 47, 1312–1318. [Google Scholar] [CrossRef]
- Shimizu, K.; Okita, R.; Saisho, S.; Yukawa, T.; Maeda, A.; Nojima, Y.; Nakata, M. Prognostic nutritional index before adjuvant chemotherapy predicts chemotherapy compliance and survival among patients with non-small-cell lung cancer. Ther. Clin. Risk Manag. 2015, 11, 1555–1561. [Google Scholar] [CrossRef]
- Go, S.I.; Park, S.; Kang, M.H.; Kim, H.G.; Kim, H.R.; Lee, G.W. Clinical impact of prognostic nutritional index in diffuse large B cell lymphoma. Ann. Hematol. 2019, 98, 401–411. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, Y.G.; Hwang, I.G.; Song, H.S.; Koh, S.J.; Ko, Y.H.; Shin, S.H.; Woo, I.S.; Hong, S.; Kim, T.Y.; et al. Predicting cumulative incidence of adverse events in older patients with cancer undergoing first-line palliative chemotherapy: Korean Cancer Study Group (KCSG) multicentre prospective study. Br. J. Cancer 2018, 118, 1169–1175. [Google Scholar] [CrossRef]
- Bohlius, J.; Herbst, C.; Reiser, M.; Schwarzer, G.; Engert, A. Granulopoiesis-stimulating factors to prevent adverse effects in the treatment of malignant lymphoma. Cochrane Database Syst. Rev. 2008, 2008, Cd003189. [Google Scholar] [CrossRef]
- Pettengell, R.; Gurney, H.; Radford, J.A.; Deakin, D.P.; James, R.; Wilkinson, P.M.; Kane, K.; Bentley, J.; Crowther, D. Granulocyte colony-stimulating factor to prevent dose-limiting neutropenia in non-Hodgkin’s lymphoma: A randomized controlled trial. Blood 1992, 80, 1430–1436. [Google Scholar] [CrossRef]
- Vollset, S.E.; Goren, E.; Yuan, C.W.; Cao, J.; Smith, A.E.; Hsiao, T.; Bisignano, C.; Azhar, G.S.; Castro, E.; Chalek, J.; et al. Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: A forecasting analysis for the Global Burden of Disease Study. Lancet 2020, 396, 1285–1306. [Google Scholar] [CrossRef]
- Carson, K.R.; Riedell, P.; Lynch, R.; Nabhan, C.; Wildes, T.M.; Liu, W.; Ganti, A.; Roop, R.; Sanfilippo, K.M.; O’Brian, K.; et al. Comparative effectiveness of anthracycline-containing chemotherapy in United States veterans age 80 and older with diffuse large B-cell lymphoma. J. Geriatr. Oncol. 2015, 6, 211–218. [Google Scholar] [CrossRef]
- Eyre, T.A.; Martinez-Calle, N.; Hildyard, C.; Eyre, D.W.; Plaschkes, H.; Griffith, J.; Wolf, J.; Fields, P.; Gunawan, A.; Oliver, R.; et al. Impact of intended and relative dose intensity of R-CHOP in a large, consecutive cohort of elderly diffuse large B-cell lymphoma patients treated with curative intent: No difference in cumulative incidence of relapse comparing patients by age. J. Intern. Med. 2019, 285, 681–692. [Google Scholar] [CrossRef]
- Juul, M.B.; Jensen, P.H.; Engberg, H.; Wehberg, S.; Dessau-Arp, A.; Haziri, D.; Kristensen, H.B.; Baech, J.; Schurmann, L.; Clausen, M.R.; et al. Treatment strategies and outcomes in diffuse large B-cell lymphoma among 1011 patients aged 75 years or older: A Danish population-based cohort study. Eur. J. Cancer 2018, 99, 86–96. [Google Scholar] [CrossRef]
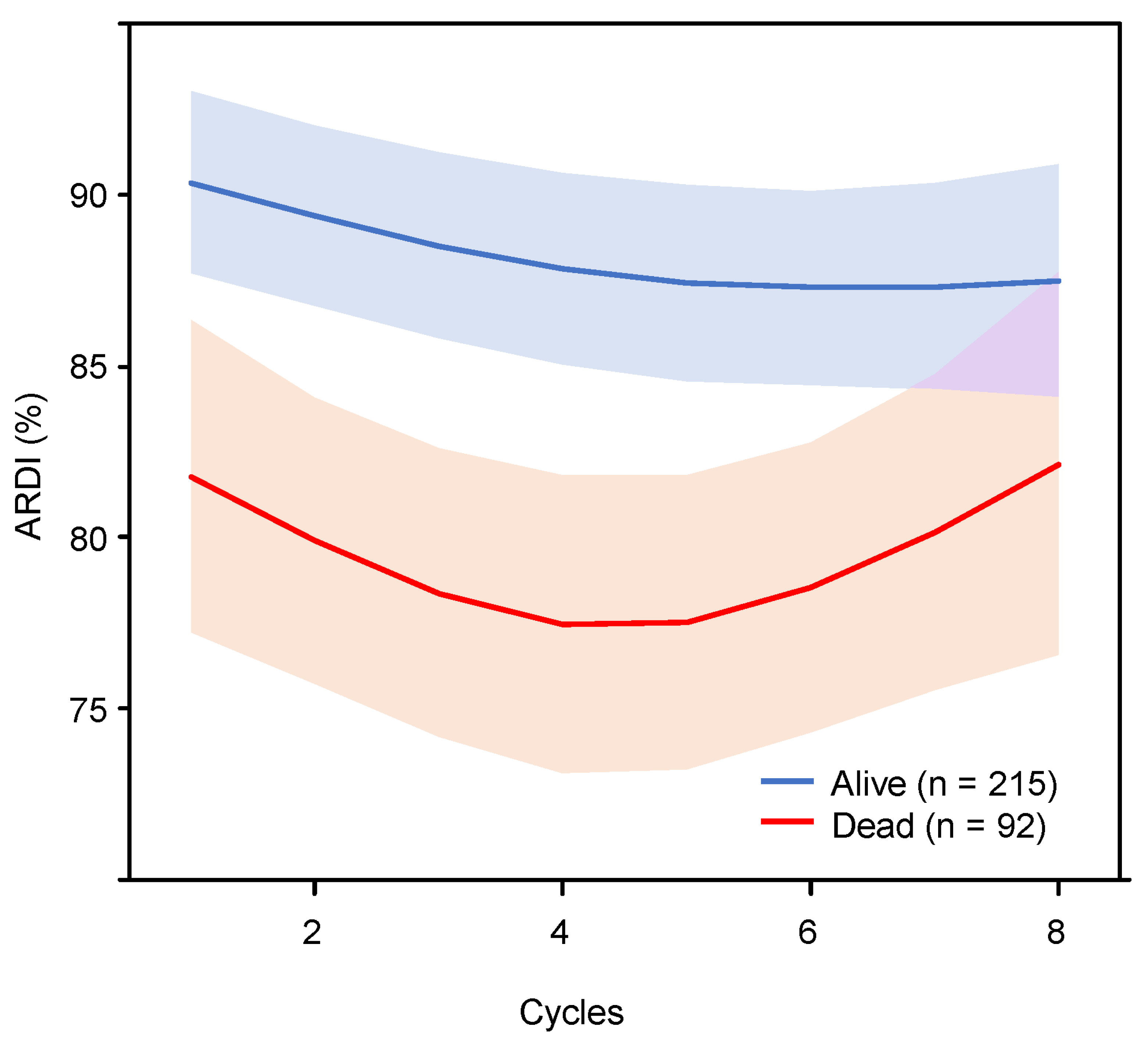

| All Patients (n = 307) | Survivor Group (n = 215) | Deceased Group (n = 92) | p Value | ||||
|---|---|---|---|---|---|---|---|
| Age, years—median, range | 71 | (16–96) | 71 | (16–89) | 73 | (46–96) | 0.016 |
| Age > 60 years | 243 | (79.2) | 162 | (75.3) | 81 | (88.0) | 0.014 |
| Male—n (%) | 162 | (52.8) | 114 | (53.0) | 48 | (52.2) | 0.901 |
| ECOG PS ≥ 2—n (%) | 74 | (24.1) | 34 | (15.8) | 40 | (43.5) | <0.001 |
| LDH > ULN—n (%) | 193 | (62.9) | 118 | (54.9) | 75 | (81.5) | <0.001 |
| Stage ≥ 3—n (%) | 206 | (67.1) | 134 | (62.3) | 75 | (81.5) | 0.008 |
| Extranodal sites ≥ 2—n (%) | 120 | (39.1) | 75 | (34.9) | 45 | (48.9) | 0.022 |
| IPI—n (%) | |||||||
| Low | 67 | (21.8) | 60 | (27.9) | 7 | (7.6) | |
| Low-intermediate | 63 | (20.5) | 51 | (23.7) | 12 | (13.0) | <0.001 |
| High-intermediate | 74 | (24.1) | 51 | (23.7) | 23 | (25.0) | |
| High | 103 | (33.6) | 53 | (24.7) | 50 | (54.4) | |
| B symptoms—n (%) | 84 | (27.4) | 51 | (23.7) | 33 | (35.9) | 0.035 |
| Bulky mass—n (%) | 70 | (22.8) | 37 | (17.2) | 33 | (35.9) | <0.001 |
| sIL-2R, U/mL—median, range | 1400 | (125–38,400) | 1160 | (125–31,000) | 2423 | (332–38,400) | <0.001 |
| tARDI, %—median, range | 100.0 | (19.2–143.3) | 101.2 | (35.4–143.3) | 91.6 | (19.2–137.8) | <0.001 |
| THP-COP as initial therapy—n (%) | 35 | (11.4) | 20 | (9.3) | 15 | (16.3) | 0.168 |
| CCI score—median, range | 1 | (0–7) | 1 | (0–7) | 1 | (0–7) | 0.012 |
| GNRI score—median, range | 95.9 | (42.5–128.8) | 98.7 | (51.5–128.8) | 90.3 | (42.5–120.8) | <0.001 |
| All Patients (n = 307) | Cluster A (n = 184) | Cluster B (n = 39) | Cluster C (n = 48) | Cluster D (n = 22) | Cluster E (n = 14) | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, years—median, range | 71 | (16–96) | 67 | (16–96) | 74 | (52–88) | 77 | (69–89) | 83 | (59–90) | 74 | (53–86) | <0.001 |
| Age > 60 years | 243 | (79.2) | 128 | (69.6) | 34 | (87.2) | 48 | (100.0) | 21 | (95.5) | 12 | (85.7) | <0.001 |
| Male—n (%) | 162 | (52.8) | 103 | (56.0) | 14 | (35.9) | 26 | (54.2) | 12 | (54.6) | 7 | (50.0) | 0.252 |
| ECOG PS ≥ 2—n (%) | 74 | (24.1) | 39 | (21.2) | 12 | (30.8) | 10 | (20.8) | 10 | (45.5) | 3 | (21.4) | 0.121 |
| LDH >ULN—n (%) | 193 | (62.9) | 115 | (62.5) | 26 | (66.7) | 29 | (60.4) | 14 | (63.6) | 9 | (64.3) | 0.987 |
| Stage ≥ 3—n (%) | 206 | (67.1) | 124 | (67.4) | 25 | (64.1) | 30 | (62.5) | 16 | (72.7) | 11 | (78.6) | 0.805 |
| Extranodal sites ≥ 2—n (%) | 120 | (39.1) | 73 | (39.7) | 19 | (48.7) | 11 | (22.9) | 11 | (50.0) | 6 | (42.9) | 0.080 |
| IPI—n (%) | |||||||||||||
| Low | 67 | (21.8) | 46 | (25.0) | 6 | (15.4) | 11 | (22.9) | 3 | (13.69 | 1 | (7.1) | |
| Low-intermediate | 63 | (20.5) | 38 | (20.7) | 9 | (23.1) | 10 | (20.8) | 2 | (9.1) | 4 | (28.6) | 0.569 |
| High-intermediate | 74 | (24.1) | 44 | (23.9) | 8 | (20.5) | 13 | (27.1) | 5 | (22.7) | 4 | (28.6) | |
| High | 103 | (33.6) | 56 | (30.4) | 16 | (41.0) | 14 | (29.2) | 12 | (54.6) | 5 | (35.7) | |
| B symptoms—n (%) | 84 | (27.4) | 52 | (28.3) | 7 | (17.9) | 16 | (33.3) | 5 | (22.7) | 4 | (28.6) | 0.573 |
| Bulky mass—n (%) | 70 | (22.8) | 44 | (23.9) | 12 | (30.8) | 6 | (12.5) | 5 | (22.7) | 3 | (21.4) | 0.300 |
| sIL-2R, U/mL—median, range | 1400 | (125–38,400) | 1429 | (125–38,400) | 1374 | (184–14,871) | 1134 | (239–28,225) | 1820 | (291–21,263) | 1570 | (416–19,054) | 0.532 |
| tARDI, %—median, range | 100.0 | (19.2–143.3) | 121.9 | (85.0–143.3) | 103.7 | (77.1–120.1) | 78.1 | (59.1–105.7) | 53.1 | (19.2–74.1) | 75.2 | (48.8–96.6) | <0.001 |
| THP-COP as initial therapy—n (%) | 35 | (11.4) | 7 | (3.8) | 2 | (5.1) | 13 | (27.1) | 11 | (50.0) | 2 | (14.3) | <0.001 |
| CCI score—median, range | 1 | (0–7) | 1 | (0–6) | 1 | (0–4) | 1 | (0–7) | 2 | (0–7) | 0 | (0–5) | 0.007 |
| GNRI score—median, range | 95.9 | (42.5–128.8) | 98.1 | (51.5–125.3) | 100.4 | (80.3–128.8) | 92.4 | (60.1–113.4) | 91.2 | (42.5–120.0) | 94.0 | (79.5–106.0) | 0.011 |
| All Patients (n = 307) | Cluster A, B, C (n = 271) | Cluster D, E (n = 36) | p Value | ||||
|---|---|---|---|---|---|---|---|
| Age, year—median, range | 71 | (16–96) | 70 | (16–96) | 81 | (53–90) | <0.001 |
| Age > 60 | 243 | (79.2) | 210 | (77.5) | 33 | (91.7) | 0.051 |
| Male—n (%) | 162 | (52.8) | 143 | (52.8) | 19 | (52.7) | 0.999 |
| ECOG PS ≥ 2—n (%) | 74 | (24.1) | 61 | (22.5) | 13 | (36.1) | 0.095 |
| LDH > ULN—n (%) | 193 | (62.9) | 170 | (62.7) | 23 | (63.9) | 0.999 |
| Stage ≥ 3—n (%) | 206 | (67.1) | 179 | (66.1) | 27 | (75.0) | 0.347 |
| Extranodal sites ≥ 2—n (%) | 120 | (39.1) | 103 | (38.0) | 17 | (47.2) | 0.363 |
| IPI—n (%) | |||||||
| Low | 67 | (21.8) | 63 | (23.3) | 4 | (11.1) | |
| Low-intermediate | 63 | (20.5) | 57 | (21.0) | 6 | (16.7) | 0.204 |
| High-intermediate | 74 | (24.1) | 65 | (24.0) | 9 | (25.0) | |
| High | 103 | (33.6) | 86 | (31.7) | 17 | (47.2) | |
| B symptoms—n (%) | 84 | (27.4) | 75 | (27.7) | 9 | (25.0) | 0.844 |
| Bulky mass—n (%) | 70 | (22.8) | 62 | (22.9) | 8 | (22.2) | 0.999 |
| sIL-2R, U/mL—median, range | 1400 | (125–38,400) | 1355 | (125–38,400) | 1815 | (291–21,263) | 0.093 |
| tARDI, %—median, range | 100.0 | (19.2–143.3) | 102.4 | (59.1–143.3) | 66.2 | (19.2–96.6) | <0.001 |
| Initial treatment with THP-COP therapy—n (%) | 35 | (11.4) | 22 | (8.1) | 13 | (36.1) | <0.001 |
| CCI score—median, range | 1 | (0–7) | 1 | (0–7) | 2 | (0–7) | 0.033 |
| GNRI score—median, range | 95.9 | (42.5–128.8) | 97.0 | (51.5–128.8) | 92.7 | (42.5–120.0) | 0.024 |
| Odds Ratio (95%CI) | p Value | ||
|---|---|---|---|
| Age > 60 years | 2.070 | (0.576–7.400) | 0.265 |
| Male | 1.040 | (0.490–2.210) | 0.920 |
| ECOG PS ≥ 2 | 1.230 | (0.551–2.760) | 0.610 |
| LDH > ULN | 0.728 | (0.319–1.660) | 0.451 |
| Stage ≥ 3 | 1.100 | (0.431–2.810) | 0.841 |
| Extranodal sites ≥ 2 | 1.190 | (0.539–2.620) | 0.668 |
| CCI category | 1.420 | (0.932–2.170) | 0.102 |
| GNRI risk | 2.540 | (1.020–6.310) | 0.044 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Fujita, K.; Morishita, T.; Negoro, E.; Tsukasaki, H.; Oiwa, K.; Hara, T.; Tsurumi, H.; Ueda, T.; Yamauchi, T. The Relative Dose Intensity Changes during Cycles of Standard Regimens in Patients with Diffuse Large B-Cell Lymphoma. Cancers 2023, 15, 4458. https://doi.org/10.3390/cancers15184458
Lee S, Fujita K, Morishita T, Negoro E, Tsukasaki H, Oiwa K, Hara T, Tsurumi H, Ueda T, Yamauchi T. The Relative Dose Intensity Changes during Cycles of Standard Regimens in Patients with Diffuse Large B-Cell Lymphoma. Cancers. 2023; 15(18):4458. https://doi.org/10.3390/cancers15184458
Chicago/Turabian StyleLee, Shin, Kei Fujita, Tetsuji Morishita, Eiju Negoro, Hikaru Tsukasaki, Kana Oiwa, Takeshi Hara, Hisashi Tsurumi, Takanori Ueda, and Takahiro Yamauchi. 2023. "The Relative Dose Intensity Changes during Cycles of Standard Regimens in Patients with Diffuse Large B-Cell Lymphoma" Cancers 15, no. 18: 4458. https://doi.org/10.3390/cancers15184458




