Accurate Detection of Urothelial Bladder Cancer Using Targeted Deep Sequencing of Urine DNA
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
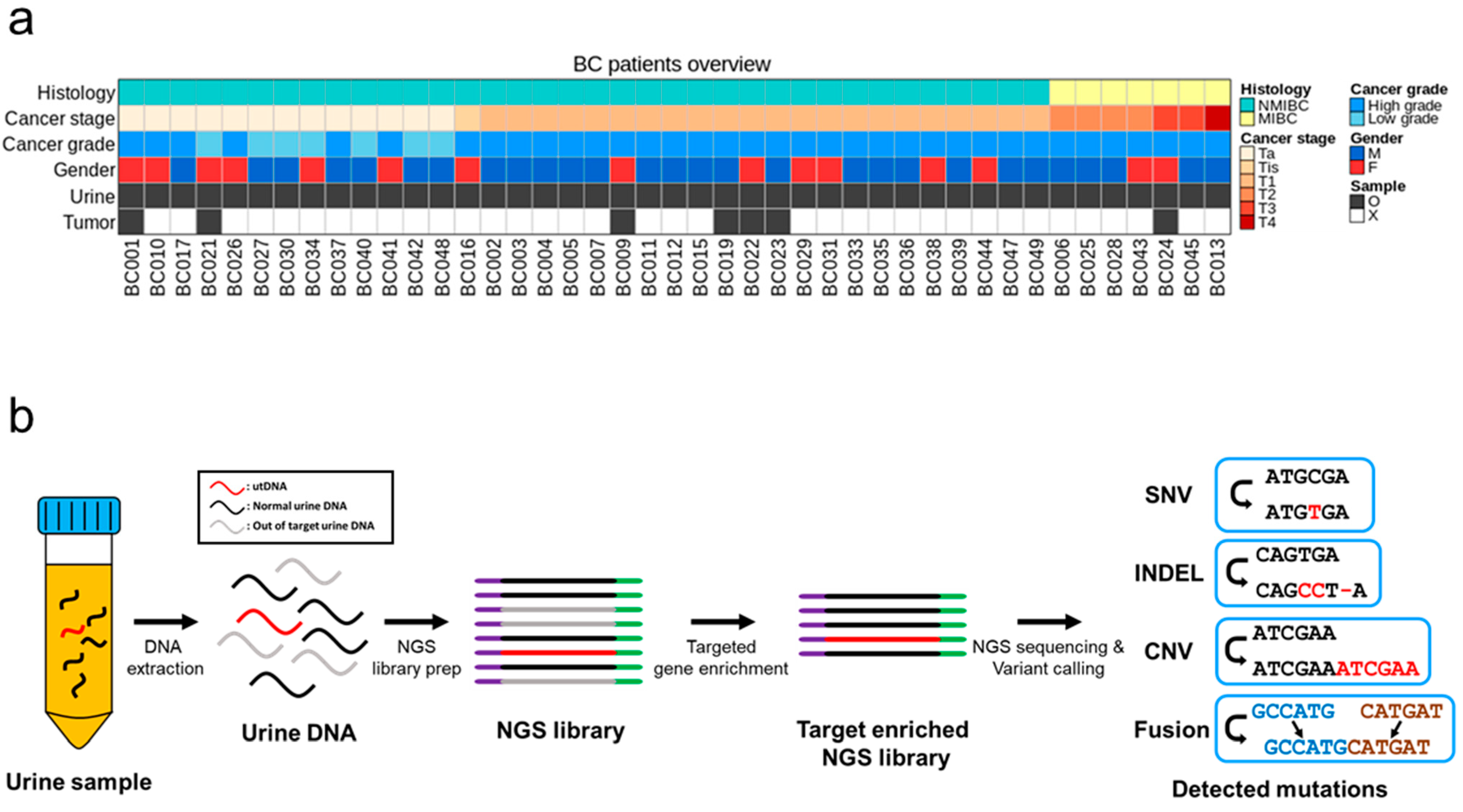
2.1. Patient Enrollment and Sample Collection
2.2. Analysis of DNA from Urine and Bladder Tumors
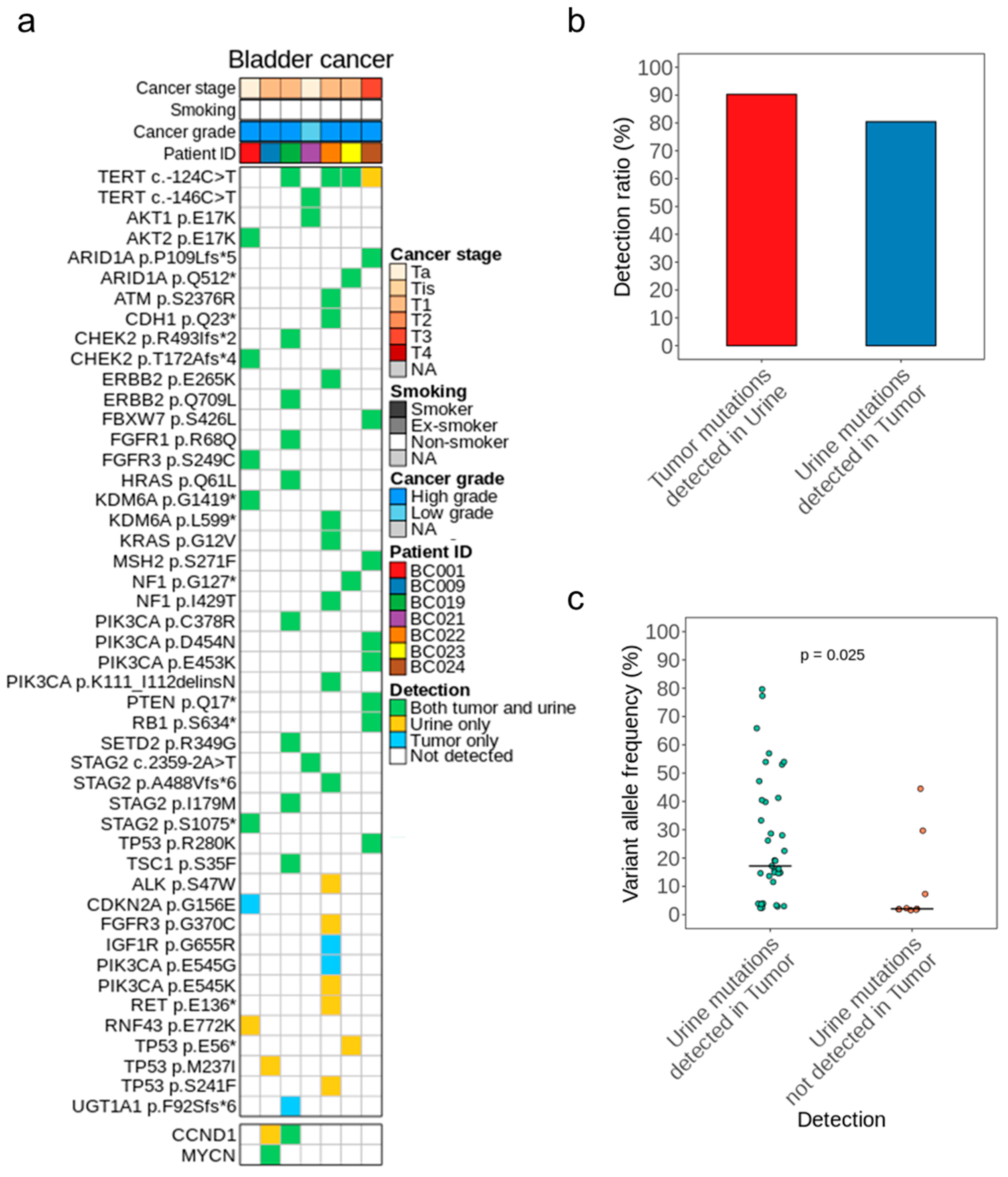
2.3. Concordance Analysis of Tumor and Urine DNA
2.4. BC Detection without Tumor Samples
3. Results
3.1. Patient Characteristics and Study Design
3.2. Mutational Concordance in Paired Samples of Tumor and Urine DNA
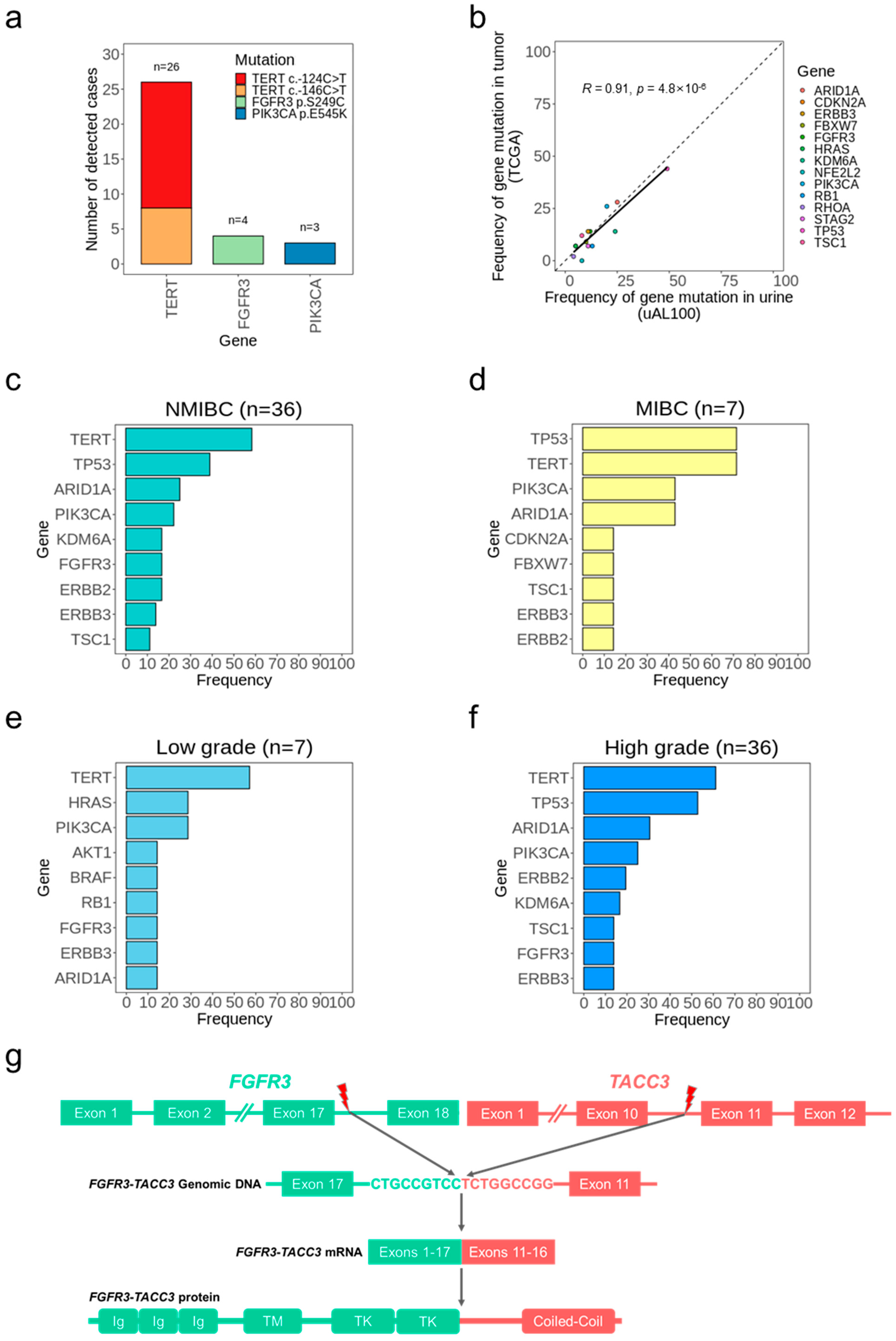
3.3. Bladder Cancer Detection without Tumor Samples
3.4. Bladder Cancer Genotypes Determined Using uAL100
3.5. Information from Peripheral Blood Mononuclear Cells in Urine-Based Bladder Cancer Detection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wallace, D.M.; Bryan, R.T.; Dunn, J.A.; Begum, G.; Bathers, S.; West Midlands Urological Research Group. Delay and survival in bladder cancer. BJU Int. 2002, 89, 868–878. [Google Scholar] [CrossRef] [PubMed]
- Edwards, T.J.; Dickinson, A.J.; Natale, S.; Gosling, J.; McGrath, J.S. A prospective analysis of the diagnostic yield resulting from the attendance of 4020 patients at a protocol-driven haematuria clinic. BJU Int. 2006, 97, 301–305; discussion 305. [Google Scholar] [CrossRef]
- Tan, W.S.; Feber, A.; Sarpong, R.; Khetrapal, P.; Rodney, S.; Jalil, R.; Mostafid, H.; Cresswell, J.; Hicks, J.; Rane, A.; et al. Who Should Be Investigated for Haematuria? Results of a Contemporary Prospective Observational Study of 3556 Patients. Eur. Urol. 2018, 74, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Lodewijk, I.; Duenas, M.; Rubio, C.; Munera-Maravilla, E.; Segovia, C.; Bernardini, A.; Teijeira, A.; Paramio, J.M.; Suarez-Cabrera, C. Liquid Biopsy Biomarkers in Bladder Cancer: A Current Need for Patient Diagnosis and Monitoring. Int. J. Mol. Sci. 2018, 19, 2514. [Google Scholar] [CrossRef] [PubMed]
- Sathianathen, N.J.; Butaney, M.; Weight, C.J.; Kumar, R.; Konety, B.R. Urinary Biomarkers in the Evaluation of Primary Hematuria: A Systematic Review and Meta-Analysis. Bladder Cancer 2018, 4, 353–363. [Google Scholar] [CrossRef]
- Chaudhuri, A.A.; Pellini, B.; Pejovic, N.; Chauhan, P.S.; Harris, P.K.; Szymanski, J.J.; Smith, Z.L.; Arora, V.K. Emerging Roles of Urine-Based Tumor DNA Analysis in Bladder Cancer Management. JCO Precis. Oncol. 2020, 4, 806–817. [Google Scholar] [CrossRef] [PubMed]
- Harsanyi, S.; Novakova, Z.V.; Bevizova, K.; Danisovic, L.; Ziaran, S. Biomarkers of Bladder Cancer: Cell-Free DNA, Epigenetic Modifications and Non-Coding RNAs. Int. J. Mol. Sci. 2022, 23, 13206. [Google Scholar] [CrossRef]
- Newman, A.M.; Lovejoy, A.F.; Klass, D.M.; Kurtz, D.M.; Chabon, J.J.; Scherer, F.; Stehr, H.; Liu, C.L.; Bratman, S.V.; Say, C.; et al. Integrated digital error suppression for improved detection of circulating tumor DNA. Nat. Biotechnol. 2016, 34, 547–555. [Google Scholar] [CrossRef]
- Phallen, J.; Sausen, M.; Adleff, V.; Leal, A.; Hruban, C.; White, J.; Anagnostou, V.; Fiksel, J.; Cristiano, S.; Papp, E.; et al. Direct detection of early-stage cancers using circulating tumor DNA. Sci. Transl. Med. 2017, 9, eaan2415. [Google Scholar] [CrossRef]
- Dudley, J.C.; Schroers-Martin, J.; Lazzareschi, D.V.; Shi, W.Y.; Chen, S.B.; Esfahani, M.S.; Trivedi, D.; Chabon, J.J.; Chaudhuri, A.A.; Stehr, H.; et al. Detection and Surveillance of Bladder Cancer Using Urine Tumor DNA. Cancer Discov. 2019, 9, 500–509. [Google Scholar] [CrossRef]
- Ward, D.G.; Baxter, L.; Ott, S.; Gordon, N.S.; Wang, J.; Patel, P.; Piechocki, K.; Silcock, L.; Sale, C.; Zeegers, M.P.; et al. Highly Sensitive and Specific Detection of Bladder Cancer via Targeted Ultra-deep Sequencing of Urinary DNA. Eur. Urol. Oncol. 2022, 6, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zang, J.; Xie, F.; Zhang, Y.; Wang, Y.; Jing, Y.; Zhang, Y.; Chen, Z.; Shahatiaili, A.; Cai, M.C.; et al. Urinary Molecular Pathology for Patients with Newly Diagnosed Urothelial Bladder Cancer. J. Urol. 2021, 206, 873–884. [Google Scholar] [CrossRef]
- Tivey, A.; Church, M.; Rothwell, D.; Dive, C.; Cook, N. Circulating tumour DNA—Looking beyond the blood. Nat. Rev. Clin. Oncol. 2022, 19, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.S.; Chen, K.; Babbra, R.K.; Feng, W.; Pejovic, N.; Nallicheri, A.; Harris, P.K.; Dienstbach, K.; Atkocius, A.; Maguire, L.; et al. Urine tumor DNA detection of minimal residual disease in muscle-invasive bladder cancer treated with curative-intent radical cystectomy: A cohort study. PLoS Med. 2021, 18, e1003732. [Google Scholar] [CrossRef]
- Springer, S.U.; Chen, C.H.; Rodriguez Pena, M.D.C.; Li, L.; Douville, C.; Wang, Y.; Cohen, J.D.; Taheri, D.; Silliman, N.; Schaefer, J.; et al. Non-invasive detection of urothelial cancer through the analysis of driver gene mutations and aneuploidy. Elife 2018, 7, e32143. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lim, Y.; Kang, J.K.; Kim, H.P.; Roh, H.; Kim, S.Y.; Lee, D.; Bang, D.; Jeong, S.Y.; Park, K.J.; et al. Dynamic changes in longitudinal circulating tumour DNA profile during metastatic colorectal cancer treatment. Br. J. Cancer 2022, 127, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Barkan, G.A.; Wojcik, E.M.; Nayar, R.; Savic-Prince, S.; Quek, M.L.; Kurtycz, D.F.; Rosenthal, D.L. The Paris System for Reporting Urinary Cytology: The Quest to Develop a Standardized Terminology. Acta Cytol. 2016, 60, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, M.; Huang, T.; Liao, W.; Xu, M.; Gu, J. GeneFuse: Detection and visualization of target gene fusions from DNA sequencing data. Int. J. Biol. Sci. 2018, 14, 843–848. [Google Scholar] [CrossRef]
- Gawronski, A.R.; Lin, Y.Y.; McConeghy, B.; LeBihan, S.; Asghari, H.; Kockan, C.; Orabi, B.; Adra, N.; Pili, R.; Collins, C.C.; et al. Structural variation and fusion detection using targeted sequencing data from circulating cell free DNA. Nucleic Acids Res. 2019, 47, e38. [Google Scholar] [CrossRef]
- Chakravarty, D.; Gao, J.; Phillips, S.M.; Kundra, R.; Zhang, H.; Wang, J.; Rudolph, J.E.; Yaeger, R.; Soumerai, T.; Nissan, M.H.; et al. OncoKB: A Precision Oncology Knowledge Base. JCO Precis Oncol. 2017, 2017, 1–16. [Google Scholar] [CrossRef]
- Tate, J.G.; Bamford, S.; Jubb, H.C.; Sondka, Z.; Beare, D.M.; Bindal, N.; Boutselakis, H.; Cole, C.G.; Creatore, C.; Dawson, E.; et al. COSMIC: The Catalogue Of Somatic Mutations In Cancer. Nucleic Acids Res. 2019, 47, D941–D947. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.T.; Bhattarai, T.S.; Schram, A.M.; Bielski, C.M.; Donoghue, M.T.A.; Jonsson, P.; Chakravarty, D.; Phillips, S.; Kandoth, C.; Penson, A.; et al. Accelerating Discovery of Functional Mutant Alleles in Cancer. Cancer Discov. 2018, 8, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.T.; Asthana, S.; Gao, S.P.; Lee, B.H.; Chapman, J.S.; Kandoth, C.; Gao, J.; Socci, N.D.; Solit, D.B.; Olshen, A.B.; et al. Identifying recurrent mutations in cancer reveals widespread lineage diversity and mutational specificity. Nat. Biotechnol. 2016, 34, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Weinhold, N.; Jacobsen, A.; Schultz, N.; Sander, C.; Lee, W. Genome-wide analysis of noncoding regulatory mutations in cancer. Nat. Genet. 2014, 46, 1160–1165. [Google Scholar] [CrossRef]
- Rachakonda, P.S.; Hosen, I.; de Verdier, P.J.; Fallah, M.; Heidenreich, B.; Ryk, C.; Wiklund, N.P.; Steineck, G.; Schadendorf, D.; Hemminki, K.; et al. TERT promoter mutations in bladder cancer affect patient survival and disease recurrence through modification by a common polymorphism. Proc. Natl. Acad. Sci. USA 2013, 110, 17426–17431. [Google Scholar] [CrossRef]
- Hayashi, T.; Fujita, K.; Hayashi, Y.; Hatano, K.; Kawashima, A.; McConkey, D.J.; Nonomura, N. Mutational Landscape and Environmental Effects in Bladder Cancer. Int. J. Mol. Sci. 2020, 21, 6072. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research, N. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014, 507, 315–322. [Google Scholar] [CrossRef]
- Knowles, M.A.; Hurst, C.D. Molecular biology of bladder cancer: New insights into pathogenesis and clinical diversity. Nat. Rev. Cancer 2015, 15, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.; Richter, J.; Wagner, U.; Fijan, A.; Bruderer, J.; Schmid, U.; Ackermann, D.; Maurer, R.; Alund, G.; Knonagel, H.; et al. High-throughput tissue microarray analysis of 3p25 (RAF1) and 8p12 (FGFR1) copy number alterations in urinary bladder cancer. Cancer Res. 2001, 61, 4514–4519. [Google Scholar]
- Bekele, R.T.; Samant, A.S.; Nassar, A.H.; So, J.; Garcia, E.P.; Curran, C.R.; Hwang, J.H.; Mayhew, D.L.; Nag, A.; Thorner, A.R.; et al. RAF1 amplification drives a subset of bladder tumors and confers sensitivity to MAPK-directed therapeutics. J. Clin. Invest. 2021, 131, e147849. [Google Scholar] [CrossRef] [PubMed]
- Chabon, J.J.; Hamilton, E.G.; Kurtz, D.M.; Esfahani, M.S.; Moding, E.J.; Stehr, H.; Schroers-Martin, J.; Nabet, B.Y.; Chen, B.; Chaudhuri, A.A.; et al. Integrating genomic features for non-invasive early lung cancer detection. Nature 2020, 580, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Razavi, P.; Li, B.T.; Brown, D.N.; Jung, B.; Hubbell, E.; Shen, R.; Abida, W.; Juluru, K.; De Bruijn, I.; Hou, C.; et al. High-intensity sequencing reveals the sources of plasma circulating cell-free DNA variants. Nat. Med. 2019, 25, 1928–1937. [Google Scholar] [CrossRef]
- Dyrskjot, L.; Ingersoll, M.A. Biology of nonmuscle-invasive bladder cancer: Pathology, genomic implications, and immunology. Curr. Opin. Urol. 2018, 28, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Lindskrog, S.V.; Prip, F.; Lamy, P.; Taber, A.; Groeneveld, C.S.; Birkenkamp-Demtroder, K.; Jensen, J.B.; Strandgaard, T.; Nordentoft, I.; Christensen, E.; et al. An integrated multi-omics analysis identifies prognostic molecular subtypes of non-muscle-invasive bladder cancer. Nat. Commun. 2021, 12, 2301. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Bohle, A.; Burger, M.; Capoun, O.; Cohen, D.; Comperat, E.M.; Hernandez, V.; Kaasinen, E.; Palou, J.; Roupret, M.; et al. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur. Urol. 2017, 71, 447–461. [Google Scholar] [CrossRef]
- Svatek, R.S.; Hollenbeck, B.K.; Holmang, S.; Lee, R.; Kim, S.P.; Stenzl, A.; Lotan, Y. The economics of bladder cancer: Costs and considerations of caring for this disease. Eur. Urol. 2014, 66, 253–262. [Google Scholar] [CrossRef] [PubMed]

| Disease Status | Total | M/F | Median Age | Cancer Stage | Cancer Grade | Cytology | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ta | Tis | T1 | T2–T4 | High Grade | Low Grade | 0 | 1 | 2 | ||||
| Bladder Cancer | 43 | 28/15 | 68 | 13 | 1 | 22 | 7 | 36 | 7 | 21 | 14 | 8 |
| Non-Bladder Cancer | 21 | 20/1 | 64 | – | – | – | – | – | – | – | – | – |
| Methods | Ward et al. (2022) [11] | Dudley et al. (2019) [10] | uAL100 |
|---|---|---|---|
| Sample Type | Urine cell-pellet DNA | Urine DNA | Urine DNA |
| Sensitivity | 87.3% | 84% | 83.7% |
| Specificity | 84.8% | 96% | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Lee, W.; Kim, H.-P.; Kim, M.; Ahn, H.K.; Bang, D.; Kim, K.H. Accurate Detection of Urothelial Bladder Cancer Using Targeted Deep Sequencing of Urine DNA. Cancers 2023, 15, 2868. https://doi.org/10.3390/cancers15102868
Lee D, Lee W, Kim H-P, Kim M, Ahn HK, Bang D, Kim KH. Accurate Detection of Urothelial Bladder Cancer Using Targeted Deep Sequencing of Urine DNA. Cancers. 2023; 15(10):2868. https://doi.org/10.3390/cancers15102868
Chicago/Turabian StyleLee, Dongin, Wookjae Lee, Hwang-Phill Kim, Myong Kim, Hyun Kyu Ahn, Duhee Bang, and Kwang Hyun Kim. 2023. "Accurate Detection of Urothelial Bladder Cancer Using Targeted Deep Sequencing of Urine DNA" Cancers 15, no. 10: 2868. https://doi.org/10.3390/cancers15102868







