How Does Environmental and Occupational Exposure Contribute to Carcinogenesis in Genitourinary and Lung Cancers?
Abstract
:Simple Summary
Abstract
1. Introduction
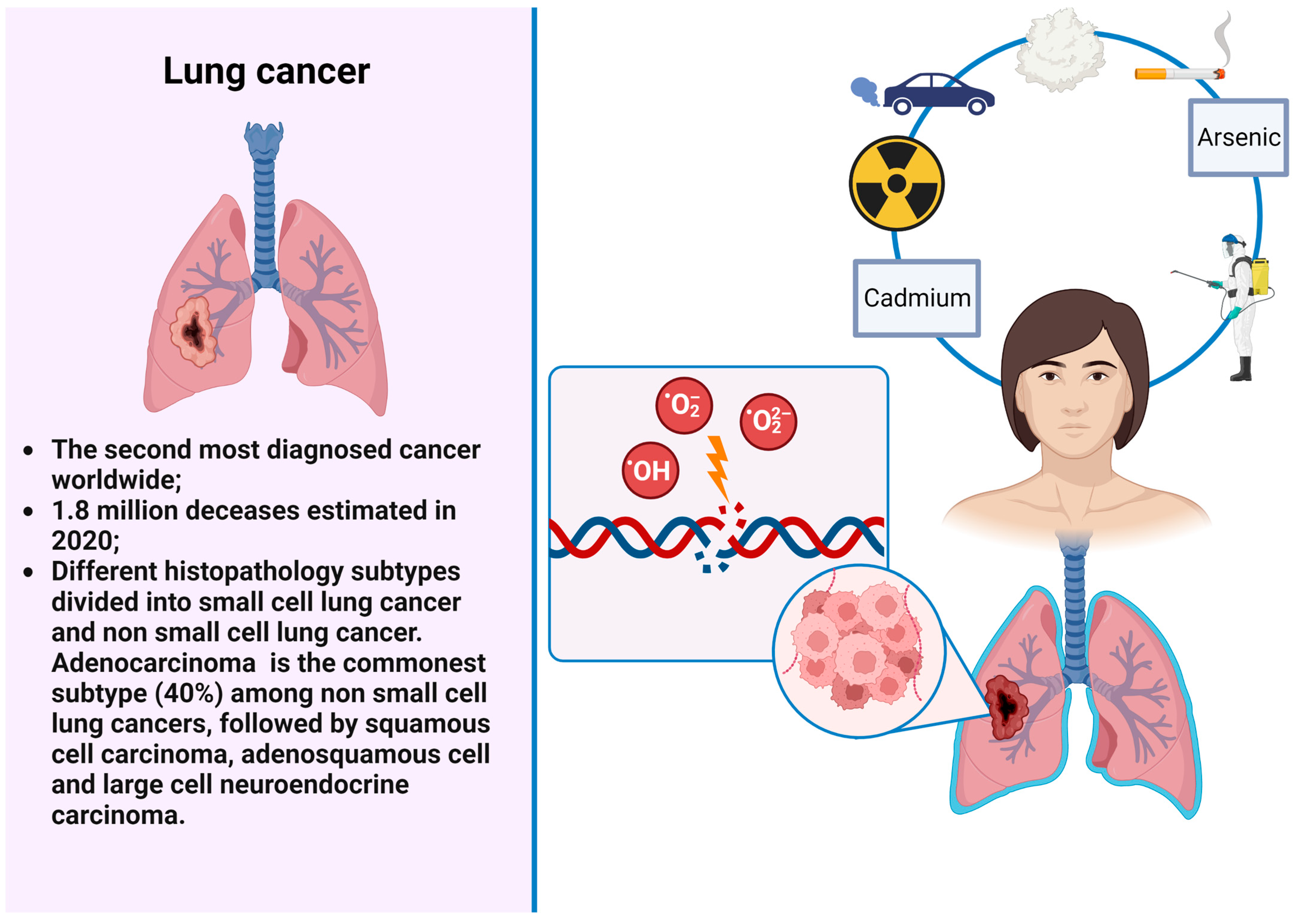
2. Tobacco Smoking
3. Indoor Air Pollution
4. Outdoor Air Pollution
5. Asbestos
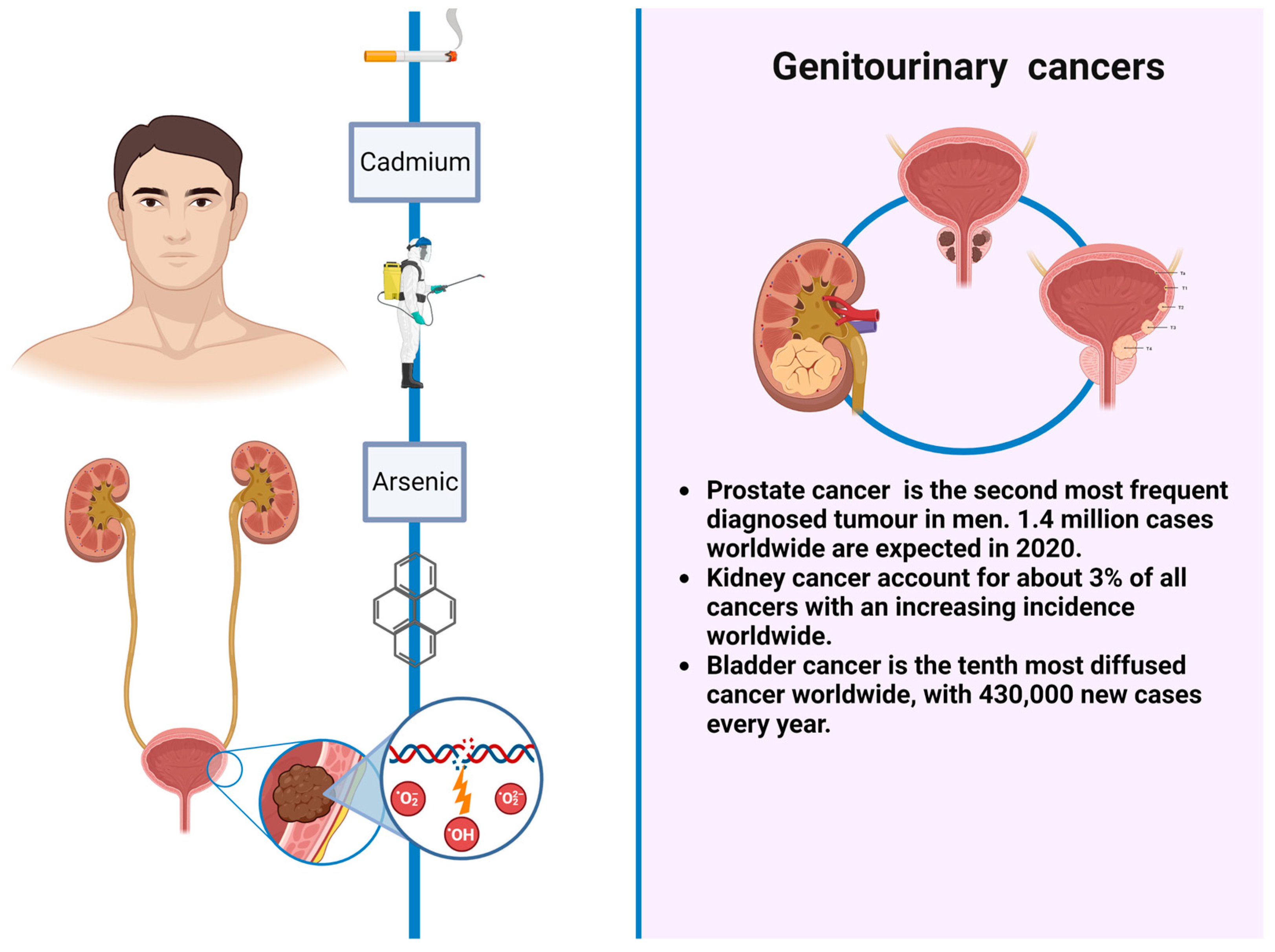
6. Cadmium
7. Arsenic
8. Chromium
9. Nickel
10. Polycyclic Aromatic Hydrocarbons (PAHs)
11. Aromatic Amines
12. Trichloroethylene
13. Pesticides
14. Diesel
15. Aristolochic Acid
16. Ionizing Radiations
17. Screening Programs
18. Discussion and Future Directions
| Risk Factor | Pathogenic Mechanisms | Sufficient Evidence | Limited Evidence | |
|---|---|---|---|---|
| Tobacco smoking | DNA adducts among tumor suppressor genes. | Oral cavity, pharynx, esophagus, stomach, colon, rectum, liver, bile duct, pancreas, nasal cavity/paranasal sinus, larynx, lung, uterine cervix, ovary, kidney, renal pelvis and ureter, urinary bladder | Breast, childhood leukemias (parental smoking) | |
| Radon | DNA base mutations and chromosomal strand breaks | Lung | Leukemias | |
| Indoor air pollution (coal combustion) | DNA adducts | Lung | - | |
| Outdoor air pollution | Induced oncogene mutations by still unclear molecular mechanisms | Lung | Urinary bladder | |
| Asbestos | Increased ROS synthesis implicated in DNA damage across tumor suppressor genes | Larynx, lung, mesothelium, ovary | Pharynx, stomach, colon, rectum | |
| Cadmium | Induction of oxidative stress and suppression of DNA repair genes. Alterations of DNA methylation | Lung | Prostate, kidney | |
| Arsenic | DNA strand breaks, chromosomal aberrations. Epigenetic alterations | Lung, skin, urinary bladder | Liver, bile duct, prostate, kidney | |
| Chromium | Damage to cellular components, generation of free radicals resulting in DNA damage. | Lung | Nasal cavity and paranasal sinus | |
| Nickel | DNA damages and epigenetic changes | Lung, nasal cavity and paranasal sinus | - | |
| PAHs (benzopyrene) | DNA adducts | Lung, bladder, esophagus, liver, lymphoid and hematopoietic tissues | - | |
| AAs (benzidine as hair dyes) | DNA adducts | Urinary bladder | - | |
| Trichloroethylene | DNA adducts synthesis by reactive intermediates | Kidney | Liver, bile duct | |
| Pesticides (DDT) | ROS induced synthesis and subsequent DNA and protein damages | - | Liver and bile duct | |
| Diesel | Chromosomal damage, altered gene expression patters, inflammation onset | Lung | Urinary bladder | |
| Ionizing radiation | DNA double-strand breaks | Salivary gland, esophagus, stomach, colon, lung, bone, skin, breast, kidney, urinary bladder, brain/central nervous system, thyroid, chronic myeloid and acute lymphocytic leukemia | Rectum, liver, bile duct, pancreas, ovary, prostate, childhood leukemia | |
| Aristolochic acid | Aristolactam—deoxyadenosine adducts synthesis and subsequent tumorigenesis. | Renal pelvis and ureter | - | |
19. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Weiderpass, E. Lifestyle and cancer risk. J. Prev. Med. Public Health 2010, 43, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Couespel, N.; Price, R. Strengthening Europe in the Fight against Cancer; European Union: Brussels, Belgium, 2020.
- Stein, C.J.; Colditz, G.A. Modifiable risk factors for cancer. Br. J. Cancer 2004, 90, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Respiratory Tract Cancers Collaborators. Global, regional, and national burden of respiratory tract cancers and associated risk factors from 1990 to 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Respir. Med. 2021, 9, 1030–1049. [Google Scholar] [CrossRef]
- Mullins, J.K.; Loeb, S. Environmental exposures and prostate cancer. Urol. Oncol. 2012, 30, 216–219. [Google Scholar] [CrossRef]
- Tahbaz, R.; Schmid, M.; Merseburger, A.S. Prevention of kidney cancer incidence and recurrence: Lifestyle, medication and nutrition. Curr. Opin. Urol. 2018, 28, 62–79. [Google Scholar] [CrossRef]
- Al-Zalabani, A.H.; Stewart, K.F.; Wesselius, A.; Schols, A.M.; Zeegers, M.P. Modifiable risk factors for the prevention of bladder cancer: A systematic review of meta-analyses. Eur. J. Epidemiol. 2016, 31, 811–851. [Google Scholar] [CrossRef]
- Pesch, B.; Taeger, D.; Johnen, G.; Gawrych, K.; Bonberg, N.; Schwentner, C.; Wellhausser, H.; Kluckert, M.; Leng, G.; Nasterlack, M.; et al. Screening for bladder cancer with urinary tumor markers in chemical workers with exposure to aromatic amines. Int. Arch. Occup. Environ. Health 2014, 87, 715–724. [Google Scholar] [CrossRef]
- Steinmaus, C.; Ferreccio, C.; Acevedo, J.; Yuan, Y.; Liaw, J.; Duran, V.; Cuevas, S.; Garcia, J.; Meza, R.; Valdes, R.; et al. Increased lung and bladder cancer incidence in adults after in utero and early-life arsenic exposure. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1529–1538. [Google Scholar] [CrossRef]
- Koutros, S.; Baris, D.; Waddell, R.; Beane Freeman, L.E.; Colt, J.S.; Schwenn, M.; Johnson, A.; Ward, M.H.; Hosain, G.M.; Moore, L.E.; et al. Potential effect modifiers of the arsenic-bladder cancer risk relationship. Int. J. Cancer 2018, 143, 2640–2646. [Google Scholar] [CrossRef] [PubMed]
- Koutros, S.; Kogevinas, M.; Friesen, M.C.; Stewart, P.A.; Baris, D.; Karagas, M.R.; Schwenn, M.; Johnson, A.; Monawar Hosain, G.M.; Serra, C.; et al. Diesel exhaust and bladder cancer risk by pathologic stage and grade subtypes. Environ. Int. 2020, 135, 105346. [Google Scholar] [CrossRef] [PubMed]
- Grollman, A.P. Aristolochic acid nephropathy: Harbinger of a global iatrogenic disease. Environ. Mol. Mutagen. 2013, 54, 1–7. [Google Scholar] [CrossRef]
- Marant Micallef, C.; Shield, K.D.; Baldi, I.; Charbotel, B.; Fervers, B.; Gilg Soit Ilg, A.; Guenel, P.; Olsson, A.; Rushton, L.; Hutchings, S.J.; et al. Occupational exposures and cancer: A review of agents and relative risk estimates. Occup. Environ. Med. 2018, 75, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Yokota, R.T.C.; Nusselder, W.J.; Robine, J.M.; Tafforeau, J.; Charafeddine, R.; Gisle, L.; Deboosere, P.; Van Oyen, H. Contribution of chronic conditions to smoking differences in life expectancy with and without disability in Belgium. Eur. J. Public Health 2018, 28, 859–863. [Google Scholar] [CrossRef]
- Taylor, R.; Najafi, F.; Dobson, A. Meta-analysis of studies of passive smoking and lung cancer: Effects of study type and continent. Int. J. Epidemiol. 2007, 36, 1048–1059. [Google Scholar] [CrossRef]
- Secretan, B.; Straif, K.; Baan, R.; Grosse, Y.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens—Part E: Tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009, 10, 1033–1034. [Google Scholar] [CrossRef]
- Stading, R.; Gastelum, G.; Chu, C.; Jiang, W.; Moorthy, B. Molecular mechanisms of pulmonary carcinogenesis by polycyclic aromatic hydrocarbons (PAHs): Implications for human lung cancer. Semin. Cancer Biol. 2021, 76, 3–16. [Google Scholar] [CrossRef]
- Zhu, K.; Xu, A.; Xia, W.; Li, P.; Zhang, B.; Jiang, H.; Zhou, S.; Wang, R. Association between NAT2 Polymorphism and Lung Cancer Risk: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 567762. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Gabrielson, E. Worldwide trends in lung cancer pathology. Respirology 2006, 11, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.; Peto, R. Global effects of smoking, of quitting, and of taxing tobacco. N. Engl. J. Med. 2014, 370, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.J.; Yu, D.; Wen, W.; Shu, X.O.; Saito, E.; Rahman, S.; Gupta, P.C.; He, J.; Tsugane, S.; Xiang, Y.B.; et al. Tobacco Smoking and Mortality in Asia: A Pooled Meta-analysis. JAMA Netw. Open 2019, 2, e191474. [Google Scholar] [CrossRef]
- Crispo, A.; Brennan, P.; Jockel, K.H.; Schaffrath-Rosario, A.; Wichmann, H.E.; Nyberg, F.; Simonato, L.; Merletti, F.; Forastiere, F.; Boffetta, P.; et al. The cumulative risk of lung cancer among current, ex- and never-smokers in European men. Br. J. Cancer 2004, 91, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Caini, S.; Del Riccio, M.; Vettori, V.; Scotti, V.; Martinoli, C.; Raimondi, S.; Cammarata, G.; Palli, D.; Banini, M.; Masala, G.; et al. Quitting Smoking at or Around Diagnosis Improves the Overall Survival of Lung Cancer Patients: A Systematic Review and Meta-Analysis. J. Thorac. Oncol. 2022, 17, 623–636. [Google Scholar] [CrossRef]
- Available online: https://www.iaslc.org/iaslc-news/press-release/iaslc-position-statement-tobacco-cessation-after-cancer-diagnosis (accessed on 19 March 2023).
- Cumberbatch, M.G.; Rota, M.; Catto, J.W.; La Vecchia, C. The Role of Tobacco Smoke in Bladder and Kidney Carcinogenesis: A Comparison of Exposures and Meta-analysis of Incidence and Mortality Risks. Eur. Urol. 2016, 70, 458–466. [Google Scholar] [CrossRef]
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef]
- Chow, W.H.; Dong, L.M.; Devesa, S.S. Epidemiology and risk factors for kidney cancer. Nat. Rev. Urol. 2010, 7, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Kiriluk, K.J.; Prasad, S.M.; Patel, A.R.; Steinberg, G.D.; Smith, N.D. Bladder cancer risk from occupational and environmental exposures. Urol. Oncol. 2012, 30, 199–211. [Google Scholar] [CrossRef]
- Moritsugu, K.P. The 2006 Report of the Surgeon General: The health consequences of involuntary exposure to tobacco smoke. Am. J. Prev. Med. 2007, 32, 542–543. [Google Scholar] [CrossRef]
- Brennan, P.; Bogillot, O.; Cordier, S.; Greiser, E.; Schill, W.; Vineis, P.; Lopez-Abente, G.; Tzonou, A.; Chang-Claude, J.; Bolm-Audorff, U.; et al. Cigarette smoking and bladder cancer in men: A pooled analysis of 11 case-control studies. Int. J. Cancer 2000, 86, 289–294. [Google Scholar] [CrossRef]
- Chen, C.H.; Shun, C.T.; Huang, K.H.; Huang, C.Y.; Tsai, Y.C.; Yu, H.J.; Pu, Y.S. Stopping smoking might reduce tumour recurrence in nonmuscle-invasive bladder cancer. BJU Int. 2007, 100, 281–286; discussion 286. [Google Scholar] [CrossRef] [PubMed]
- De Nunzio, C.; Tema, G.; Lombardo, R.; Trucchi, A.; Bellangino, M.; Esperto, F.; Deroma, M.; Proietti, F.; Vecchione, A.; Tubaro, A. Cigarette smoking is not associated with prostate cancer diagnosis and aggressiveness: A cross sectional Italian study. Minerva Urol. Nefrol. 2018, 70, 598–605. [Google Scholar] [CrossRef]
- Ngo, T.C.; Lee, J.J.; Brooks, J.D.; Nolley, R.; Ferrari, M.; Presti, J.C., Jr. Smoking and adverse outcomes at radical prostatectomy. Urol. Oncol. 2013, 31, 749–754. [Google Scholar] [CrossRef]
- Enokida, H.; Shiina, H.; Urakami, S.; Terashima, M.; Ogishima, T.; Li, L.C.; Kawahara, M.; Nakagawa, M.; Kane, C.J.; Carroll, P.R.; et al. Smoking influences aberrant CpG hypermethylation of multiple genes in human prostate carcinoma. Cancer 2006, 106, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Prueitt, R.L.; Wallace, T.A.; Glynn, S.A.; Yi, M.; Tang, W.; Luo, J.; Dorsey, T.H.; Stagliano, K.E.; Gillespie, J.W.; Hudson, R.S.; et al. An Immune-Inflammation Gene Expression Signature in Prostate Tumors of Smokers. Cancer Res. 2016, 76, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Samet, J.M.; Avila-Tang, E.; Boffetta, P.; Hannan, L.M.; Olivo-Marston, S.; Thun, M.J.; Rudin, C.M. Lung cancer in never smokers: Clinical epidemiology and environmental risk factors. Clin. Cancer Res. 2009, 15, 5626–5645. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Tobacco Smoke and Involuntary Smoking; IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Lyon, France, 2004; Volume 83, pp. 1–1438. [Google Scholar]
- Alberg, A.J.; Kouzis, A.; Genkinger, J.M.; Gallicchio, L.; Burke, A.E.; Hoffman, S.C.; Diener-West, M.; Helzlsouer, K.J.; Comstock, G.W. A prospective cohort study of bladder cancer risk in relation to active cigarette smoking and household exposure to secondhand cigarette smoke. Am. J. Epidemiol. 2007, 165, 660–666. [Google Scholar] [CrossRef]
- Wilhelm-Benartzi, C.S.; Christensen, B.C.; Koestler, D.C.; Houseman, E.A.; Schned, A.R.; Karagas, M.R.; Kelsey, K.T.; Marsit, C.J. Association of secondhand smoke exposures with DNA methylation in bladder carcinomas. Cancer Causes Control 2011, 22, 1205–1213. [Google Scholar] [CrossRef]
- Torres-Duran, M.; Ruano-Ravina, A.; Parente-Lamelas, I.; Leiro-Fernandez, V.; Abal-Arca, J.; Montero-Martinez, C.; Pena-Alvarez, C.; Gonzalez-Barcala, F.J.; Castro-Anon, O.; Golpe-Gomez, A.; et al. Lung cancer in never-smokers: A case-control study in a radon-prone area (Galicia, Spain). Eur. Respir. J. 2014, 44, 994–1001. [Google Scholar] [CrossRef]
- United States Enviromental Protection Agency. A Citizen’s Guide to Radon: The Guide to Protecting Yourself and Your Family from Radon; Indoor Enviroments Division, United States Enviromental Protection Agency: Washington, DC, USA, 2016.
- Darby, S.; Hill, D.; Auvinen, A.; Barros-Dios, J.M.; Baysson, H.; Bochicchio, F.; Deo, H.; Falk, R.; Forastiere, F.; Hakama, M.; et al. Radon in homes and risk of lung cancer: Collaborative analysis of individual data from 13 European case-control studies. BMJ 2005, 330, 223. [Google Scholar] [CrossRef] [PubMed]
- Krewski, D.; Lubin, J.H.; Zielinski, J.M.; Alavanja, M.; Catalan, V.S.; Field, R.W.; Klotz, J.B.; Letourneau, E.G.; Lynch, C.F.; Lyon, J.I.; et al. Residential radon and risk of lung cancer: A combined analysis of 7 North American case-control studies. Epidemiology 2005, 16, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Torres-Duran, M.; Barros-Dios, J.M.; Fernandez-Villar, A.; Ruano-Ravina, A. Residential radon and lung cancer in never smokers. A systematic review. Cancer Lett. 2014, 345, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Riudavets, M.; Garcia de Herreros, M.; Besse, B.; Mezquita, L. Radon and Lung Cancer: Current Trends and Future Perspectives. Cancers 2022, 14, 3142. [Google Scholar] [CrossRef]
- Barros-Dios, J.M.; Ruano-Ravina, A.; Gastelu-Iturri, J.; Figueiras, A. Factors underlying residential radon concentration: Results from Galicia, Spain. Environ. Res. 2007, 103, 185–190. [Google Scholar] [CrossRef]
- Mezquita, L.; Barlesi, F.; Ielsch, G.; Merlio, J.; Debieuvre, D.; Mosser, J.; Ricordel, C.; Ouafik, L.; Rouquette, I.; Monnet, I.; et al. FP09.05 Driver Oncogenic Alterations and Indoor Radon in NSCLC Patients from the IFCT Biomarker Cohort: Bioradon France Study. J. Thorac. Oncol. 2021, 16, S214. [Google Scholar] [CrossRef]
- SPECTA: Screening Cancer Patients for Efficient Clinical Trial Access (SPECTA). Available online: https://clinicaltrials.gov/ct2/show/results/NCT02834884?view=results (accessed on 15 February 2023).
- Barbosa-Lorenzo, R.; Barros-Dios, J.M.; Raices Aldrey, M.; Cerdeira Carames, S.; Ruano-Ravina, A. Residential radon and cancers other than lung cancer: A cohort study in Galicia, a Spanish radon-prone area. Eur. J. Epidemiol. 2016, 31, 437–441. [Google Scholar] [CrossRef]
- Mozzoni, P.; Pinelli, S.; Corradi, M.; Ranzieri, S.; Cavallo, D.; Poli, D. Environmental/Occupational Exposure to Radon and Non-Pulmonary Neoplasm Risk: A Review of Epidemiologic Evidence. Int. J. Environ. Res. Public Health 2021, 18, 10466. [Google Scholar] [CrossRef]
- McCunney, R.J.; Yong, M. Coal Miners and Lung Cancer: Can Mortality Studies Offer a Perspective on Rat Inhalation Studies of Poorly Soluble Low Toxicity Particles? Front. Public Health 2022, 10, 907157. [Google Scholar] [CrossRef]
- Zhang, J.J.; Smith, K.R. Household air pollution from coal and biomass fuels in China: Measurements, health impacts, and interventions. Environ. Health Perspect. 2007, 115, 848–855. [Google Scholar] [CrossRef]
- Hosgood, H.D., 3rd; Boffetta, P.; Greenland, S.; Lee, Y.C.; McLaughlin, J.; Seow, A.; Duell, E.J.; Andrew, A.S.; Zaridze, D.; Szeszenia-Dabrowska, N.; et al. In-home coal and wood use and lung cancer risk: A pooled analysis of the International Lung Cancer Consortium. Environ. Health Perspect. 2010, 118, 1743–1747. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, G.; Lei, Y.; Yang, K.; Niu, H.; Zhao, J.; He, R.; Ning, H.; Huang, Q.; Zhou, Q.; et al. Lung cancer family history and exposure to occupational/domestic coal combustion contribute to variations in clinicopathologic features and gene fusion patterns in non-small cell lung cancer. Thorac. Cancer 2019, 10, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.H.; Jaakkola, J.J.; Chuang, C.Y.; Liou, S.H.; Lung, S.C.; Loh, C.H.; Yu, D.S.; Strickland, P.T. Exposure to cooking oil fumes and oxidative damages: A longitudinal study in Chinese military cooks. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 94–100. [Google Scholar] [CrossRef]
- Ko, Y.C.; Cheng, L.S.; Lee, C.H.; Huang, J.J.; Huang, M.S.; Kao, E.L.; Wang, H.Z.; Lin, H.J. Chinese food cooking and lung cancer in women nonsmokers. Am. J. Epidemiol. 2000, 151, 140–147. [Google Scholar] [CrossRef]
- Yu, I.T.; Chiu, Y.L.; Au, J.S.; Wong, T.W.; Tang, J.L. Dose-response relationship between cooking fumes exposures and lung cancer among Chinese nonsmoking women. Cancer Res. 2006, 66, 4961–4967. [Google Scholar] [CrossRef]
- Xue, Y.; Jiang, Y.; Jin, S.; Li, Y. Association between cooking oil fume exposure and lung cancer among Chinese nonsmoking women: A meta-analysis. Onco Targets Ther. 2016, 9, 2987–2992. [Google Scholar] [CrossRef]
- Ganesan, K.; Sukalingam, K.; Xu, B. Impact of consumption of repeatedly heated cooking oils on the incidence of various cancers- A critical review. Crit. Rev. Food Sci. Nutr. 2019, 59, 488–505. [Google Scholar] [CrossRef] [PubMed]
- Elangovan, I.; Thirugnanam, S.; Chen, A.; Zheng, G.; Bosland, M.C.; Kajdacsy-Balla, A.; Gnanasekar, M. Targeting receptor for advanced glycation end products (RAGE) expression induces apoptosis and inhibits prostate tumor growth. Biochem. Biophys. Res. Commun. 2012, 417, 1133–1138. [Google Scholar] [CrossRef]
- Lyon, F. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; World Health Organization: Geneva, Switzerland; International Agency for Research on Cancer: Lyon, France, 2014. Available online: https://monographs.iarc.who.int/wp-content/uploads/2018/08/14-002.pdf (accessed on 2 February 2023).
- Nafstad, P.; Haheim, L.L.; Oftedal, B.; Gram, F.; Holme, I.; Hjermann, I.; Leren, P. Lung cancer and air pollution: A 27 year follow up of 16 209 Norwegian men. Thorax 2003, 58, 1071–1076. [Google Scholar] [CrossRef]
- Lepeule, J.; Laden, F.; Dockery, D.; Schwartz, J. Chronic exposure to fine particles and mortality: An extended follow-up of the Harvard Six Cities study from 1974 to 2009. Environ. Health Perspect. 2012, 120, 965–970. [Google Scholar] [CrossRef]
- Heinrich, J.; Thiering, E.; Rzehak, P.; Kramer, U.; Hochadel, M.; Rauchfuss, K.M.; Gehring, U.; Wichmann, H.E. Long-term exposure to NO2 and PM10 and all-cause and cause-specific mortality in a prospective cohort of women. Occup. Environ. Med. 2013, 70, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A., 3rd; Burnett, R.T.; Thun, M.J.; Calle, E.E.; Krewski, D.; Ito, K.; Thurston, G.D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 2002, 287, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Katanoda, K.; Sobue, T.; Satoh, H.; Tajima, K.; Suzuki, T.; Nakatsuka, H.; Takezaki, T.; Nakayama, T.; Nitta, H.; Tanabe, K.; et al. An association between long-term exposure to ambient air pollution and mortality from lung cancer and respiratory diseases in Japan. J. Epidemiol. 2011, 21, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Gharibvand, L.; Shavlik, D.; Ghamsary, M.; Beeson, W.L.; Soret, S.; Knutsen, R.; Knutsen, S.F. The Association between Ambient Fine Particulate Air Pollution and Lung Cancer Incidence: Results from the AHSMOG-2 Study. Environ. Health Perspect. 2017, 125, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Raaschou-Nielsen, O.; Andersen, Z.J.; Beelen, R.; Samoli, E.; Stafoggia, M.; Weinmayr, G.; Hoffmann, B.; Fischer, P.; Nieuwenhuijsen, M.J.; Brunekreef, B.; et al. Air pollution and lung cancer incidence in 17 European cohorts: Prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol. 2013, 14, 813–822. [Google Scholar] [CrossRef]
- Liu, X.; Mubarik, S.; Wang, F.; Yu, Y.; Wang, Y.; Shi, F.; Wen, H.; Yu, C. Lung Cancer Death Attributable to Long-Term Ambient Particulate Matter (PM(2.5)) Exposure in East Asian Countries During 1990–2019. Front. Med. 2021, 8, 742076. [Google Scholar] [CrossRef]
- Swanton, C.; Hill, W.; Lim, E.; Lee, C.; Weeden, C.; Augustine, M.; Chen, K.; Kuan, F.; Marongiu, F.; Rodrigues, F. LBA1 Mechanism of action and an actionable inflammatory axis for air pollution induced non-small cell lung cancer: Towards molecular cancer prevention. Ann. Oncol. 2022, 33, S1413. [Google Scholar] [CrossRef]
- Youogo, L.M.K.; Parent, M.E.; Hystad, P.; Villeneuve, P.J. Ambient air pollution and prostate cancer risk in a population-based Canadian case-control study. Environ. Epidemiol. 2022, 6, e219. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Arsenic, Metals, Fibres, and Dusts; IARC Monogr IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Lyon, France, 2012; Volume 100, pp. 11–465. [Google Scholar]
- Paris, C.; Thaon, I.; Herin, F.; Clin, B.; Lacourt, A.; Luc, A.; Coureau, G.; Brochard, P.; Chamming’s, S.; Gislard, A.; et al. Occupational Asbestos Exposure and Incidence of Colon and Rectal Cancers in French Men: The Asbestos-Related Diseases Cohort (ARDCo-Nut). Environ. Health Perspect. 2017, 125, 409–415. [Google Scholar] [CrossRef]
- Omenn, G.S.; Merchant, J.; Boatman, E.; Dement, J.M.; Kuschner, M.; Nicholson, W.; Peto, J.; Rosenstock, L. Contribution of environmental fibers to respiratory cancer. Environ. Health Perspect. 1986, 70, 51–56. [Google Scholar] [CrossRef]
- Klebe, S.; Leigh, J.; Henderson, D.W.; Nurminen, M. Asbestos, Smoking and Lung Cancer: An Update. Int. J. Environ. Res. Public Health 2019, 17, 258. [Google Scholar] [CrossRef] [PubMed]
- Kamp, D.W.; Weitzman, S.A. The molecular basis of asbestos induced lung injury. Thorax 1999, 54, 638–652. [Google Scholar] [CrossRef] [PubMed]
- Gulino, G.R.; Polimeni, M.; Prato, M.; Gazzano, E.; Kopecka, J.; Colombatto, S.; Ghigo, D.; Aldieri, E. Effects of Chrysotile Exposure in Human Bronchial Epithelial Cells: Insights into the Pathogenic Mechanisms of Asbestos-Related Diseases. Environ. Health Perspect. 2016, 124, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, S.B.; Levin, S.M.; Miller, A.; Morabia, A. Asbestos, asbestosis, smoking, and lung cancer. New findings from the North American insulator cohort. Am. J. Respir. Crit. Care Med. 2013, 188, 90–96. [Google Scholar] [CrossRef]
- Dutheil, F.; Zaragoza-Civale, L.; Pereira, B.; Mermillod, M.; Baker, J.S.; Schmidt, J.; Moustafa, F.; Navel, V. Prostate Cancer and Asbestos: A Systematic Review and Meta-Analysis. Perm. J. 2020, 24, 19.086. [Google Scholar] [CrossRef]
- Peng, R.; Fang, F.; Chen, Z.; Yang, S.; Dai, C.; Wang, C.; Guan, H.; Li, Q. Does exposure to asbestos cause prostate cancer? A systematic literature review and meta-analysis. Medicine 2019, 98, e14108. [Google Scholar] [CrossRef]
- Godono, A.; Clari, M.; Franco, N.; Ciocan, C.; Mansour, I.; Zunarelli, C.; Pira, E.; Boffetta, P. The association between occupational asbestos exposure with the risk of incidence and mortality from prostate cancer: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2022, 25, 604–614. [Google Scholar] [CrossRef]
- Zunarelli, C.; Godono, A.; Visci, G.; Violante, F.S.; Boffetta, P. Occupational exposure to asbestos and risk of kidney cancer: An updated meta-analysis. Eur. J. Epidemiol. 2021, 36, 927–936. [Google Scholar] [CrossRef]
- Sali, D.; Boffetta, P. Kidney cancer and occupational exposure to asbestos: A meta-analysis of occupational cohort studies. Cancer Causes Control 2000, 11, 37–47. [Google Scholar] [CrossRef]
- Kwak, K.; Kang, D.; Paek, D. Environmental exposure to asbestos and the risk of lung cancer: A systematic review and meta-analysis. Occup. Environ. Med. 2022, 79, 207–214. [Google Scholar] [CrossRef]
- Ju-Kun, S.; Yuan, D.B.; Rao, H.F.; Chen, T.F.; Luan, B.S.; Xu, X.M.; Jiang, F.N.; Zhong, W.D.; Zhu, J.G. Association between Cd Exposure and Risk of Prostate Cancer: A PRISMA-Compliant Systematic Review and Meta-Analysis. Medicine 2016, 95, e2708. [Google Scholar] [CrossRef] [PubMed]
- Rapisarda, V.; Miozzi, E.; Loreto, C.; Matera, S.; Fenga, C.; Avola, R.; Ledda, C. Cadmium exposure and prostate cancer: Insights, mechanisms and perspectives. Front. Biosci. 2018, 23, 1687–1700. [Google Scholar] [CrossRef]
- Zimta, A.A.; Schitcu, V.; Gurzau, E.; Stavaru, C.; Manda, G.; Szedlacsek, S.; Berindan-Neagoe, I. Biological and molecular modifications induced by cadmium and arsenic during breast and prostate cancer development. Environ. Res. 2019, 178, 108700. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xun, P.; Nishijo, M.; He, K. Cadmium exposure and risk of lung cancer: A meta-analysis of cohort and case-control studies among general and occupational populations. J. Expo. Sci. Environ. Epidemiol. 2016, 26, 437–444. [Google Scholar] [CrossRef]
- Nawrot, T.S.; Martens, D.S.; Hara, A.; Plusquin, M.; Vangronsveld, J.; Roels, H.A.; Staessen, J.A. Association of total cancer and lung cancer with environmental exposure to cadmium: The meta-analytical evidence. Cancer Causes Control 2015, 26, 1281–1288. [Google Scholar] [CrossRef]
- Nurchi, V.M.; Djordjevic, A.B.; Crisponi, G.; Alexander, J.; Bjorklund, G.; Aaseth, J. Arsenic Toxicity: Molecular Targets and Therapeutic Agents. Biomolecules 2020, 10, 235. [Google Scholar] [CrossRef]
- Martinez, V.D.; Buys, T.P.; Adonis, M.; Benitez, H.; Gallegos, I.; Lam, S.; Lam, W.L.; Gil, L. Arsenic-related DNA copy-number alterations in lung squamous cell carcinomas. Br. J. Cancer 2010, 103, 1277–1283. [Google Scholar] [CrossRef]
- Hubaux, R.; Becker-Santos, D.D.; Enfield, K.S.; Lam, S.; Lam, W.L.; Martinez, V.D. Arsenic, asbestos and radon: Emerging players in lung tumorigenesis. Environ. Health 2012, 11, 89. [Google Scholar] [CrossRef]
- Bustaffa, E.; Stoccoro, A.; Bianchi, F.; Migliore, L. Genotoxic and epigenetic mechanisms in arsenic carcinogenicity. Arch. Toxicol. 2014, 88, 1043–1067. [Google Scholar] [CrossRef]
- Soza-Ried, C.; Bustamante, E.; Caglevic, C.; Rolfo, C.; Sirera, R.; Marsiglia, H. Oncogenic role of arsenic exposure in lung cancer: A forgotten risk factor. Crit. Rev. Oncol. Hematol. 2019, 139, 128–133. [Google Scholar] [CrossRef]
- Yang, Y.; McDonald, A.C.; Wang, X.; Pan, Y.; Wang, M. Arsenic exposures and prostate cancer risk: A multilevel meta-analysis. J. Trace Elem. Med. Biol. 2022, 72, 126992. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Sun, R.; Hu, Y.; Wang, H.; Guo, Y.; Yang, B.; Pi, J.; Xu, Y. miRNA-182-5p, via HIF2alpha, contributes to arsenic carcinogenesis: Evidence from human renal epithelial cells. Metallomics 2018, 10, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.D.; Vucic, E.A.; Lam, S.; Lam, W.L. Arsenic and lung cancer in never-smokers: Lessons from Chile. Am. J. Respir. Crit. Care Med. 2012, 185, 1131–1132. [Google Scholar] [CrossRef] [PubMed]
- Podgorski, J.; Berg, M. Global threat of arsenic in groundwater. Science 2020, 368, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Baszuk, P.; Janasik, B.; Pietrzak, S.; Marciniak, W.; Reszka, E.; Bialkowska, K.; Jablonska, E.; Muszynska, M.; Lesicka, M.; Derkacz, R.; et al. Lung Cancer Occurrence-Correlation with Serum Chromium Levels and Genotypes. Biol. Trace Elem. Res. 2021, 199, 1228–1236. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, X.; Chen, H.; Li, Q.; Costa, M. Modulation of histone methylation and MLH1 gene silencing by hexavalent chromium. Toxicol. Appl. Pharmacol. 2009, 237, 258–266. [Google Scholar] [CrossRef]
- Kondo, K.; Takahashi, Y.; Hirose, Y.; Nagao, T.; Tsuyuguchi, M.; Hashimoto, M.; Ochiai, A.; Monden, Y.; Tangoku, A. The reduced expression and aberrant methylation of p16(INK4a) in chromate workers with lung cancer. Lung Cancer 2006, 53, 295–302. [Google Scholar] [CrossRef]
- Wise, S.S.; Holmes, A.L.; Liou, L.; Adam, R.M.; Wise, J.P., Sr. Hexavalent chromium induces chromosome instability in human urothelial cells. Toxicol. Appl. Pharmacol. 2016, 296, 54–60. [Google Scholar] [CrossRef]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Nickel and Nickel Compounds; IARC Monogr IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Lyon, France, 2012; Volume 100, pp. 169–218. [Google Scholar]
- Chen, K.; Liao, Q.L.; Ma, Z.W.; Jin, Y.; Hua, M.; Bi, J.; Huang, L. Association of soil arsenic and nickel exposure with cancer mortality rates, a town-scale ecological study in Suzhou, China. Environ. Sci. Pollut. Res. Int. 2015, 22, 5395–5404. [Google Scholar] [CrossRef]
- Pesch, B.; Kendzia, B.; Pohlabeln, H.; Ahrens, W.; Wichmann, H.E.; Siemiatycki, J.; Taeger, D.; Zschiesche, W.; Behrens, T.; Jockel, K.H.; et al. Exposure to Welding Fumes, Hexavalent Chromium, or Nickel and Risk of Lung Cancer. Am. J. Epidemiol. 2019, 188, 1984–1993. [Google Scholar] [CrossRef] [PubMed]
- Behrens, T.; Ge, C.; Vermeulen, R.; Kendzia, B.; Olsson, A.; Schuz, J.; Kromhout, H.; Pesch, B.; Peters, S.; Portengen, L.; et al. Occupational exposure to nickel and hexavalent chromium and the risk of lung cancer in a pooled analysis of case-control studies (SYNERGY). Int. J. Cancer 2023, 152, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, S.; Inoue, S.; Oikawa, S.; Yamashita, N.; Toyokuni, S.; Kawanishi, M.; Nishino, K. Oxidative DNA damage in cultured cells and rat lungs by carcinogenic nickel compounds. Free. Radic. Biol. Med. 2001, 31, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Ellen, T.P.; Kluz, T.; Harder, M.E.; Xiong, J.; Costa, M. Heterochromatinization as a Potential Mechanism of Nickel-Induced Carcinogenesis. Biochemistry 2009, 48, 4626–4632. [Google Scholar] [CrossRef] [PubMed]
- Seilkop, S.K.; Oller, A.R. Respiratory cancer risks associated with low-level nickel exposure: An integrated assessment based on animal, epidemiological, and mechanistic data. Regul. Toxicol. Pharmacol. 2003, 37, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Sciannameo, V.; Ricceri, F.; Soldati, S.; Scarnato, C.; Gerosa, A.; Giacomozzi, G.; d’Errico, A. Cancer mortality and exposure to nickel and chromium compounds in a cohort of Italian electroplaters. Am. J. Ind. Med. 2019, 62, 99–110. [Google Scholar] [CrossRef]
- Shannon, H.S.; Julian, J.A.; Roberts, R.S. A mortality study of 11,500 nickel workers. J. Natl. Cancer Inst. 1984, 73, 1251–1258. [Google Scholar]
- Michalek, I.M.; Martinsen, J.I.; Weiderpass, E.; Hansen, J.; Sparen, P.; Tryggvadottir, L.; Pukkala, E. Heavy metals, welding fumes, and other occupational exposures, and the risk of kidney cancer: A population-based nested case-control study in three Nordic countries. Environ. Res. 2019, 173, 117–123. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some Non-heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures; IARC Monogr IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Lyon, France, 2010; Volume 92, pp. 1–853. [Google Scholar]
- Olsson, A.; Guha, N.; Bouaoun, L.; Kromhout, H.; Peters, S.; Siemiatycki, J.; Ho, V.; Gustavsson, P.; Boffetta, P.; Vermeulen, R.; et al. Occupational Exposure to Polycyclic Aromatic Hydrocarbons and Lung Cancer Risk: Results from a Pooled Analysis of Case-Control Studies (SYNERGY). Cancer Epidemiol. Biomark. Prev. 2022, 31, 1433–1441. [Google Scholar] [CrossRef]
- Moubarz, G.; Saad-Hussein, A.; Shahy, E.M.; Mahdy-Abdallah, H.; Mohammed, A.M.F.; Saleh, I.A.; Abo-Zeid, M.A.M.; Abo-Elfadl, M.T. Lung cancer risk in workers occupationally exposed to polycyclic aromatic hydrocarbons with emphasis on the role of DNA repair gene. Int. Arch. Occup. Environ. Health 2023, 96, 313–329. [Google Scholar] [CrossRef]
- Petit, P.; Maitre, A.; Persoons, R.; Bicout, D.J. Lung cancer risk assessment for workers exposed to polycyclic aromatic hydrocarbons in various industries. Environ. Int. 2019, 124, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Gelboin, H.V. Benzo[alpha]pyrene metabolism, activation and carcinogenesis: Role and regulation of mixed-function oxidases and related enzymes. Physiol. Rev. 1980, 60, 1107–1166. [Google Scholar] [CrossRef] [PubMed]
- Meehan, T.; Straub, K.; Calvin, M. Benzo[alpha]pyrene diol epoxide covalently binds to deoxyguanosine and deoxyadenosine in DNA. Nature 1977, 269, 725–727. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, G.P.; Besaratinia, A. Mutational spectra of human cancer. Hum. Genet. 2009, 125, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Tsay, J.J.; Tchou-Wong, K.M.; Greenberg, A.K.; Pass, H.; Rom, W.N. Aryl hydrocarbon receptor and lung cancer. Anticancer. Res. 2013, 33, 1247–1256. [Google Scholar]
- Chappell, G.; Pogribny, I.P.; Guyton, K.Z.; Rusyn, I. Epigenetic alterations induced by genotoxic occupational and environmental human chemical carcinogens: A systematic literature review. Mutat. Res. Rev. Mutat. Res. 2016, 768, 27–45. [Google Scholar] [CrossRef]
- Rota, M.; Bosetti, C.; Boccia, S.; Boffetta, P.; La Vecchia, C. Occupational exposures to polycyclic aromatic hydrocarbons and respiratory and urinary tract cancers: An updated systematic review and a meta-analysis to 2014. Arch. Toxicol. 2014, 88, 1479–1490. [Google Scholar] [CrossRef]
- Barul, C.; Parent, M.E. Occupational exposure to polycyclic aromatic hydrocarbons and risk of prostate cancer. Environ. Health 2021, 20, 71. [Google Scholar] [CrossRef]
- Karami, S.; Lan, Q.; Rothman, N.; Stewart, P.A.; Lee, K.M.; Vermeulen, R.; Moore, L.E. Occupational trichloroethylene exposure and kidney cancer risk: A meta-analysis. Occup. Environ. Med. 2012, 69, 858–867. [Google Scholar] [CrossRef]
- Dumas, O.; Despreaux, T.; Perros, F.; Lau, E.; Andujar, P.; Humbert, M.; Montani, D.; Descatha, A. Respiratory effects of trichloroethylene. Respir. Med. 2018, 134, 47–53. [Google Scholar] [CrossRef]
- Silva, J.F.; Mattos, I.E.; Luz, L.L.; Carmo, C.N.; Aydos, R.D. Exposure to pesticides and prostate cancer: Systematic review of the literature. Rev. Environ. Health 2016, 31, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Lewis-Mikhael, A.M.; Bueno-Cavanillas, A.; Ofir Giron, T.; Olmedo-Requena, R.; Delgado-Rodriguez, M.; Jimenez-Moleon, J.J. Occupational exposure to pesticides and prostate cancer: A systematic review and meta-analysis. Occup. Environ. Med. 2016, 73, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Andreotti, G.; Beane Freeman, L.E.; Shearer, J.J.; Lerro, C.C.; Koutros, S.; Parks, C.G.; Blair, A.; Lynch, C.F.; Lubin, J.H.; Sandler, D.P.; et al. Occupational Pesticide Use and Risk of Renal Cell Carcinoma in the Agricultural Health Study. Environ. Health Perspect. 2020, 128, 67011. [Google Scholar] [CrossRef]
- Karami, S.; Boffetta, P.; Rothman, N.; Hung, R.J.; Stewart, T.; Zaridze, D.; Navritalova, M.; Mates, D.; Janout, V.; Kollarova, H.; et al. Renal cell carcinoma, occupational pesticide exposure and modification by glutathione S-transferase polymorphisms. Carcinogenesis 2008, 29, 1567–1571. [Google Scholar] [CrossRef]
- Mink, P.J.; Adami, H.O.; Trichopoulos, D.; Britton, N.L.; Mandel, J.S. Pesticides and prostate cancer: A review of epidemiologic studies with specific agricultural exposure information. Eur. J. Cancer Prev. 2008, 17, 97–110. [Google Scholar] [CrossRef]
- Bonner, M.R.; Freeman, L.E.; Hoppin, J.A.; Koutros, S.; Sandler, D.P.; Lynch, C.F.; Hines, C.J.; Thomas, K.; Blair, A.; Alavanja, M.C. Occupational Exposure to Pesticides and the Incidence of Lung Cancer in the Agricultural Health Study. Environ. Health Perspect. 2017, 125, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Silver, S.R.; Bertke, S.J.; Hines, C.J.; Alavanja, M.C.; Hoppin, J.A.; Lubin, J.H.; Rusiecki, J.A.; Sandler, D.P.; Beane Freeman, L.E. Cancer incidence and metolachlor use in the Agricultural Health Study: An update. Int. J. Cancer 2015, 137, 2630–2643. [Google Scholar] [CrossRef]
- Kim, B.; Park, E.Y.; Kim, J.; Park, E.; Oh, J.K.; Lim, M.K. Occupational Exposure to Pesticides and Lung Cancer Risk: A Propensity Score Analyses. Cancer Res. Treat. 2022, 54, 130–139. [Google Scholar] [CrossRef]
- Olsson, A.C.; Gustavsson, P.; Kromhout, H.; Peters, S.; Vermeulen, R.; Bruske, I.; Pesch, B.; Siemiatycki, J.; Pintos, J.; Bruning, T.; et al. Exposure to diesel motor exhaust and lung cancer risk in a pooled analysis from case-control studies in Europe and Canada. Am. J. Respir. Crit. Care Med. 2011, 183, 941–948. [Google Scholar] [CrossRef]
- Cosyns, J.P. Aristolochic acid and ‘Chinese herbs nephropathy’: A review of the evidence to date. Drug Saf. 2003, 26, 33–48. [Google Scholar] [CrossRef]
- Rosenquist, T.A.; Grollman, A.P. Mutational signature of aristolochic acid: Clue to the recognition of a global disease. DNA Repair 2016, 44, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Jelakovic, B.; Karanovic, S.; Vukovic-Lela, I.; Miller, F.; Edwards, K.L.; Nikolic, J.; Tomic, K.; Slade, N.; Brdar, B.; Turesky, R.J.; et al. Aristolactam-DNA adducts are a biomarker of environmental exposure to aristolochic acid. Kidney Int. 2012, 81, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.B.; Cardis, E.; Daniels, R.D.; Gillies, M.; Haylock, R.; Leuraud, K.; Laurier, D.; Moissonnier, M.; Schubauer-Berigan, M.K.; Thierry-Chef, I.; et al. Site-specific Solid Cancer Mortality after Exposure to Ionizing Radiation: A Cohort Study of Workers (INWORKS). Epidemiology 2018, 29, 31–40. [Google Scholar] [CrossRef]
- Richardson, D.B.; Cardis, E.; Daniels, R.D.; Gillies, M.; O’Hagan, J.A.; Hamra, G.B.; Haylock, R.; Laurier, D.; Leuraud, K.; Moissonnier, M.; et al. Risk of cancer from occupational exposure to ionising radiation: Retrospective cohort study of workers in France, the United Kingdom, and the United States (INWORKS). BMJ 2015, 351, h5359. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, E.L.; Jacobson, J.S.; Hershman, D.L.; Desai, M.; Neugut, A.I. Effect of breast cancer radiotherapy and cigarette smoking on risk of second primary lung cancer. J. Clin. Oncol. 2008, 26, 392–398. [Google Scholar] [CrossRef] [PubMed]
- The National Lung Screening Trial Research Team; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef] [PubMed]
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef]
- Humphrey, L.L.; Deffebach, M.; Pappas, M.; Baumann, C.; Artis, K.; Mitchell, J.P.; Zakher, B.; Fu, R.; Slatore, C.G. Screening for lung cancer with low-dose computed tomography: A systematic review to update the US Preventive services task force recommendation. Ann. Intern. Med. 2013, 159, 411–420. [Google Scholar] [CrossRef]
- Printz, C. US Preventive Services Task Force issues new draft recommendation statement regarding lung cancer screening. Cancer 2020, 126, 4269. [Google Scholar] [CrossRef]
- Veronesi, G.; Baldwin, D.R.; Henschke, C.I.; Ghislandi, S.; Iavicoli, S.; Oudkerk, M.; De Koning, H.J.; Shemesh, J.; Field, J.K.; Zulueta, J.J.; et al. Recommendations for Implementing Lung Cancer Screening with Low-Dose Computed Tomography in Europe. Cancers 2020, 12, 1672. [Google Scholar] [CrossRef]
- Ten Haaf, K.; van der Aalst, C.M.; de Koning, H.J.; Kaaks, R.; Tammemagi, M.C. Personalising lung cancer screening: An overview of risk-stratification opportunities and challenges. Int. J. Cancer 2021, 149, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Potter, A.L.; Rosenstein, A.L.; Kiang, M.V.; Shah, S.A.; Gaissert, H.A.; Chang, D.C.; Fintelmann, F.J.; Yang, C.J. Association of computed tomography screening with lung cancer stage shift and survival in the United States: Quasi-experimental study. BMJ 2022, 376, e069008. [Google Scholar] [CrossRef] [PubMed]
- Passiglia, F.; Cinquini, M.; Bertolaccini, L.; Del Re, M.; Facchinetti, F.; Ferrara, R.; Franchina, T.; Larici, A.R.; Malapelle, U.; Menis, J.; et al. Benefits and Harms of Lung Cancer Screening by Chest Computed Tomography: A Systematic Review and Meta-Analysis. J. Clin. Oncol. 2021, 39, 2574–2585. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Q.; Yuan, S.; Xie, W.; Liu, Y.; Xiang, Y.; Wu, N.; Wu, L.; Ma, X.; Cai, T.; et al. Genetic predisposition to lung cancer: Comprehensive literature integration, meta-analysis, and multiple evidence assessment of candidate-gene association studies. Sci. Rep. 2017, 7, 8371. [Google Scholar] [CrossRef]
- Brown, K.F.; Rumgay, H.; Dunlop, C.; Ryan, M.; Quartly, F.; Cox, A.; Deas, A.; Elliss-Brookes, L.; Gavin, A.; Hounsome, L.; et al. The fraction of cancer attributable to modifiable risk factors in England, Wales, Scotland, Northern Ireland, and the United Kingdom in 2015. Br. J. Cancer 2018, 118, 1130–1141. [Google Scholar] [CrossRef]
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Aluru, J.S.; Barsouk, A. Epidemiology of lung cancer. Contemp. Oncol. 2021, 25, 45–52. [Google Scholar] [CrossRef]
- World Health Organization. WHO Report on the Global Tobacco Epidemic, 2021: Addressing New and Emerging Products; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Franklin, P.; Gladfelter, A.; Meek, M.; Steliga, M. P1. 01-019 Integration of Tobacco Cessation Counseling in a Lung Screening Program: Topic: Protective Factors, Risk Reduction, Smoking Cessation. J. Thorac. Oncol. 2017, 12, S459–S460. [Google Scholar] [CrossRef]
- Bade, M.; Bahr, V.; Brandt, U.; Eigentopf, A.; Bruchert, T.; Gross, M.L.; Motsch, E.; Becker, N. Effect of smoking cessation counseling within a randomised study on early detection of lung cancer in Germany. J. Cancer Res. Clin. Oncol. 2016, 142, 959–968. [Google Scholar] [CrossRef]
- Pozzi, P.; Munarini, E.; Bravi, F.; Rossi, M.; La Vecchia, C.; Boffi, R.; Pastorino, U. A combined smoking cessation intervention within a lung cancer screening trial: A pilot observational study. Tumori 2015, 101, 306–311. [Google Scholar] [CrossRef]
- Pastorino, U.; Ladisa, V.; Trussardo, S.; Sabia, F.; Rolli, L.; Valsecchi, C.; Ledda, R.E.; Milanese, G.; Suatoni, P.; Boeri, M.; et al. Cytisine Therapy Improved Smoking Cessation in the Randomized Screening and Multiple Intervention on Lung Epidemics Lung Cancer Screening Trial. J. Thorac. Oncol. 2022, 17, 1276–1286. [Google Scholar] [CrossRef]
- Lancaster, T.; Stead, L.F. Individual behavioural counselling for smoking cessation. Cochrane Database Syst. Rev. 2017, 3, CD001292. [Google Scholar] [CrossRef] [PubMed]
| Group | Meaning | Examples |
|---|---|---|
| GROUP 1 Carcinogenic to humans | Enough evidence for a proven association with human cancer. | Tobacco smoking, outdoor air pollution, alcoholic beverages, asbestos, arsenic, benzene, formaldehyde, engine exhaust and diesel, ionizing radiation, coal as indoor emissions from household, nickel compounds, welding fumes, chromium-VI compounds, aristolochic acid, cadmium, trichloroethylene, radon, aluminum, iron and steel founding, mineral oils, soot, wood dust |
| GROUP 2A Probably carcinogenic to humans | Limited evidence for an association with human cancer. Sufficient data of cancer in experimental animals. | High temperature frying, red meat, petroleum refining (only occupational exposure), hairdresser or barber (aromatic amines) as occupational exposure, glyphosate, N-nitrosodiethylamine (NDMA), 4,4’-dichlorodiphenyltrichloroethane (DDT) |
| GROUP 2B Possibly carcinogenic to humans | Limited data for an association with human cancer, but insufficient evidence of cancer in experimental animals. | Dry cleaning (occupational exposure), magnetic fields (extremely low frequency), styrene, coffee and pickled vegetables |
| GROUP 3 Not classifiable as human carcinogens | Evidence is inadequate in humans and inadequate or limited in animals. | Acrylic acid, chlorinated drinking water, electric field, fluorescent lighting, hair coloring products (personal use) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cani, M.; Turco, F.; Butticè, S.; Vogl, U.M.; Buttigliero, C.; Novello, S.; Capelletto, E. How Does Environmental and Occupational Exposure Contribute to Carcinogenesis in Genitourinary and Lung Cancers? Cancers 2023, 15, 2836. https://doi.org/10.3390/cancers15102836
Cani M, Turco F, Butticè S, Vogl UM, Buttigliero C, Novello S, Capelletto E. How Does Environmental and Occupational Exposure Contribute to Carcinogenesis in Genitourinary and Lung Cancers? Cancers. 2023; 15(10):2836. https://doi.org/10.3390/cancers15102836
Chicago/Turabian StyleCani, Massimiliano, Fabio Turco, Simona Butticè, Ursula Maria Vogl, Consuelo Buttigliero, Silvia Novello, and Enrica Capelletto. 2023. "How Does Environmental and Occupational Exposure Contribute to Carcinogenesis in Genitourinary and Lung Cancers?" Cancers 15, no. 10: 2836. https://doi.org/10.3390/cancers15102836






