Age- and Sex-Matched Normal Leukocyte Subset Ranges in the General Population Defined with the EuroFlow Lymphocyte Screening Tube (LST) for Monoclonal B-Cell Lymphocytosis (MBL) vs. Non-MBL Subjects
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Donors and Samples
2.2. Flow Cytometry Immunophenotypic Studies
2.3. Statistical Analyses
3. Results
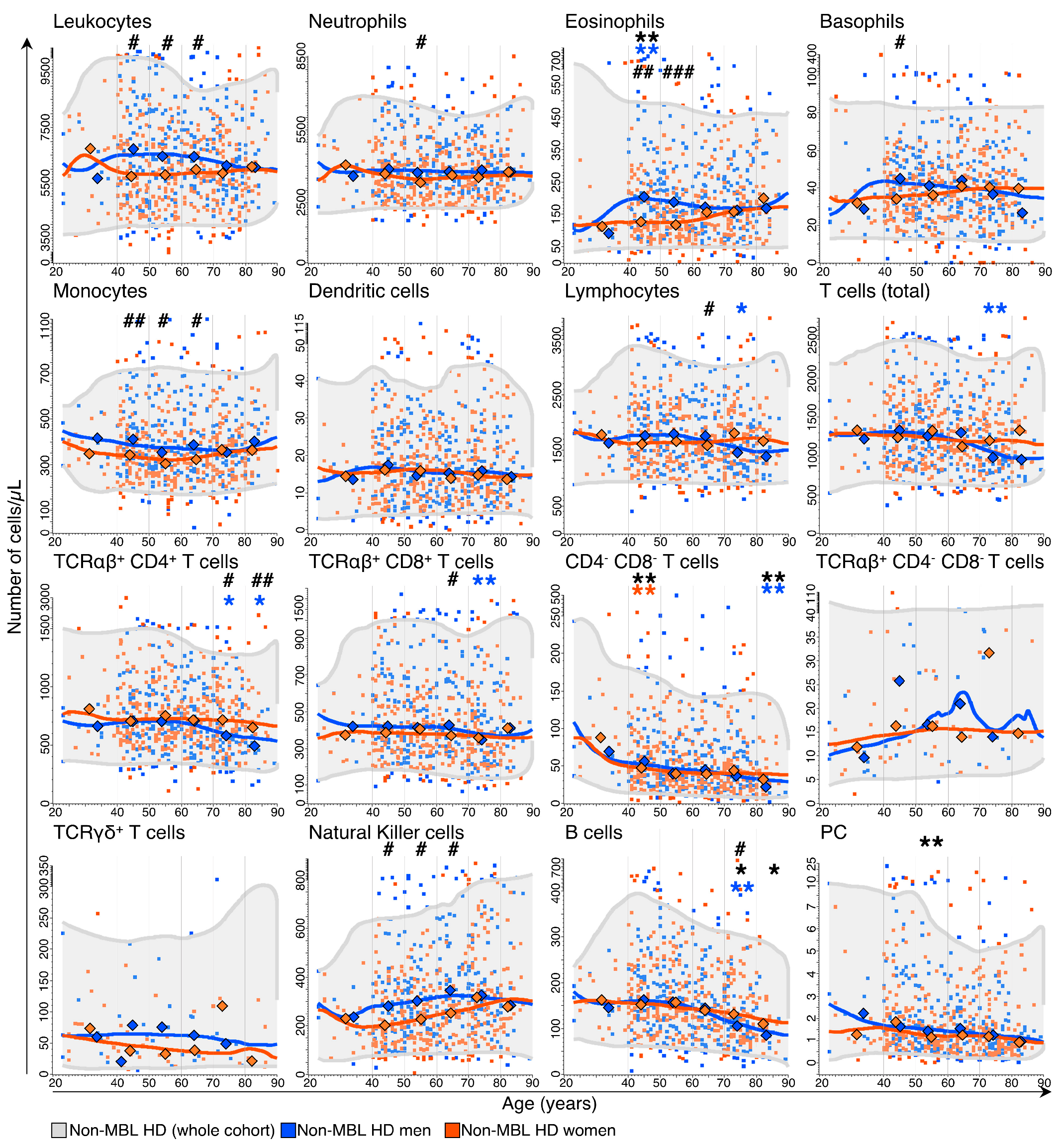
3.1. Kinetics of the Major Blood Leukocyte Populations in Non-MBL Healthy Donors (HD) through Adulthood, According to Age and Sex
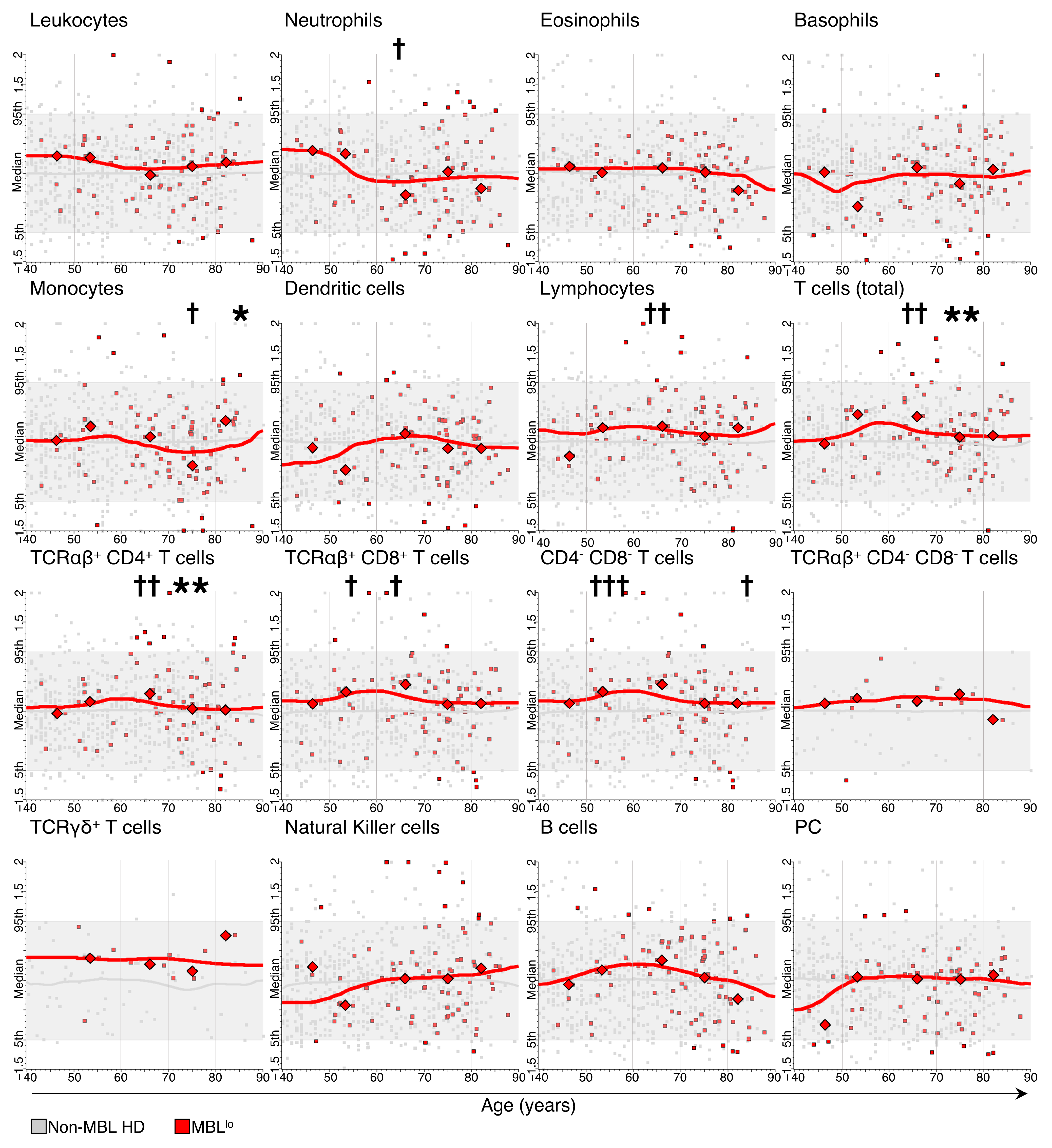
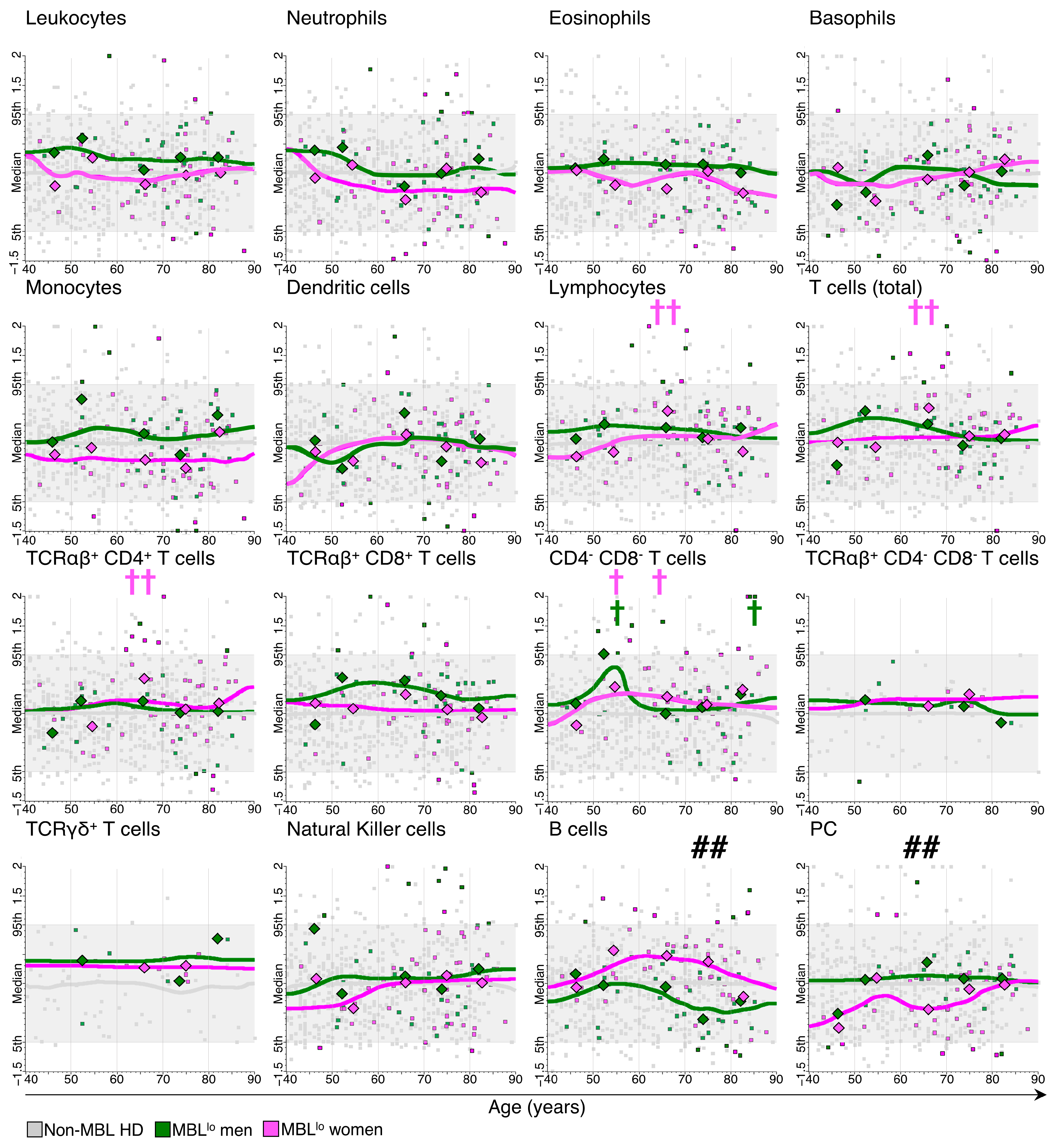
3.2. Kinetics of the Major Blood Leukocyte Populations in MBLlo Subjects through Adulthood According to Age and Sex
3.3. Distribution of Major Leukocyte Populations in MBLlo vs. Non-MBL Subjects through Adulthood
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perez-Andres, M.; Paiva, B.; Nieto, W.G.; Caraux, A.; Schmitz, A.; Almeida, J.; Vogt, R.F.; Marti, G.E.; Rawstron, A.C.; Van Zelm, M.C.; et al. Human Peripheral Blood B-Cell Compartments: A Crossroad in B-Cell Traffic. Cytom. B Clin. Cytom. 2010, 78 (Suppl. 1), S47–S60. [Google Scholar] [CrossRef] [PubMed]
- Cossarizza, A.; Chang, H.-D.; Radbruch, A.; Acs, A.; Adam, D.; Adam-Klages, S.; Agace, W.W.; Aghaeepour, N.; Akdis, M.; Allez, M.; et al. Guidelines for the Use of Flow Cytometry and Cell Sorting in Immunological Studies (Second Edition). Eur. J. Immunol. 2019, 49, 1457–1973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jentsch-Ullrich, K.; Koenigsmann, M.; Mohren, M.; Franke, A. Lymphocyte Subsets’ Reference Ranges in an Age- and Gender-Balanced Population of 100 Healthy Adults—A Monocentric German Study. Clin. Immunol. 2005, 116, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lee, S.J.; Lee, Y.A.; Maeng, H.G.; Lee, J.K.; Kang, Y.W. Reference Values for Peripheral Blood Lymphocyte Subsets in a Healthy Korean Population. Immune Netw. 2014, 14, 289–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valiathan, R.; Deeb, K.; Diamante, M.; Ashman, M.; Sachdeva, N.; Asthana, D. Reference Ranges of Lymphocyte Subsets in Healthy Adults and Adolescents with Special Mention of T Cell Maturation Subsets in Adults of South Florida. Immunobiology 2014, 219, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Melzer, S.; Zachariae, S.; Bocsi, J.; Engel, C.; Löffler, M.; Tárnok, A. Reference Intervals for Leukocyte Subsets in Adults: Results from a Population-Based Study Using 10-Color Flow Cytometry. Cytom. Part B Clin. Cytom. 2015, 88, 270–281. [Google Scholar] [CrossRef]
- Kverneland, A.H.; Streitz, M.; Geissler, E.; Hutchinson, J.; Vogt, K.; Boës, D.; Niemann, N.; Pedersen, A.E.; Schlickeiser, S.; Sawitzki, B. Age and Gender Leucocytes Variances and References Values Generated Using the Standardized ONE-Study Protocol. Cytom. A 2016, 89, 543–564. [Google Scholar] [CrossRef] [Green Version]
- Blanco, E.; Pérez-Andrés, M.; Arriba-Méndez, S.; Contreras-Sanfeliciano, T.; Criado, I.; Pelak, O.; Serra-Caetano, A.; Romero, A.; Puig, N.; Remesal, A.; et al. Age-Associated Distribution of Normal B-Cell and Plasma Cell Subsets in Peripheral Blood. J. Allergy Clin. Immunol. 2018, 141, 2208–2219. [Google Scholar] [CrossRef] [Green Version]
- Damasceno, D.; Teodosio, C.; van den Bossche, W.B.L.; Perez-Andres, M.; Arriba-Méndez, S.; Muñoz-Bellvis, L.; Romero, A.; Blanco, J.F.; Remesal, A.; Puig, N.; et al. Distribution of Subsets of Blood Monocytic Cells throughout Life. J. Allergy Clin. Immunol. 2019, 144, 320–323.e6. [Google Scholar] [CrossRef] [Green Version]
- Louati, N.; Rekik, T.; Menif, H.; Gargouri, J. Blood Lymphocyte T Subsets Reference Values in Blood Donors by Flow Cytometry. Tunis Med. 2019, 97, 327–334. [Google Scholar]
- Magierowicz, M.; Lechevalier, N.; Freynet, N.; Pastoret, C.; Badaoui, B.; Ly-Sunnaram, B.; Fest, T.; Lacombe, F.; Wagner-Ballon, O.; Roussel, M. Reference Values for WBC Differential by Hematoflow Analysis. Am. J. Clin. Pathol. 2019, 151, 324–327. [Google Scholar] [CrossRef] [PubMed]
- van Dongen, J.J.M.; van der Burg, M.; Kalina, T.; Perez-Andres, M.; Mejstrikova, E.; Vlkova, M.; Lopez-Granados, E.; Wentink, M.; Kienzler, A.-K.; Philippé, J.; et al. EuroFlow-Based Flowcytometric Diagnostic Screening and Classification of Primary Immunodeficiencies of the Lymphoid System. Front. Immunol. 2019, 10, 1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botafogo, V.; Pérez-Andres, M.; Jara-Acevedo, M.; Bárcena, P.; Grigore, G.; Hernández-Delgado, A.; Damasceno, D.; Comans, S.; Blanco, E.; Romero, A.; et al. Age Distribution of Multiple Functionally Relevant Subsets of CD4+ T Cells in Human Blood Using a Standardized and Validated 14-Color EuroFlow Immune Monitoring Tube. Front. Immunol. 2020, 11, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawstron, A.C.; Green, M.J.; Kuzmicki, A.; Kennedy, B.; Fenton, J.A.L.; Evans, P.A.S.; O’Connor, S.J.M.; Richards, S.J.; Morgan, G.J.; Jack, A.S.; et al. Monoclonal B Lymphocytes with the Characteristics of “Indolent” Chronic Lymphocytic Leukemia Are Present in 3.5% of Adults with Normal Blood Counts. Blood 2002, 100, 635–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghia, P.; Prato, G.; Scielzo, C.; Stella, S.; Geuna, M.; Guida, G.; Caligaris-Cappio, F. Monoclonal CD5+ and CD5− B-Lymphocyte Expansions Are Frequent in the Peripheral Blood of the Elderly. Blood 2004, 103, 2337–2342. [Google Scholar] [CrossRef] [Green Version]
- Nieto, W.G.; Almeida, J.; Romero, A.; Teodosio, C.; López, A.; Henriques, A.F.; Sánchez, M.L.; Jara-Acevedo, M.; Rasillo, A.; González, M.; et al. Increased Frequency (12%) of Circulating Chronic Lymphocytic Leukemia-like B-Cell Clones in Healthy Subjects Using a Highly Sensitive Multicolor Flow Cytometry Approach. Blood 2009, 114, 33–37. [Google Scholar] [CrossRef]
- Dagklis, A.; Fazi, C.; Sala, C.; Cantarelli, V.; Scielzo, C.; Massacane, R.; Toniolo, D.; Caligaris-Cappio, F.; Stamatopoulos, K.; Ghia, P. The Immunoglobulin Gene Repertoire of Low-Count Chronic Lymphocytic Leukemia (CLL)-like Monoclonal B Lymphocytosis Is Different from CLL: Diagnostic Implications for Clinical Monitoring. Blood 2009, 114, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Shim, Y.K.; Rachel, J.M.; Ghia, P.; Boren, J.; Abbasi, F.; Dagklis, A.; Venable, G.; Kang, J.; Degheidy, H.; Plapp, F.V.; et al. Monoclonal B-Cell Lymphocytosis in Healthy Blood Donors: An Unexpectedly Common Finding. Blood 2014, 123, 1319–1326. [Google Scholar] [CrossRef] [Green Version]
- Rawstron, A.C.; Ssemaganda, A.; de Tute, R.; Doughty, C.; Newton, D.; Vardi, A.; Evans, P.A.S.; Stamatopoulos, K.; Owen, R.G.; Lightfoot, T.; et al. Monoclonal B-Cell Lymphocytosis in a Hospital-Based UK Population and a Rural Ugandan Population: A Cross-Sectional Study. Lancet Haematol. 2017, 4, e334–e340. [Google Scholar] [CrossRef] [Green Version]
- de Faria-Moss, M.; Yamamoto, M.; Arrais-Rodrigues, C.; Criado, I.; Gomes, C.P.; de Lourdes Chauffaille, M.; Gonçalves, M.V.; Kimura, E.; Koulieris, E.; Borges, F.; et al. High Frequency of Chronic Lymphocytic Leukemia-like Low-Count Monoclonal B-Cell Lymphocytosis in Japanese Descendants Living in Brazil. Haematologica 2020, 105, e298–e301. [Google Scholar] [CrossRef]
- Slager, S.L.; Parikh, S.A.; Achenbach, S.J.; Norman, A.D.; Rabe, K.G.; Boddicker, N.J.; Olson, J.E.; Kleinstern, G.; Lesnick, C.; Call, T.G.; et al. Progression and Survival of Monoclonal B-Cell Lymphocytosis (MBL): A Screening Study of 10,139 Individuals. Blood 2022, 140, 1702–1709. [Google Scholar] [CrossRef] [PubMed]
- Hauswirth, A.W.; Almeida, J.; Nieto, W.G.; Teodosio, C.; Rodriguez-Caballero, A.; Romero, A.; López, A.; Fernandez-Navarro, P.; Vega, T.; Perez-Andres, M.; et al. Monoclonal B-Cell Lymphocytosis (MBL) with Normal Lymphocyte Counts Is Associated with Decreased Numbers of Normal Circulating B-Cell Subsets. Am. J. Hematol. 2012, 87, 721–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Criado, I.; Blanco, E.; Rodríguez-Caballero, A.; Alcoceba, M.; Contreras, T.; Gutiérrez, M.L.; Romero, A.; Fernández-Navarro, P.; González, M.; Solano, F.; et al. Residual Normal B-Cell Profiles in Monoclonal B-Cell Lymphocytosis versus Chronic Lymphocytic Leukemia. Leukemia 2018, 32, 2701–2705. [Google Scholar] [CrossRef] [PubMed]
- Marti, G.E.; Rawstron, A.C.; Ghia, P.; Hillmen, P.; Houlston, R.S.; Kay, N.; Schleinitz, T.A.; Caporaso, N.; Consortium, T.I.F.C. Diagnostic Criteria for Monoclonal B-Cell Lymphocytosis. Br. J. Haematol. 2005, 130, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; de Oliveira Araujo, I.B.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th Edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef] [PubMed]
- Rawstron, A.C.; Shanafelt, T.; Lanasa, M.C.; Landgren, O.; Hanson, C.; Orfao, A.; Hillmen, P.; Ghia, P. Different Biology and Clinical Outcome According to the Absolute Numbers of Clonal B-Cells in Monoclonal B-Cell Lymphocytosis (MBL). Cytom. B Clin. Cytom. 2010, 78 (Suppl. 1), S19–S23. [Google Scholar] [CrossRef]
- Criado, I.; Rodríguez-Caballero, A.; Gutiérrez, M.L.; Pedreira, C.E.; Alcoceba, M.; Nieto, W.; Teodosio, C.; Bárcena, P.; Romero, A.; Fernández-Navarro, P.; et al. Low-Count Monoclonal B-Cell Lymphocytosis Persists after Seven Years of Follow up and Is Associated with a Poorer Outcome. Haematologica 2018, 103, 1198–1208. [Google Scholar] [CrossRef] [Green Version]
- van Dongen, J.J.M.; Lhermitte, L.; Böttcher, S.; Almeida, J.; van der Velden, V.H.J.; Flores-Montero, J.; Rawstron, A.; Asnafi, V.; Lécrevisse, Q.; Lucio, P.; et al. EuroFlow Antibody Panels for Standardized N-Dimensional Flow Cytometric Immunophenotyping of Normal, Reactive and Malignant Leukocytes. Leukemia 2012, 26, 1908–1975. [Google Scholar] [CrossRef] [Green Version]
- Flores-Montero, J.; Sanoja-Flores, L.; Paiva, B.; Puig, N.; García-Sánchez, O.; Böttcher, S.; van der Velden, V.H.J.; Pérez-Morán, J.-J.; Vidriales, M.-B.; García-Sanz, R.; et al. Next Generation Flow for Highly Sensitive and Standardized Detection of Minimal Residual Disease in Multiple Myeloma. Leukemia 2017, 31, 2094–2103. [Google Scholar] [CrossRef] [Green Version]
- Flores-Montero, J.; Grigore, G.; Fluxá, R.; Hernández, J.; Fernandez, P.; Almeida, J.; Muñoz, N.; Böttcher, S.; Sedek, L.; van der Velden, V.; et al. EuroFlow Lymphoid Screening Tube (LST) Data Base for Automated Identification of Blood Lymphocyte Subsets. J. Immunol. Methods 2019, 475, 112662. [Google Scholar] [CrossRef]
- Hultin, L.E.; Chow, M.; Jamieson, B.D.; O’Gorman, M.R.G.; Menendez, F.A.; Borowski, L.; Denny, T.N.; Margolick, J.B. Comparison of Interlaboratory Variation in Absolute T-Cell Counts by Single-Platform and Optimized Dual-Platform Methods. Cytom. B Clin. Cytom. 2010, 78, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 Revision of the World Health Organization Classification of Lymphoid Neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [Green Version]
- van der Velden, V.H.J.; Flores-Montero, J.; Perez-Andres, M.; Martin-Ayuso, M.; Crespo, O.; Blanco, E.; Kalina, T.; Philippé, J.; Bonroy, C.; de Bie, M.; et al. Optimization and Testing of Dried Antibody Tube: The EuroFlow LST and PIDOT Tubes as Examples. J. Immunol. Methods 2019, 475, 112287. [Google Scholar] [CrossRef]
- Center for Disease Control and prevention (CDC) Total White Blood Cell Counts for Persons Ages 1–74 Years with Differential Leukocyte Counts for Adults Ages 25–74 Years: United States, 1971–75. Available online: https://www.cdc.gov/nchs/data/series/sr_11/sr11_220.pdf (accessed on 25 February 2022).
- Chen, Y.; Zhang, Y.; Zhao, G.; Chen, C.; Yang, P.; Ye, S.; Tan, X. Difference in Leukocyte Composition between Women before and after Menopausal Age, and Distinct Sexual Dimorphism. PLoS ONE 2016, 11, e0162953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nah, E.-H.; Kim, S.; Cho, S.; Cho, H.-I. Complete Blood Count Reference Intervals and Patterns of Changes Across Pediatric, Adult, and Geriatric Ages in Korea. Ann. Lab. Med. 2018, 38, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Hartl, S.; Breyer, M.-K.; Burghuber, O.C.; Ofenheimer, A.; Schrott, A.; Urban, M.H.; Agusti, A.; Studnicka, M.; Wouters, E.F.M.; Breyer-Kohansal, R. Blood Eosinophil Count in the General Population: Typical Values and Potential Confounders. Eur. Respir. J. 2020, 55, 1901874. [Google Scholar] [CrossRef] [PubMed]
- Andreu-Ballester, J.C.; García-Ballesteros, C.; Benet-Campos, C.; Amigó, V.; Almela-Quilis, A.; Mayans, J.; Ballester, F. Values for Aβ and Γδ T-Lymphocytes and CD4+, CD8+, and CD56+ Subsets in Healthy Adult Subjects: Assessment by Age and Gender. Cytom. B Clin. Cytom. 2012, 82, 238–244. [Google Scholar] [CrossRef]
- Conte, M.; Martucci, M.; Chiariello, A.; Franceschi, C.; Salvioli, S. Mitochondria, Immunosenescence and Inflammaging: A Role for Mitokines? Semin. Immunopathol. 2020, 42, 607–617. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, C.; Han, Y.; Gu, Z.; Sun, C. Immunosenescence, Aging and Successful Aging. Front. Immunol. 2022, 13, 942796. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex Differences in Immune Responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Whitacre, C.C. Sex Differences in Autoimmune Disease. Nat. Immunol. 2001, 2, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.J.; Bugiardini, R.; Merz, C.N.B. Women and Ischemic Heart Disease: Evolving Knowledge. J. Am. Coll Cardiol. 2009, 54, 1561–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.; Zheng, Y.; Sheng, J.; Han, Y.; Yang, Y.; Pan, H.; Yao, J. CD3+CD4−CD8− (Double-Negative) T Cells in Inflammation, Immune Disorders and Cancer. Front. Immunol. 2022, 13, 816005. [Google Scholar] [CrossRef]
- Zaborsky, N.; Gassner, F.J.; Asslaber, D.; Reinthaler, P.; Denk, U.; Flenady, S.; Hofbauer, J.P.; Danner, B.; Rebhandl, S.; Harrer, A.; et al. CD1d Expression on Chronic Lymphocytic Leukemia B Cells Affects Disease Progression and Induces T Cell Skewing in CD8 Positive and CD4CD8 Double Negative T Cells. Oncotarget 2016, 7, 49459–49469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, M.; Liu, H.; Malafa, M.; Centeno, B.; Hodul, P.J.; Pimiento, J.; Pilon-Thomas, S.; Sarnaik, A.A. Expansion of Tumor-Infiltrating Lymphocytes (TIL) from Human Pancreatic Tumors. J. Immunother Cancer 2016, 4, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, L.; Ly, D.; Wang, S.; Lee, J.B.; Kang, H.; Xu, H.; Yao, J.; Tsao, M.; Liu, W.; Zhang, L. Targeting Late-Stage Non-Small Cell Lung Cancer with a Combination of DNT Cellular Therapy and PD-1 Checkpoint Blockade. J. Exp. Clin. Cancer Res. 2019, 38, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenplate, A.R.; McClanahan, D.D.; Oberholtzer, B.K.; Doxie, D.B.; Roe, C.E.; Diggins, K.E.; Leelatian, N.; Rasmussen, M.L.; Kelley, M.C.; Gama, V.; et al. Computational Immune Monitoring Reveals Abnormal Double-Negative T Cells Present across Human Tumor Types. Cancer Immunol. Res. 2019, 7, 86–99. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Criado, I.; Nieto, W.G.; Oliva-Ariza, G.; Fuentes-Herrero, B.; Teodosio, C.; Lecrevisse, Q.; Lopez, A.; Romero, A.; Almeida, J.; Orfao, A.; et al. Age- and Sex-Matched Normal Leukocyte Subset Ranges in the General Population Defined with the EuroFlow Lymphocyte Screening Tube (LST) for Monoclonal B-Cell Lymphocytosis (MBL) vs. Non-MBL Subjects. Cancers 2023, 15, 58. https://doi.org/10.3390/cancers15010058
Criado I, Nieto WG, Oliva-Ariza G, Fuentes-Herrero B, Teodosio C, Lecrevisse Q, Lopez A, Romero A, Almeida J, Orfao A, et al. Age- and Sex-Matched Normal Leukocyte Subset Ranges in the General Population Defined with the EuroFlow Lymphocyte Screening Tube (LST) for Monoclonal B-Cell Lymphocytosis (MBL) vs. Non-MBL Subjects. Cancers. 2023; 15(1):58. https://doi.org/10.3390/cancers15010058
Chicago/Turabian StyleCriado, Ignacio, Wendy G. Nieto, Guillermo Oliva-Ariza, Blanca Fuentes-Herrero, Cristina Teodosio, Quentin Lecrevisse, Antonio Lopez, Alfonso Romero, Julia Almeida, Alberto Orfao, and et al. 2023. "Age- and Sex-Matched Normal Leukocyte Subset Ranges in the General Population Defined with the EuroFlow Lymphocyte Screening Tube (LST) for Monoclonal B-Cell Lymphocytosis (MBL) vs. Non-MBL Subjects" Cancers 15, no. 1: 58. https://doi.org/10.3390/cancers15010058




