The E3 Ubiquitin Ligase Fbxo4 Functions as a Tumor Suppressor: Its Biological Importance and Therapeutic Perspectives
Abstract
:Simple Summary
Abstract
1. Introduction
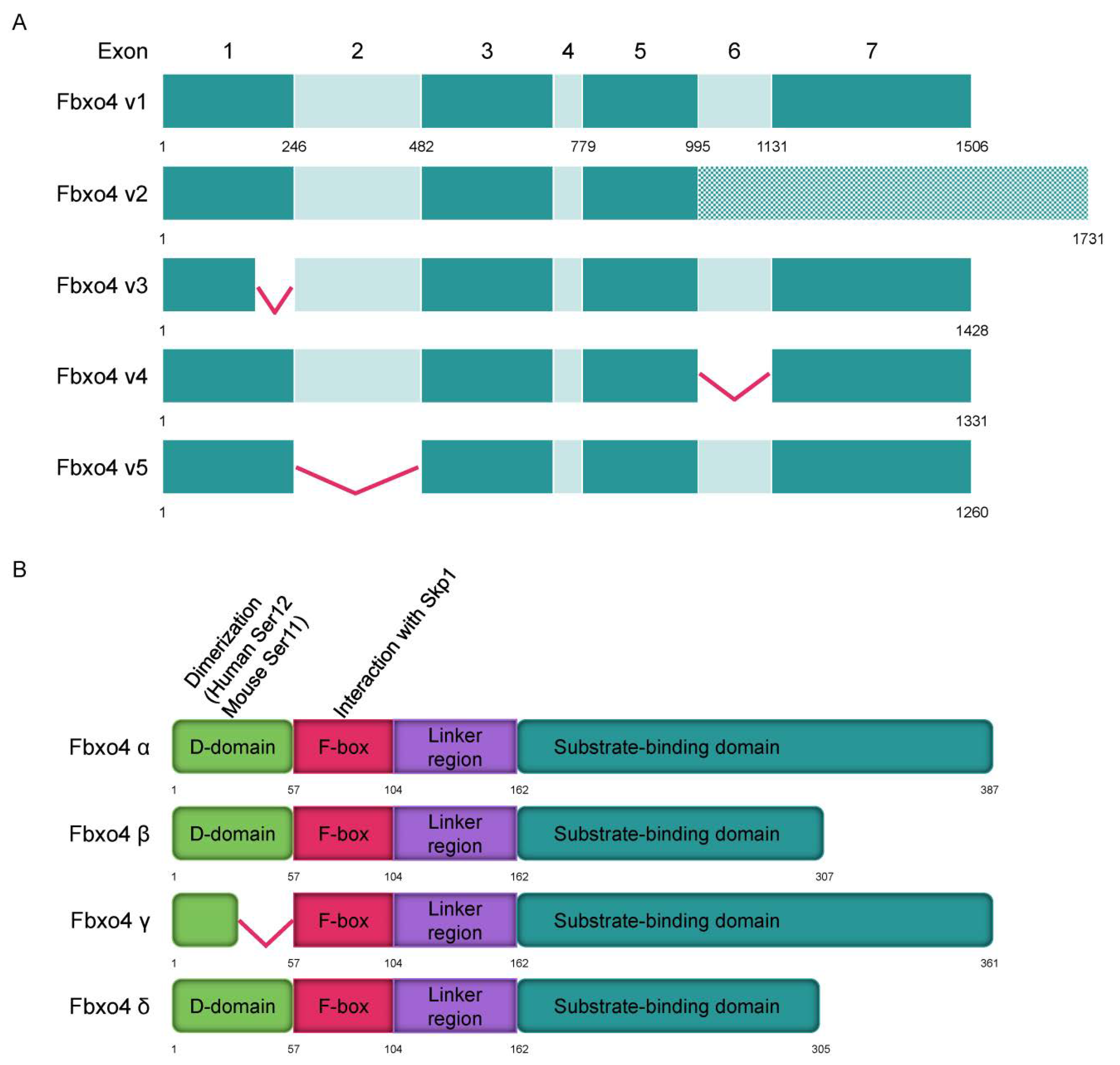
2. The FBXO4 Gene and Protein Structure
3. The Regulation of Fbxo4 Expression and Activity
3.1. Translational Regulation of Fbxo4
3.2. αB-Crystallin Functions as a Co-Factor of Fbxo4
3.3. Phosphorylation and Dimerization of Fbox4
4. The Identified Substrates of Fbxo4
4.1. Cyclin D1
4.2. Trf1/Pin2
4.3. p53
4.4. Fxr1
4.5. Mcl-1
4.6. ICAM-1
4.7. PPARγ
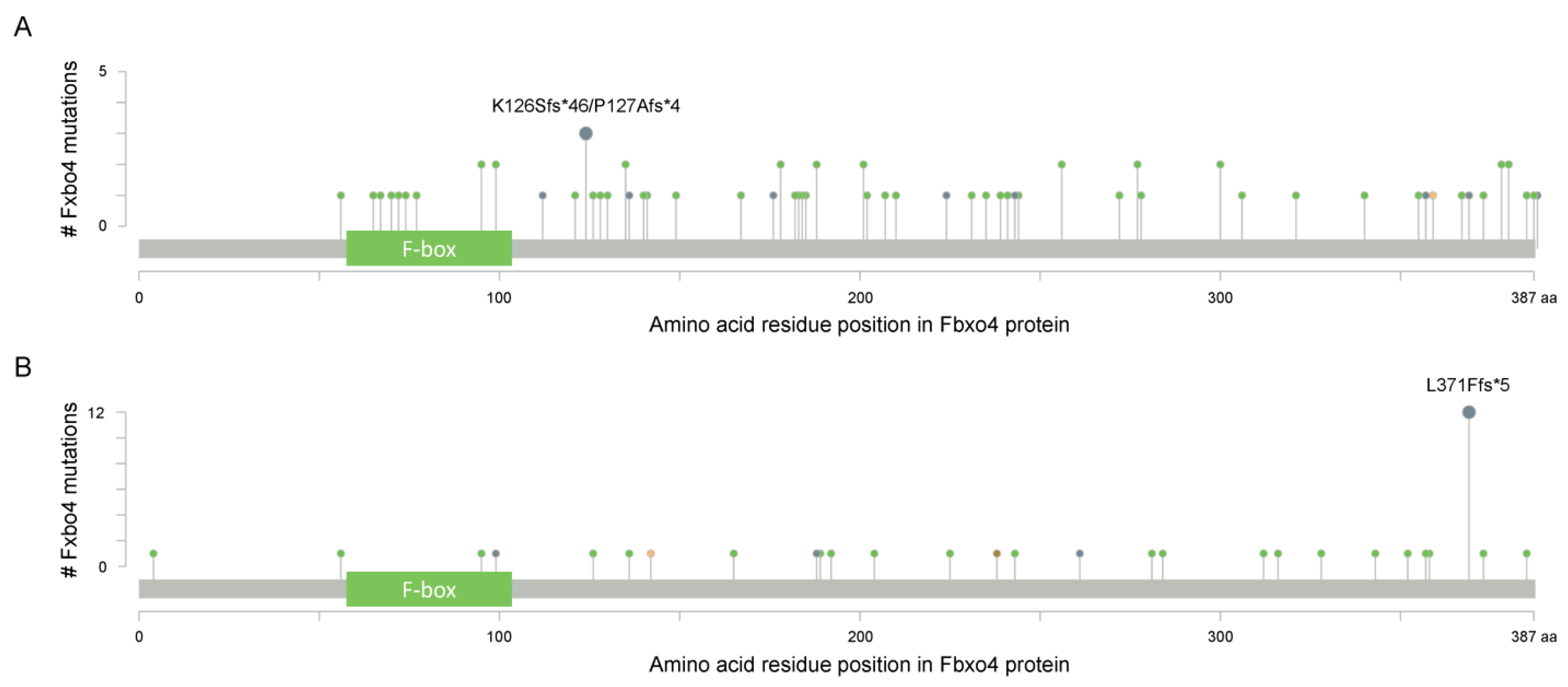
5. The Clinicopathological Importance of Fbxo4
6. The Biological Functions of Fbxo4
6.1. Cell Cycle
6.2. DNA Damage Response
6.3. Tumor Metabolism
6.4. Cellular Senescence
6.5. Other Biological Functions
7. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qie, S.; Majumder, M.; Mackiewicz, K.; Howley, B.V.; Peterson, Y.K.; Howe, P.H.; Palanisamy, V.; Diehl, J.A. Fbxo4-mediated degradation of Fxr1 suppresses tumorigenesis in head and neck squamous cell carcinoma. Nat. Commun. 2017, 8, 1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebeaud, M.E.; Mallik, S.; Goloubinoff, P.; Tawfik, D.S. On the evolution of chaperones and cochaperones and the expansion of proteomes across the Tree of Life. Proc. Natl. Acad. Sci. USA 2021, 118, e2020885118. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Meng, F.; Park, K.S.; Storey, A.J.; Gong, W.; Tsai, Y.H.; Gibson, E.; Byrum, S.D.; Li, D.; Edmondson, R.D.; et al. A NSD3-targeted PROTAC suppresses NSD3 and cMyc oncogenic nodes in cancer cells. Cell Chem. Biol. 2021, 29, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kim, J.; Alexander, A.; Cai, S.; Tripathi, D.N.; Dere, R.; Tee, A.R.; Tait-Mulder, J.; Di Nardo, A.; Han, J.M.; et al. A tuberous sclerosis complex signalling node at the peroxisome regulates mTORC1 and autophagy in response to ROS. Nat. Cell Biol. 2013, 15, 1186–1196. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wu, S.; Jia, L.; Cao, W.; Yao, Y.; Zhao, G.; Li, H. CUL4 E3 ligase regulates the proliferation and apoptosis of lung squamous cell carcinoma and small cell lung carcinoma. Cancer Biol. Med. 2020, 17, 357–370. [Google Scholar] [CrossRef]
- Grabarczyk, D.B.; Petrova, O.A.; Deszcz, L.; Kurzbauer, R.; Murphy, P.; Ahel, J.; Vogel, A.; Gogova, R.; Faas, V.; Kordic, D.; et al. HUWE1 employs a giant substrate-binding ring to feed and regulate its HECT E3 domain. Nat. Chem. Biol. 2021, 17, 1084–1092. [Google Scholar] [CrossRef]
- Qie, S.; Diehl, J.A. Cyclin D degradation by E3 ligases in cancer progression and treatment. Semin. Cancer Biol. 2020, 67, 159–170. [Google Scholar] [CrossRef]
- Chan, C.H.; Li, C.F.; Yang, W.L.; Gao, Y.; Lee, S.W.; Feng, Z.; Huang, H.Y.; Tsai, K.K.; Flores, L.G.; Shao, Y.; et al. The Skp2-SCF E3 ligase regulates Akt ubiquitination, glycolysis, herceptin sensitivity, and tumorigenesis. Cell 2012, 149, 1098–1111. [Google Scholar] [CrossRef] [Green Version]
- Yao, F.; Zhou, Z.; Kim, J.; Hang, Q.; Xiao, Z.; Ton, B.N.; Chang, L.; Liu, N.; Zeng, L.; Wang, W.; et al. SKP2- and OTUD1-regulated non-proteolytic ubiquitination of YAP promotes YAP nuclear localization and activity. Nat. Commun. 2018, 9, 2269. [Google Scholar] [CrossRef]
- Hoeller, D.; Dikic, I. Targeting the ubiquitin system in cancer therapy. Nature 2009, 458, 438–444. [Google Scholar] [CrossRef]
- Ernst, A.; Avvakumov, G.; Tong, J.; Fan, Y.; Zhao, Y.; Alberts, P.; Persaud, A.; Walker, J.R.; Neculai, A.M.; Neculai, D.; et al. A strategy for modulation of enzymes in the ubiquitin system. Science 2013, 339, 590–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson, K.D. Protein ubiquitination: A regulatory post-translational modification. Anticancer Drug Des. 1987, 2, 211–229. [Google Scholar] [PubMed]
- Williams, K.M.; Qie, S.; Atkison, J.H.; Salazar-Arango, S.; Alan Diehl, J.; Olsen, S.K. Structural insights into E1 recognition and the ubiquitin-conjugating activity of the E2 enzyme Cdc34. Nat. Commun. 2019, 10, 3296. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Dai, X.; Jiang, W.; Li, Y.; Wei, W. RBR E3 ubiquitin ligases in tumorigenesis. Semin. Cancer Biol. 2020, 67, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Horn-Ghetko, D.; Krist, D.T.; Prabu, J.R.; Baek, K.; Mulder, M.P.C.; Klugel, M.; Scott, D.C.; Ovaa, H.; Kleiger, G.; Schulman, B.A. Ubiquitin ligation to F-box protein targets by SCF-RBR E3-E3 super-assembly. Nature 2021, 590, 671–676. [Google Scholar] [CrossRef]
- Skaar, J.R.; Pagan, J.K.; Pagano, M. Mechanisms and function of substrate recruitment by F-box proteins. Nat. Rev. Mol. Cell. Biol. 2013, 14, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Qie, S.; Diehl, J.A. Cyclin D1, cancer progression, and opportunities in cancer treatment. J. Mol. Med. 2016, 94, 1313–1326. [Google Scholar] [CrossRef] [Green Version]
- Chiaur, D.S.; Murthy, S.; Cenciarelli, C.; Parks, W.; Loda, M.; Inghirami, G.; Demetrick, D.; Pagano, M. Five human genes encoding F-box proteins: Chromosome mapping and analysis in human tumors. Cytogenet Cell. Genet. 2000, 88, 255–258. [Google Scholar] [CrossRef]
- Karlsson, M.; Zhang, C.; Mear, L.; Zhong, W.; Digre, A.; Katona, B.; Sjostedt, E.; Butler, L.; Odeberg, J.; Dusart, P.; et al. A single-cell type transcriptomics map of human tissues. Sci. Adv. 2021, 7, eabh2169. [Google Scholar] [CrossRef]
- Ponten, F.; Jirstrom, K.; Uhlen, M. The Human Protein Atlas—A tool for pathology. J. Pathol. 2008, 216, 387–393. [Google Scholar] [CrossRef]
- Barbash, O.; Zamfirova, P.; Lin, D.I.; Chen, X.; Yang, K.; Nakagawa, H.; Lu, F.; Rustgi, A.K.; Diehl, J.A. Mutations in Fbx4 inhibit dimerization of the SCF(Fbx4) ligase and contribute to cyclin D1 overexpression in human cancer. Cancer Cell 2008, 14, 68–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, C.; Yang, F.; Wang, J. FBXO4 inhibits lung cancer cell survival by targeting Mcl-1 for degradation. Cancer Gene Ther. 2017, 24, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Her, Y.R.; Chung, I.K. Ubiquitin Ligase RLIM Modulates Telomere Length Homeostasis through a Proteolysis of TRF1. J. Biol. Chem. 2009, 284, 8557–8566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, X.; Moskophidis, D.; Hu, Y.; Phillips, A.; Mivechi, N.F. Heat shock factor 1 deficiency via its downstream target gene alphaB-crystallin (Hspb5) impairs p53 degradation. J. Cell Biochem. 2009, 107, 504–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.H.; Choi, M.Y.; Cui, Y.H.; Kaushik, N.; Uddin, N.; Yoo, K.C.; Kim, M.J.; Lee, S.J. Regulation of FBXO4-mediated ICAM-1 protein stability in metastatic breast cancer. Oncotarget 2017, 8, 83100–83113. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.H.; Perrem, K.; Harper, J.W.; Lu, K.P.; Zhou, X.Z. The F-box protein FBX4 targets PIN2/TRF1 for ubiquitin-mediated degradation and regulates telomere maintenance. J. Biol. Chem. 2006, 281, 759–768. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.I.; Barbash, O.; Kumar, K.G.; Weber, J.D.; Harper, J.W.; Klein-Szanto, A.J.; Rustgi, A.; Fuchs, S.Y.; Diehl, J.A. Phosphorylation-dependent ubiquitination of cyclin D1 by the SCF(FBX4-alphaB crystallin) complex. Mol. Cell 2006, 24, 355–366. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Li, Y.; Wang, X.; Deng, S.; Holland, J.; Yates, E.; Chen, J.; Gu, H.; Essandoh, K.; Mu, X.; et al. An Hsp20-FBXO4 Axis Regulates Adipocyte Function through Modulating PPARgamma Ubiquitination. Cell Rep. 2018, 23, 3607–3620. [Google Scholar] [CrossRef]
- Zhu, Q.; Meng, L.; Hsu, J.K.; Lin, T.; Teishima, J.; Tsai, R.Y. GNL3L stabilizes the TRF1 complex and promotes mitotic transition. J. Cell Biol. 2009, 185, 827–839. [Google Scholar] [CrossRef] [Green Version]
- Radke, S.; Pirkmaier, A.; Germain, D. Differential expression of the F-box proteins Skp2 and Skp2B in breast cancer. Oncogene 2005, 24, 3448–3458. [Google Scholar] [CrossRef] [Green Version]
- Seo, E.; Kim, H.; Kim, R.; Yun, S.; Kim, M.; Han, J.K.; Costantini, F.; Jho, E.H. Multiple isoforms of beta-TrCP display differential activities in the regulation of Wnt signaling. Cell Signal 2009, 21, 43–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Koepp, D.M. Fbw7 isoform interaction contributes to cyclin E proteolysis. Mol. Cancer Res. 2006, 4, 935–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, X.; Zhang, T.; Wang, J.; Li, M.; Zhang, X.; Tu, J.; Sun, S.; Chen, X.; Lu, F. Alternative splicing variants of human Fbx4 disturb cyclin D1 proteolysis in human cancer. Biochem. Biophys. Res. Commun. 2014, 447, 158–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbash, O.; Diehl, J.A. SCF(Fbx4/alphaB-crystallin) E3 ligase: When one is not enough. Cell Cycle 2008, 7, 2983–2986. [Google Scholar] [CrossRef]
- Li, Y.; Hao, B. Structural basis of dimerization-dependent ubiquitination by the SCF(Fbx4) ubiquitin ligase. J. Biol. Chem. 2010, 285, 13896–13906. [Google Scholar] [CrossRef] [Green Version]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal 2013, 6, pl1. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Gao, R.; Yu, C.; Chen, L.; Feng, Y. The RNA-binding protein FXR1 modulates prostate cancer progression by regulating FBXO4. Funct. Integr. Genomics 2019, 19, 487–496. [Google Scholar] [CrossRef]
- Majumder, M.; Johnson, R.H.; Palanisamy, V. Fragile X-related protein family: A double-edged sword in neurodevelopmental disorders and cancer. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 409–424. [Google Scholar] [CrossRef]
- Herzog, R.; Sacnun, J.M.; Gonzalez-Mateo, G.; Bartosova, M.; Bialas, K.; Wagner, A.; Unterwurzacher, M.; Sobieszek, I.J.; Daniel-Fischer, L.; Rusai, K.; et al. Lithium preserves peritoneal membrane integrity by suppressing mesothelial cell alphaB-crystallin. Sci. Transl. Med. 2021, 13, eaaz9705. [Google Scholar] [CrossRef]
- Mishra, S.; Wu, S.Y.; Fuller, A.W.; Wang, Z.; Rose, K.L.; Schey, K.L.; McHaourab, H.S. Loss of alphaB-crystallin function in zebrafish reveals critical roles in the development of the lens and stress resistance of the heart. J. Biol. Chem. 2018, 293, 740–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- den Engelsman, J.; Bennink, E.J.; Doerwald, L.; Onnekink, C.; Wunderink, L.; Andley, U.P.; Kato, K.; de Jong, W.W.; Boelens, W.C. Mimicking phosphorylation of the small heat-shock protein alphaB-crystallin recruits the F-box protein FBX4 to nuclear SC35 speckles. Eur. J. Biochem. 2004, 271, 4195–4203. [Google Scholar] [CrossRef] [PubMed]
- den Engelsman, J.; Keijsers, V.; de Jong, W.W.; Boelens, W.C. The small heat-shock protein alpha B-crystallin promotes FBX4-dependent ubiquitination. J. Biol. Chem. 2003, 278, 4699–4704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbash, O.; Egan, E.; Pontano, L.L.; Kosak, J.; Diehl, J.A. Lysine 269 is essential for cyclin D1 ubiquitylation by the SCF(Fbx4/alphaB-crystallin) ligase and subsequent proteasome-dependent degradation. Oncogene 2009, 28, 4317–4325. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.I.; Lessie, M.D.; Gladden, A.B.; Bassing, C.H.; Wagner, K.U.; Diehl, J.A. Disruption of cyclin D1 nuclear export and proteolysis accelerates mammary carcinogenesis. Oncogene 2008, 27, 1231–1242. [Google Scholar] [CrossRef] [Green Version]
- Barbash, O.; Lee, E.K.; Diehl, J.A. Phosphorylation-dependent regulation of SCF(Fbx4) dimerization and activity involves a novel component, 14-3-3varepsilon. Oncogene 2011, 30, 1995–2002. [Google Scholar] [CrossRef] [Green Version]
- Oughtred, R.; Rust, J.; Chang, C.; Breitkreutz, B.J.; Stark, C.; Willems, A.; Boucher, L.; Leung, G.; Kolas, N.; Zhang, F.; et al. The BioGRID database: A comprehensive biomedical resource of curated protein, genetic, and chemical interactions. Protein Sci. 2021, 30, 187–200. [Google Scholar] [CrossRef]
- Sherr, C.J. Cancer cell cycles. Science 1996, 274, 1672–1677. [Google Scholar] [CrossRef] [Green Version]
- Sherr, C.J. Principles of tumor suppression. Cell 2004, 116, 235–246. [Google Scholar] [CrossRef] [Green Version]
- Sherr, C.J.; Roberts, J.M. CDK inhibitors: Positive and negative regulators of G1-phase progression. Genes Dev. 1999, 13, 1501–1512. [Google Scholar] [CrossRef] [Green Version]
- Qie, S.; Yoshida, A.; Parnham, S.; Oleinik, N.; Beeson, G.C.; Beeson, C.C.; Ogretmen, B.; Bass, A.J.; Wong, K.K.; Rustgi, A.K.; et al. Targeting glutamine-addiction and overcoming CDK4/6 inhibitor resistance in human esophageal squamous cell carcinoma. Nat. Commun. 2019, 10, 1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcea, G.; Neal, C.P.; Pattenden, C.J.; Steward, W.P.; Berry, D.P. Molecular prognostic markers in pancreatic cancer: A systematic review. Eur. J. Cancer 2005, 41, 2213–2236. [Google Scholar] [CrossRef]
- Gautschi, O.; Ratschiller, D.; Gugger, M.; Betticher, D.C.; Heighway, J. Cyclin D1 in non-small cell lung cancer: A key driver of malignant transformation. Lung Cancer 2007, 55, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; An, S.J.; Chen, Z.H.; Zhang, G.C.; Zhu, J.Q.; Nie, Q.; Xie, Z.; Guo, A.L.; Mok, T.S.; Wu, Y.L. Expression of cyclin D1 splice variants is differentially associated with outcome in non-small cell lung cancer patients. Hum. Pathol. 2008, 39, 1792–1801. [Google Scholar] [CrossRef] [PubMed]
- Niederst, M.J.; Sequist, L.V.; Poirier, J.T.; Mermel, C.H.; Lockerman, E.L.; Garcia, A.R.; Katayama, R.; Costa, C.; Ross, K.N.; Moran, T.; et al. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat. Commun. 2015, 6, 6377. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.; Papanikolaou, A. Cyclin D1 in breast cancer pathogenesis. J. Clin. Oncol. 2005, 23, 4215–4224. [Google Scholar] [CrossRef]
- Bertoni, F.; Rinaldi, A.; Zucca, E.; Cavalli, F. Update on the molecular biology of mantle cell lymphoma. Hematol. Oncol. 2006, 24, 22–27. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Integrated genomic characterization of oesophageal carcinoma. Nature 2017, 541, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Dey, B.; Raphael, V.; Khonglah, Y.; GiriLynrah, K. Expression of Cyclin D1 and P16 in Esophageal Squamous Cell Carcinoma. Middle East J. Dig. Dis. 2015, 7, 220–225. [Google Scholar]
- Hardisson, D. Molecular pathogenesis of head and neck squamous cell carcinoma. Eur. Arch. Otorhinolaryngol. 2003, 260, 502–508. [Google Scholar] [CrossRef]
- Li, W.; Sanki, A.; Karim, R.Z.; Thompson, J.F.; Soon Lee, C.; Zhuang, L.; McCarthy, S.W.; Scolyer, R.A. The role of cell cycle regulatory proteins in the pathogenesis of melanoma. Pathology 2006, 38, 287–301. [Google Scholar] [CrossRef] [PubMed]
- McKay, J.A.; Douglas, J.J.; Ross, V.G.; Curran, S.; Murray, G.I.; Cassidy, J.; McLeod, H.L. Cyclin D1 protein expression and gene polymorphism in colorectal cancer. Aberdeen Colorectal Initiative. Int. J. Cancer 2000, 88, 77–81. [Google Scholar] [CrossRef]
- Moreno-Bueno, G.; Rodriguez-Perales, S.; Sanchez-Estevez, C.; Hardisson, D.; Sarrio, D.; Prat, J.; Cigudosa, J.C.; Matias-Guiu, X.; Palacios, J. Cyclin D1 gene (CCND1) mutations in endometrial cancer. Oncogene 2003, 22, 6115–6118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Bueno, G.; Rodriguez-Perales, S.; Sanchez-Estevez, C.; Marcos, R.; Hardisson, D.; Cigudosa, J.C.; Palacios, J. Molecular alterations associated with cyclin D1 overexpression in endometrial cancer. Int. J. Cancer 2004, 110, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Ogino, S.; Shima, K.; Baba, Y.; Nosho, K.; Irahara, N.; Kure, S.; Chen, L.; Toyoda, S.; Kirkner, G.J.; Wang, Y.L.; et al. Colorectal cancer expression of peroxisome proliferator-activated receptor gamma (PPARG, PPARgamma) is associated with good prognosis. Gastroenterology 2009, 136, 1242–1250. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Li, L.; Ou, Y.; Gao, Z.; Li, E.; Li, X.; Zhang, W.; Wang, J.; Xu, L.; Zhou, Y.; et al. Identification of genomic alterations in oesophageal squamous cell cancer. Nature 2014, 509, 91–95. [Google Scholar] [CrossRef]
- Qie, S.; Diehl, J.A. Glutamine addiction: An Achilles heel in esophageal cancers with dysregulation of CDK4/6. Mol. Cell. Oncol. 2019, 6, 1610257. [Google Scholar] [CrossRef]
- Diehl, J.A.; Cheng, M.; Roussel, M.F.; Sherr, C.J. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998, 12, 3499–3511. [Google Scholar] [CrossRef] [Green Version]
- Diehl, J.A.; Zindy, F.; Sherr, C.J. Inhibition of cyclin D1 phosphorylation on threonine-286 prevents its rapid degradation via the ubiquitin-proteasome pathway. Genes Dev. 1997, 11, 957–972. [Google Scholar] [CrossRef] [Green Version]
- Augello, M.A.; Berman-Booty, L.D.; Carr, R., 3rd; Yoshida, A.; Dean, J.L.; Schiewer, M.J.; Feng, F.Y.; Tomlins, S.A.; Gao, E.; Koch, W.J.; et al. Consequence of the tumor-associated conversion to cyclin D1b. EMBO Mol. Med. 2015, 7, 628–647. [Google Scholar] [CrossRef]
- Knudsen, K.E.; Diehl, J.A.; Haiman, C.A.; Knudsen, E.S. Cyclin D1: Polymorphism, aberrant splicing and cancer risk. Oncogene 2006, 25, 1620–1628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaikovsky, A.C.; Li, C.; Jeng, E.E.; Loebell, S.; Lee, M.C.; Murray, C.W.; Cheng, R.; Demeter, J.; Swaney, D.L.; Chen, S.H.; et al. The AMBRA1 E3 ligase adaptor regulates the stability of cyclin D. Nature 2021, 592, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Maiani, E.; Milletti, G.; Nazio, F.; Holdgaard, S.G.; Bartkova, J.; Rizza, S.; Cianfanelli, V.; Lorente, M.; Simoneschi, D.; Di Marco, M.; et al. AMBRA1 regulates cyclin D to guard S-phase entry and genomic integrity. Nature 2021, 592, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Okabe, H.; Lee, S.H.; Phuchareon, J.; Albertson, D.G.; McCormick, F.; Tetsu, O. A critical role for FBXW8 and MAPK in cyclin D1 degradation and cancer cell proliferation. PLoS ONE 2006, 1, e128. [Google Scholar] [CrossRef] [Green Version]
- Santra, M.K.; Wajapeyee, N.; Green, M.R. F-box protein FBXO31 mediates cyclin D1 degradation to induce G1 arrest after DNA damage. Nature 2009, 459, 722–725. [Google Scholar] [CrossRef]
- Simoneschi, D.; Rona, G.; Zhou, N.; Jeong, Y.T.; Jiang, S.; Milletti, G.; Arbini, A.A.; O’Sullivan, A.; Wang, A.A.; Nithikasem, S.; et al. CRL4(AMBRA1) is a master regulator of D-type cyclins. Nature 2021, 592, 789–793. [Google Scholar] [CrossRef]
- Wei, S.; Yang, H.C.; Chuang, H.C.; Yang, J.; Kulp, S.K.; Lu, P.J.; Lai, M.D.; Chen, C.S. A novel mechanism by which thiazolidinediones facilitate the proteasomal degradation of cyclin D1 in cancer cells. J. Biol. Chem. 2008, 283, 26759–26770. [Google Scholar] [CrossRef] [Green Version]
- Yamano, H. APC/C: Current understanding and future perspectives. F1000Res 2019, 8, 725. [Google Scholar] [CrossRef]
- Lee, E.K.; Lian, Z.; D’Andrea, K.; Letrero, R.; Sheng, W.; Liu, S.; Diehl, J.N.; Pytel, D.; Barbash, O.; Schuchter, L.; et al. The FBXO4 tumor suppressor functions as a barrier to BRAFV600E-dependent metastatic melanoma. Mol. Cell. Biol. 2013, 33, 4422–4433. [Google Scholar] [CrossRef] [Green Version]
- Myler, L.R.; Kinzig, C.G.; Sasi, N.K.; Zakusilo, G.; Cai, S.W.; de Lange, T. The evolution of metazoan shelterin. Genes Dev. 2021, 35, 1625–1641. [Google Scholar] [CrossRef]
- Zeng, Z.; Wang, W.; Yang, Y.; Chen, Y.; Yang, X.; Diehl, J.A.; Liu, X.; Lei, M. Structural basis of selective ubiquitination of TRF1 by SCFFbx4. Dev. Cell 2010, 18, 214–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, R.; Nakajima, S.; Wang, Q.; Sun, H.; Xue, J.; Wu, J.; Hellwig, S.; Zeng, X.; Yates, N.A.; Smithgall, T.E.; et al. Nek7 Protects Telomeres from Oxidative DNA Damage by Phosphorylation and Stabilization of TRF1. Mol. Cell 2017, 65, 818–831.e815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Xiao, H.; Ma, J.; Zhu, Y.; Yu, J.; Sun, L.; Sun, H.; Liu, Y.; Jin, C.; Huang, H. The F-box protein beta-TrCP promotes ubiquitination of TRF1 and regulates the ALT-associated PML bodies formation in U2OS cells. Biochem. Biophys. Res. Commun. 2013, 434, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Chung, I.K. The splicing factor U2AF65 stabilizes TRF1 protein by inhibiting its ubiquitin-dependent proteolysis. Biochem. Biophys. Res. Commun. 2014, 443, 1124–1130. [Google Scholar] [CrossRef]
- Kastenhuber, E.R.; Lowe, S.W. Putting p53 in Context. Cell 2017, 170, 1062–1078. [Google Scholar] [CrossRef] [Green Version]
- Redman-Rivera, L.N.; Shaver, T.M.; Jin, H.; Marshall, C.B.; Schafer, J.M.; Sheng, Q.; Hongo, R.A.; Beckermann, K.E.; Wheeler, F.C.; Lehmann, B.D.; et al. Acquisition of aneuploidy drives mutant p53-associated gain-of-function phenotypes. Nat. Commun. 2021, 12, 5184. [Google Scholar] [CrossRef]
- Tang, Q.; Su, Z.; Gu, W.; Rustgi, A.K. Mutant p53 on the Path to Metastasis. Trends Cancer 2020, 6, 62–73. [Google Scholar] [CrossRef]
- Sun, X.; Ou, Z.; Xie, M.; Kang, R.; Fan, Y.; Niu, X.; Wang, H.; Cao, L.; Tang, D. HSPB1 as a novel regulator of ferroptotic cancer cell death. Oncogene 2015, 34, 5617–5625. [Google Scholar] [CrossRef] [Green Version]
- Kanagasabai, R.; Krishnamurthy, K.; Druhan, L.J.; Ilangovan, G. Forced expression of heat shock protein 27 (Hsp27) reverses P-glycoprotein (ABCB1)-mediated drug efflux and MDR1 gene expression in Adriamycin-resistant human breast cancer cells. J. Biol. Chem. 2011, 286, 33289–33300. [Google Scholar] [CrossRef] [Green Version]
- McClure, J.J.; Palanisamy, V. Muscle-Specific FXR1 Isoforms in Squamous Cell Cancer. Trends Cancer 2019, 5, 82–84. [Google Scholar] [CrossRef]
- Thomsen, R.; Pallesen, J.; Daugaard, T.F.; Borglum, A.D.; Nielsen, A.L. Genome wide assessment of mRNA in astrocyte protrusions by direct RNA sequencing reveals mRNA localization for the intermediate filament protein nestin. Glia 2013, 61, 1922–1937. [Google Scholar] [CrossRef] [PubMed]
- Majumder, M.; House, R.; Palanisamy, N.; Qie, S.; Day, T.A.; Neskey, D.; Diehl, J.A.; Palanisamy, V. RNA-Binding Protein FXR1 Regulates p21 and TERC RNA to Bypass p53-Mediated Cellular Senescence in OSCC. PLoS Genet. 2016, 12, e1006306. [Google Scholar] [CrossRef] [Green Version]
- Qian, J.; Hassanein, M.; Hoeksema, M.D.; Harris, B.K.; Zou, Y.; Chen, H.; Lu, P.; Eisenberg, R.; Wang, J.; Espinosa, A.; et al. The RNA binding protein FXR1 is a new driver in the 3q26-29 amplicon and predicts poor prognosis in human cancers. Proc. Natl. Acad. Sci. USA 2015, 112, 3469–3474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, J.; Li, Y.; Kadamberi, I.P.; Parashar, D.; Tsaih, S.W.; Gupta, P.; Geethadevi, A.; Chen, C.; Ghosh, C.; Sun, Y.; et al. RNA-binding protein FXR1 drives cMYC translation by recruiting eIF4F complex to the translation start site. Cell Rep. 2021, 37, 109934. [Google Scholar] [CrossRef] [PubMed]
- Senichkin, V.V.; Streletskaia, A.Y.; Zhivotovsky, B.; Kopeina, G.S. Molecular Comprehension of Mcl-1: From Gene Structure to Cancer Therapy. Trends Cell. Biol. 2019, 29, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Daniels, V.W.; Zoeller, J.J.; van Gastel, N.; McQueeney, K.E.; Parvin, S.; Potter, D.S.; Fell, G.G.; Ferreira, V.G.; Yilma, B.; Gupta, R.; et al. Metabolic perturbations sensitize triple-negative breast cancers to apoptosis induced by BH3 mimetics. Sci. Signal 2021, 14, eabc7405. [Google Scholar] [CrossRef] [PubMed]
- Alcon, C.; Manzano-Munoz, A.; Prada, E.; Mora, J.; Soriano, A.; Guillen, G.; Gallego, S.; Roma, J.; Samitier, J.; Villanueva, A.; et al. Sequential combinations of chemotherapeutic agents with BH3 mimetics to treat rhabdomyosarcoma and avoid resistance. Cell Death Dis. 2020, 11, 634. [Google Scholar] [CrossRef]
- Flores, M.L.; Castilla, C.; Gasca, J.; Medina, R.; Perez-Valderrama, B.; Romero, F.; Japon, M.A.; Saez, C. Loss of PKCdelta Induces Prostate Cancer Resistance to Paclitaxel through Activation of Wnt/beta-Catenin Pathway and Mcl-1 Accumulation. Mol. Cancer Ther. 2016, 15, 1713–1725. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Yang, D.; Sabbatini, M.E.; Colby, A.H.; Grinstaff, M.W.; Oberlies, N.H.; Pearce, C.; Liu, K. Contrasting roles of H3K4me3 and H3K9me3 in regulation of apoptosis and gemcitabine resistance in human pancreatic cancer cells. BMC Cancer 2018, 18, 149. [Google Scholar] [CrossRef] [Green Version]
- Michels, J.; Obrist, F.; Vitale, I.; Lissa, D.; Garcia, P.; Behnam-Motlagh, P.; Kohno, K.; Wu, G.S.; Brenner, C.; Castedo, M.; et al. MCL-1 dependency of cisplatin-resistant cancer cells. Biochem. Pharmacol. 2014, 92, 55–61. [Google Scholar] [CrossRef]
- Abbas, R.; Larisch, S. Killing by Degradation: Regulation of Apoptosis by the Ubiquitin-Proteasome-System. Cells 2021, 10, 3465. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.M.; Wiesolek, H.L.; Sumagin, R. ICAM-1: A master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J. Leukoc. Biol. 2020, 108, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Roebuck, K.A.; Finnegan, A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J. Leukoc. Biol. 1999, 66, 876–888. [Google Scholar] [CrossRef] [PubMed]
- Shiau, C.W.; Yang, C.C.; Kulp, S.K.; Chen, K.F.; Chen, C.S.; Huang, J.W.; Chen, C.S. Thiazolidenediones mediate apoptosis in prostate cancer cells in part through inhibition of Bcl-xL/Bcl-2 functions independently of PPARgamma. Cancer Res. 2005, 65, 1561–1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frkic, R.L.; Richter, K.; Bruning, J.B. The therapeutic potential of inhibiting PPARgamma phosphorylation to treat type 2 diabetes. J. Biol. Chem. 2021, 297, 101030. [Google Scholar] [CrossRef] [PubMed]
- Kajimura, S.; Spiegelman, B.M.; Seale, P. Brown and Beige Fat: Physiological Roles beyond Heat Generation. Cell Metab. 2015, 22, 546–559. [Google Scholar] [CrossRef] [Green Version]
- DeLany, J.P.; Floyd, Z.E.; Zvonic, S.; Smith, A.; Gravois, A.; Reiners, E.; Wu, X.; Kilroy, G.; Lefevre, M.; Gimble, J.M. Proteomic analysis of primary cultures of human adipose-derived stem cells: Modulation by Adipogenesis. Mol. Cell. Proteomics 2005, 4, 731–740. [Google Scholar] [CrossRef] [Green Version]
- Vaites, L.P.; Lee, E.K.; Lian, Z.; Barbash, O.; Roy, D.; Wasik, M.; Klein-Szanto, A.J.; Rustgi, A.K.; Diehl, J.A. The Fbx4 tumor suppressor regulates cyclin D1 accumulation and prevents neoplastic transformation. Mol. Cell Biol. 2011, 31, 4513–4523. [Google Scholar] [CrossRef] [Green Version]
- Korcheva, V.B.; Levine, J.; Beadling, C.; Warrick, A.; Countryman, G.; Olson, N.R.; Heinrich, M.C.; Corless, C.L.; Troxell, M.L. Immunohistochemical and molecular markers in breast phyllodes tumors. Appl. Immunohistochem. Mol. Morphol. 2011, 19, 119–125. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, T.; Zhang, S.; Shan, J. Prognostic values of F-box members in breast cancer: An online database analysis and literature review. Biosci. Rep. 2019, 39, BSR20180949. [Google Scholar] [CrossRef] [Green Version]
- Lian, Z.; Lee, E.K.; Bass, A.J.; Wong, K.K.; Klein-Szanto, A.J.; Rustgi, A.K.; Diehl, J.A. FBXO4 loss facilitates carcinogen induced papilloma development in mice. Cancer Biol. Ther. 2015, 16, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Pontano, L.L.; Aggarwal, P.; Barbash, O.; Brown, E.J.; Bassing, C.H.; Diehl, J.A. Genotoxic stress-induced cyclin D1 phosphorylation and proteolysis are required for genomic stability. Mol. Cell Biol. 2008, 28, 7245–7258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.H.; Liu, S.; Zhou, H.; Qu, L.H.; Yang, J.H. starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014, 42, D92–D97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbash, O.; Lin, D.I.; Diehl, J.A. SCF Fbx4/alphaB-crystallin cyclin D1 ubiquitin ligase: A license to destroy. Cell Div. 2007, 2, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, P.; Lessie, M.D.; Lin, D.I.; Pontano, L.; Gladden, A.B.; Nuskey, B.; Goradia, A.; Wasik, M.A.; Klein-Szanto, A.J.; Rustgi, A.K.; et al. Nuclear accumulation of cyclin D1 during S phase inhibits Cul4-dependent Cdt1 proteolysis and triggers p53-dependent DNA rereplication. Genes Dev. 2007, 21, 2908–2922. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, P.; Vaites, L.P.; Kim, J.K.; Mellert, H.; Gurung, B.; Nakagawa, H.; Herlyn, M.; Hua, X.; Rustgi, A.K.; McMahon, S.B.; et al. Nuclear cyclin D1/CDK4 kinase regulates CUL4 expression and triggers neoplastic growth via activation of the PRMT5 methyltransferase. Cancer Cell 2010, 18, 329–340. [Google Scholar] [CrossRef] [Green Version]
- Qian, L.; Zhang, F.; Yin, M.; Lei, Q. Cancer metabolism and dietary interventions. Cancer Biol. Med. 2021, 19, 163. [Google Scholar] [CrossRef]
- Qie, S.; Chu, C.; Li, W.; Wang, C.; Sang, N. ErbB2 activation upregulates glutaminase 1 expression which promotes breast cancer cell proliferation. J. Cell Biochem. 2014, 115, 498–509. [Google Scholar] [CrossRef] [Green Version]
- Qie, S.; Liang, D.; Yin, C.; Gu, W.; Meng, M.; Wang, C.; Sang, N. Glutamine depletion and glucose depletion trigger growth inhibition via distinctive gene expression reprogramming. Cell Cycle 2012, 11, 3679–3690. [Google Scholar] [CrossRef] [Green Version]
- Dong, M.; Miao, L.; Zhang, F.; Li, S.; Han, J.; Yu, R.; Qie, S. Nuclear factor-kappaB p65 regulates glutaminase 1 expression in human hepatocellular carcinoma. Onco Targets Ther. 2018, 11, 3721–3729. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Yang, X.; Zhang, Q.; Sun, L.; Yuan, S.; Xin, Y. Targeting GLS1 to cancer therapy through glutamine metabolism. Clin. Transl. Oncol. 2021, 23, 2253–2268. [Google Scholar] [CrossRef] [PubMed]
- Rufini, A.; Tucci, P.; Celardo, I.; Melino, G. Senescence and aging: The critical roles of p53. Oncogene 2013, 32, 5129–5143. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018, 28, 436–453. [Google Scholar] [CrossRef] [PubMed]
- Rayess, H.; Wang, M.B.; Srivatsan, E.S. Cellular senescence and tumor suppressor gene p16. Int. J. Cancer 2012, 130, 1715–1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Cancers | SubStrates | Biological Functions of Fbxo4 | Tumor Progression | References |
|---|---|---|---|---|
| ESCC | Cyclin D1 | Fbxo4 compromises cell cycle progression, colony formation and oncogene-induced transformation | Various Fbxo4 mutations are identified in human ESCC samples | [21,27,44,108,112] |
| Melanoma | Cyclin D1 | Fbxo4 I377M mutation causes the accumulation of cyclin D1 in melanoma cells | Loss of Fbxo4 increases tumor aggressiveness and reduces survival in BrafV600E/+ melanoma mouse model | [79] |
| HCC | Cyclin D1 | Different Fbxo4 transcript variants perform different functions in regulating cyclin D1 expression | Fbxo4 is downregulated in human HCC tissues comparing to normal liver | [33] |
| Breast phyllodes tumors | Cyclin D1 | Patients with Fbxo4 S8R mutation have elevated cyclin D1 levels | - | [109] |
| HNSCC | Fxr1 | Fbxo4 suppresses cell proliferation and cellular senescence | Tumor tissues have low Fbxo4 levels relative to normal counterparts | [1] |
| Lung cancer | Mcl-1 | Fbxo4 reduces cell survival | Reduced Fbxo4 expression leads to acquired resistance to chemotherapeutic compounds | [22] |
| Breast cancer | ICAM-1 | Fbxo4 compromises EMT and tumor metastasis | Low Fbxo4 expression is associated with poor prognosis | [25,110] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qie, S. The E3 Ubiquitin Ligase Fbxo4 Functions as a Tumor Suppressor: Its Biological Importance and Therapeutic Perspectives. Cancers 2022, 14, 2133. https://doi.org/10.3390/cancers14092133
Qie S. The E3 Ubiquitin Ligase Fbxo4 Functions as a Tumor Suppressor: Its Biological Importance and Therapeutic Perspectives. Cancers. 2022; 14(9):2133. https://doi.org/10.3390/cancers14092133
Chicago/Turabian StyleQie, Shuo. 2022. "The E3 Ubiquitin Ligase Fbxo4 Functions as a Tumor Suppressor: Its Biological Importance and Therapeutic Perspectives" Cancers 14, no. 9: 2133. https://doi.org/10.3390/cancers14092133






