Pharmacological Regulation of Tumor Hypoxia in Model Murine Tumors and Spontaneous Canine Tumors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Construction, Tumor Growth, and Visualization of pODD-Luc-Expressing Cells
- ODD-luciferase-AscI-F ATATAggcgcgccgaattcgccaccatggaatt
- ODD-luciferase-PmeI-R ATATAgtttaaacattacacggcgatctttccg
2.2. Orthotopic Pancreatic Xenografts
2.3. Western Blotting
2.4. In Vitro Luciferase Assay
2.5. In Vivo Bioluminescent Imaging
2.6. BOLD MRI Analysis of Canine Tumors
2.7. Statistics
3. Results
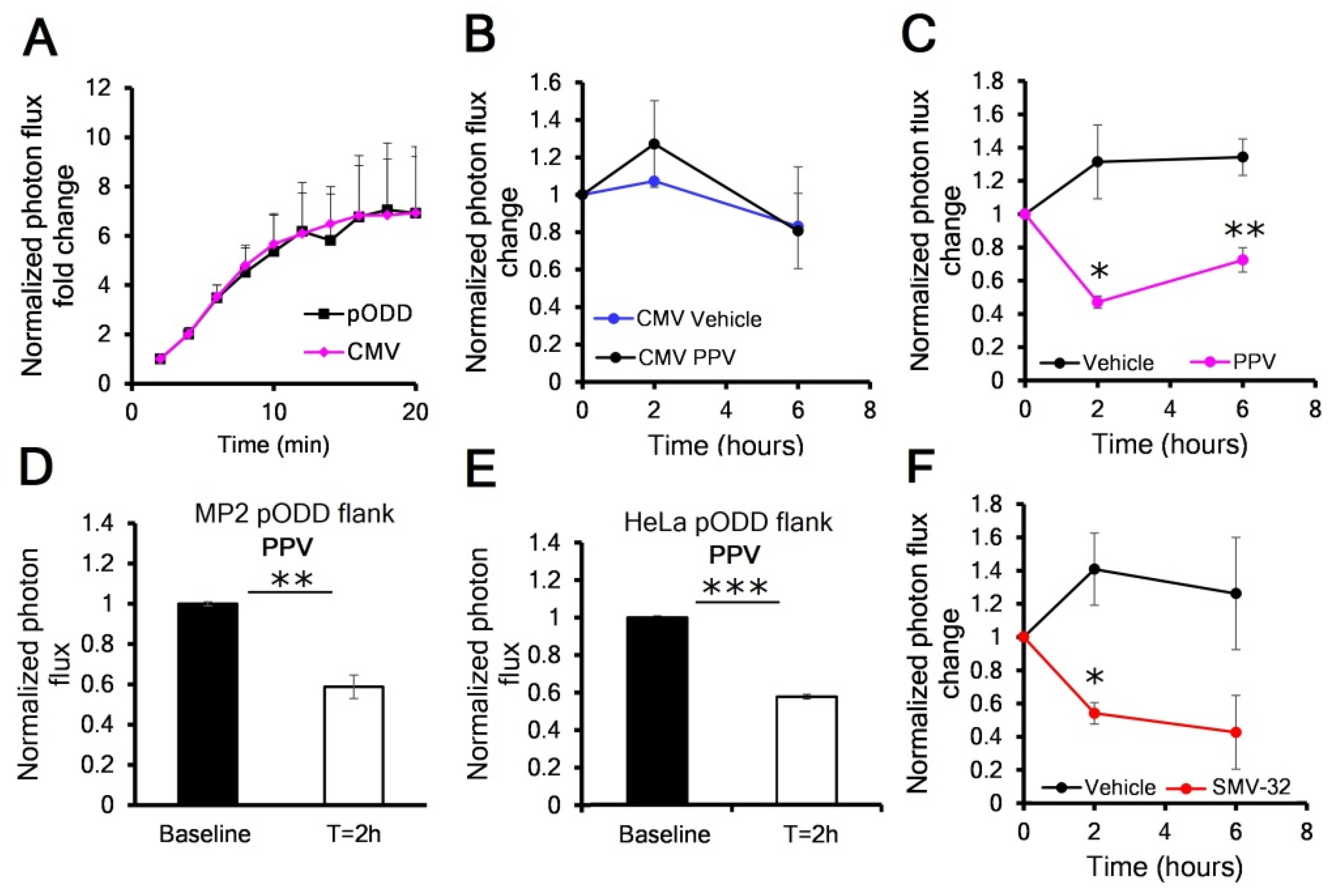
3.1. PODD-Luc Signal Increases under Hypoxia In Vitro and In Vivo
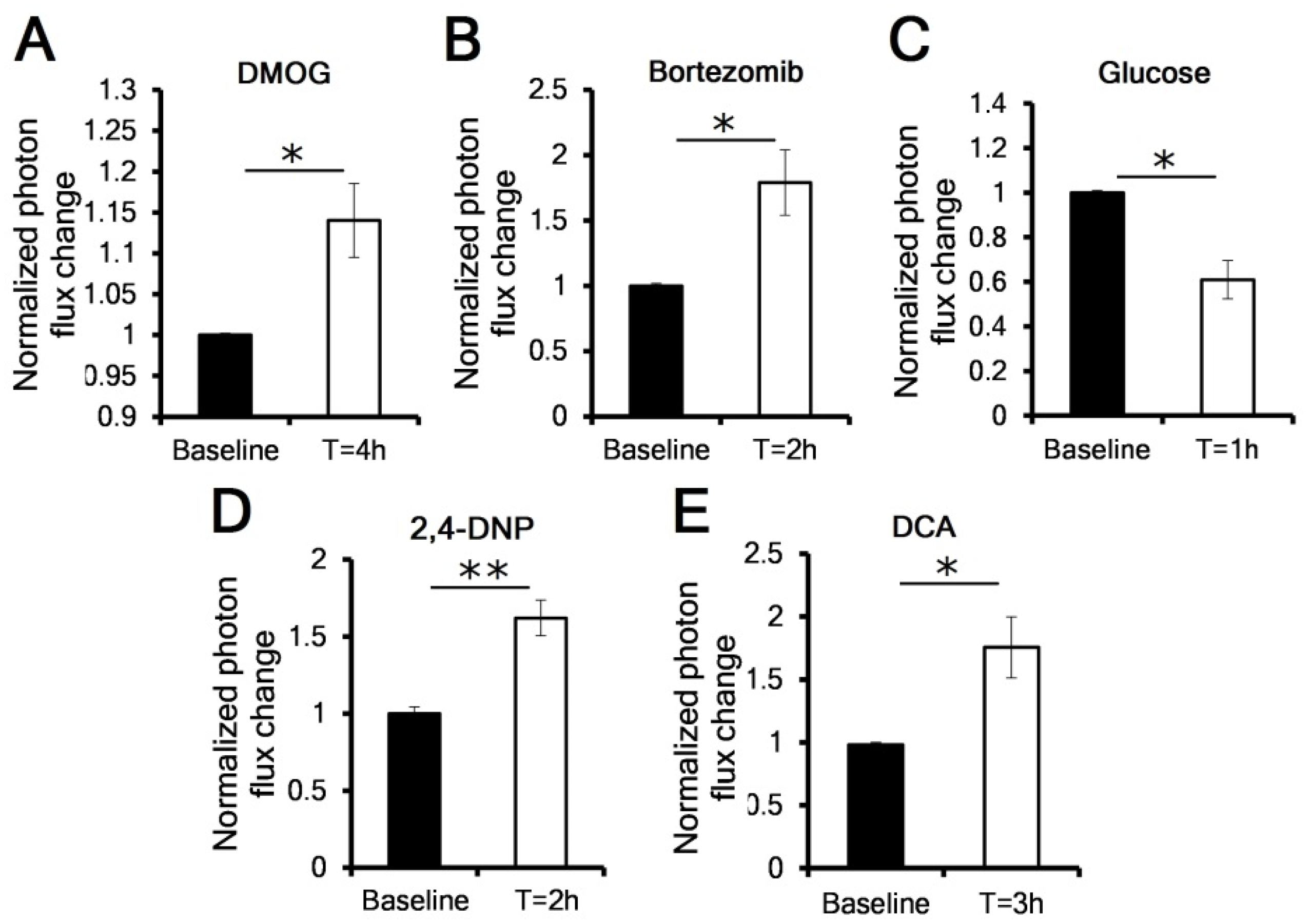
3.2. OCR Manipulation Modulates Levels of Luciferase Activity In Vivo
3.3. Mitochondrial Complex I Inhibitors Papaverine and SMV-32 Effectively Reduce Hypoxia in Orthotopic Pancreas Cancers
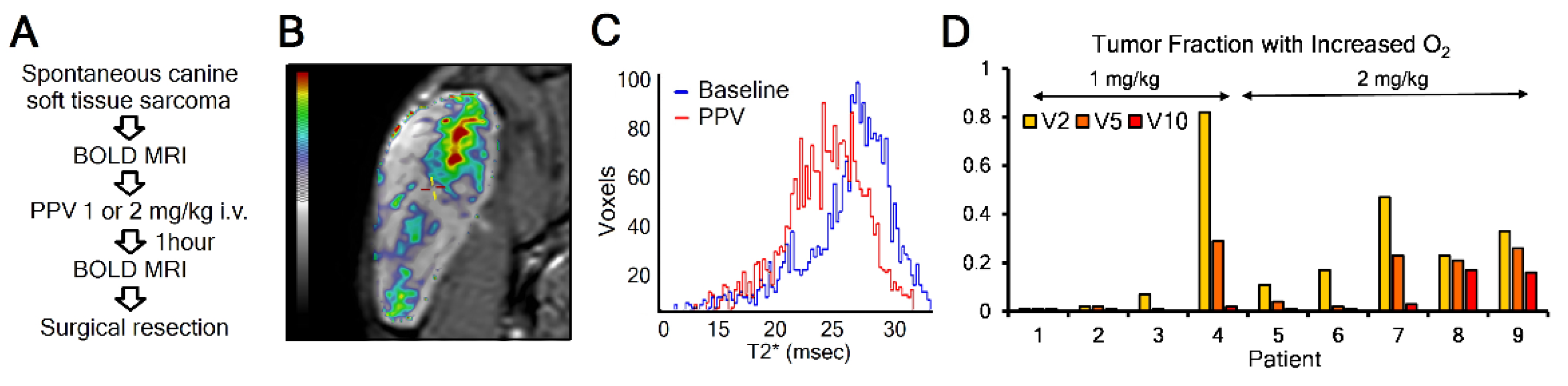
3.4. Mitochondrial Complex I Inhibitor Papaverine Increases Oxygenation in Spontaneous Canine Sarcomas
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thomlinson, R.H.; Gray, L.H. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br. J. Cancer 1955, 9, 539–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, H.; Braun, R.D.; Ong, E.T.; Hsu, R.; Secomb, T.W.; Papahadjopoulos, D.; Hong, K.; Dewhirst, M.W. Fluctuations in red cell flux in tumor microvessels can lead to transient hypoxia and reoxygenation in tumor parenchyma. Cancer Res. 1996, 56, 5522–5528. [Google Scholar]
- Bader, S.B.; Dewhirst, M.W.; Hammond, E.M. Cyclic Hypoxia: An Update on Its Characteristics, Methods to Measure It and Biological Implications in Cancer. Cancers 2021, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Wykoff, C.C.; Beasley, N.J.; Watson, P.H.; Turner, K.J.; Pastorek, J.; Sibtain, A.; Wilson, G.D.; Turley, H.; Talks, K.L.; Maxwell, P.H.; et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000, 60, 7075–7083. [Google Scholar] [PubMed]
- Wang, G.L.; Semenza, G.L. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc. Natl. Acad. Sci. USA. 1993, 90, 4304–4308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aprelikova, O.; Chandramouli, G.V.; Wood, M.; Vasselli, J.R.; Riss, J.; Maranchie, J.K.; Linehan, W.M.; Barrett, J.C. Regulation of HIF prolyl hydroxylases by hypoxia-inducible factors. J. Cell. Biochem. 2004, 92, 491–501. [Google Scholar] [CrossRef]
- Wang, G.L.; Semenza, G.L. Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J. Biol. Chem. 1993, 268, 21513–21518. [Google Scholar] [CrossRef]
- Papandreou, I.; Cairns, R.A.; Fontana, L.; Lim, A.L.; Denko, N.C. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006, 3, 187–197. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.W.; Tchernyshyov, I.; Semenza, G.L.; Dang, C.V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006, 3, 177–185. [Google Scholar] [CrossRef] [Green Version]
- Vaupel, P. Hypoxia and aggressive tumor phenotype: Implications for therapy and prognosis. Oncologist 2008, 13 (Suppl. 3), 21–26. [Google Scholar] [CrossRef] [Green Version]
- Gomez, C.R. Editorial: Tumor Hypoxia: Impact in Tumorigenesis, Diagnosis, Prognosis, and Therapeutics. Front. Oncol. 2016, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overgaard, J. Hypoxic radiosensitization: Adored and ignored. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 4066–4074. [Google Scholar] [CrossRef] [Green Version]
- Sørensen, B.S.; Horsman, M.R. Tumor Hypoxia: Impact on Radiation Therapy and Molecular Pathways. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Snyder, C.M.; Chandel, N.S. Mitochondrial regulation of cell survival and death during low-oxygen conditions. Antioxid. Redox Signal. 2009, 11, 2673–2683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, P. Coupling of Phosphorylation to Electron and Hydrogen Transfer by a Chemi-Osmotic type of Mechanism. Nature 1961, 191, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Zannella, V.E.; Dal Pra, A.; Muaddi, H.; McKee, T.D.; Stapleton, S.; Sykes, J.; Glicksman, R.; Chaib, S.; Zamiara, P.; Milosevic, M.; et al. Reprogramming metabolism with metformin improves tumor oxygenation and radiotherapy response. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 6741–6750. [Google Scholar] [CrossRef] [Green Version]
- Snyder, S.A.; Lanzen, J.L.; Braun, R.D.; Rosner, G.; Secomb, T.W.; Biaglow, J.; Brizel, D.M.; Dewhirst, M.W. Simultaneous administration of glucose and hyperoxic gas achieves greater improvement in tumor oxygenation than hyperoxic gas alone. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 494–506. [Google Scholar] [CrossRef]
- Molina, J.R.; Sun, Y.; Protopopova, M.; Gera, S.; Bandi, M.; Bristow, C.; McAfoos, T.; Morlacchi, P.; Ackroyd, J.; Agip, A.A.; et al. An inhibitor of oxidative phosphorylation exploits cancer vulnerability. Nat. Med. 2018, 24, 1036–1046. [Google Scholar] [CrossRef] [Green Version]
- Benej, M.; Hong, X.; Vibhute, S.; Scott, S.; Wu, J.; Graves, E.; Le, Q.T.; Koong, A.C.; Giaccia, A.J.; Yu, B.; et al. Papaverine and its derivatives radiosensitize solid tumors by inhibiting mitochondrial metabolism. Proc. Natl. Acad. Sci. USA 2018, 115, 10756–10761. [Google Scholar] [CrossRef] [Green Version]
- Ashton, T.M.; Fokas, E.; Kunz-Schughart, L.A.; Folkes, L.K.; Anbalagan, S.; Huether, M.; Kelly, C.J.; Pirovano, G.; Buffa, F.M.; Hammond, E.M.; et al. The anti-malarial atovaquone increases radiosensitivity by alleviating tumour hypoxia. Nat. Commun. 2016, 7, 12308. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, J.P.B.; Robinson, S.P.; Waterton, J.C. Imaging tumour hypoxia with oxygen-enhanced MRI and BOLD MRI. Br. J. Radiol. 2019, 92, 20180642. [Google Scholar] [CrossRef] [PubMed]
- Padhani, A.R.; Krohn, K.A.; Lewis, J.S.; Alber, M. Imaging oxygenation of human tumours. Eur. Radiol. 2007, 17, 861–872. [Google Scholar] [CrossRef] [Green Version]
- Euhus, D.M.; Hudd, C.; LaRegina, M.C.; Johnson, F.E. Tumor measurement in the nude mouse. J. Surg. Oncol. 1986, 31, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Safran, M.; Kim, W.Y.; O’Connell, F.; Flippin, L.; Günzler, V.; Horner, J.W.; Depinho, R.A.; Kaelin, W.G., Jr. Mouse model for noninvasive imaging of HIF prolyl hydroxylase activity: Assessment of an oral agent that stimulates erythropoietin production. Proc. Natl. Acad. Sci. USA 2006, 103, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Papandreou, I.; Goliasova, T.; Denko, N.C. Anticancer drugs that target metabolism: Is dichloroacetate the new paradigm? Int. J. Cancer 2011, 128, 1001–1008. [Google Scholar] [CrossRef]
- Baudelet, C.; Gallez, B. How does blood oxygen level-dependent (BOLD) contrast correlate with oxygen partial pressure (pO2) inside tumors? Magn. Reson. Med. 2002, 48, 980–986. [Google Scholar] [CrossRef]
- Bruehlmeier, M.; Kaser-Hotz, B.; Achermann, R.; Bley, C.R.; Wergin, M.; Schubiger, P.A.; Ametamey, S.M. Measurement of tumor hypoxia in spontaneous canine sarcomas. Vet. Radiol. Ultrasound Off. J. Am. Coll. Vet. Radiol. Int. Vet. Radiol. Assoc. 2005, 46, 348–354. [Google Scholar] [CrossRef]
- Thrall, D.E.; Rosner, G.L.; Azuma, C.; McEntee, M.C.; Raleigh, J.A. Hypoxia marker labeling in tumor biopsies: Quantification of labeling variation and criteria for biopsy sectioning. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 1997, 44, 171–176. [Google Scholar] [CrossRef]
- Klibanov, A.L.; Hu, S. Monitoring Oxygenation Levels Deep in the Tumor Core: Noninvasive Imaging of Hypoxia, Now in Real-Time 3D. Cancer Res. 2019, 79, 4577–4579. [Google Scholar] [CrossRef] [Green Version]
- Varia, M.A.; Calkins-Adams, D.P.; Rinker, L.H.; Kennedy, A.S.; Novotny, D.B.; Fowler, W.C., Jr.; Raleigh, J.A. Pimonidazole: A novel hypoxia marker for complementary study of tumor hypoxia and cell proliferation in cervical carcinoma. Gynecol. Oncol. 1998, 71, 270–277. [Google Scholar] [CrossRef]
- Ziemer, L.S.; Evans, S.M.; Kachur, A.V.; Shuman, A.L.; Cardi, C.A.; Jenkins, W.T.; Karp, J.S.; Alavi, A.; Dolbier, W.R., Jr.; Koch, C.J. Noninvasive imaging of tumor hypoxia in rats using the 2-nitroimidazole 18F-Efeuropean. J. Nucl. Med. Mol. Imaging 2003, 30, 259–266. [Google Scholar] [CrossRef]
- Brown, J.M. Evidence for acutely hypoxic cells in mouse tumours, and a possible mechanism of reoxygenation. Br. J. Radiol. 1979, 52, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Gray, L.H.; Conger, A.D.; Ebert, M.; Hornsey, S.; Scott, O.C. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br. J. Radiol. 1953, 26, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, F.; Vicario, N.; Spitale, F.M.; Cammarata, F.P.; Minafra, L.; Salvatorelli, L.; Russo, G.; Cuttone, G.; Valable, S.; Gulino, R.; et al. The Role of Hypoxia and SRC Tyrosine Kinase in Glioblastoma Invasiveness and Radioresistance. Cancers 2020, 12, 2860. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benej, M.; Wu, J.; Kreamer, M.; Kery, M.; Corrales-Guerrero, S.; Papandreou, I.; Williams, T.M.; Li, Z.; Graves, E.E.; Selmic, L.E.; et al. Pharmacological Regulation of Tumor Hypoxia in Model Murine Tumors and Spontaneous Canine Tumors. Cancers 2021, 13, 1696. https://doi.org/10.3390/cancers13071696
Benej M, Wu J, Kreamer M, Kery M, Corrales-Guerrero S, Papandreou I, Williams TM, Li Z, Graves EE, Selmic LE, et al. Pharmacological Regulation of Tumor Hypoxia in Model Murine Tumors and Spontaneous Canine Tumors. Cancers. 2021; 13(7):1696. https://doi.org/10.3390/cancers13071696
Chicago/Turabian StyleBenej, Martin, Jinghai Wu, McKenzie Kreamer, Martin Kery, Sergio Corrales-Guerrero, Ioanna Papandreou, Terence M. Williams, Zihai Li, Edward E. Graves, Laura E. Selmic, and et al. 2021. "Pharmacological Regulation of Tumor Hypoxia in Model Murine Tumors and Spontaneous Canine Tumors" Cancers 13, no. 7: 1696. https://doi.org/10.3390/cancers13071696





