Surveillance as Determinant of Long-Term Survival in Non-Transplanted Hepatocellular Carcinoma Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Multivariable Logistic Regression Analysis
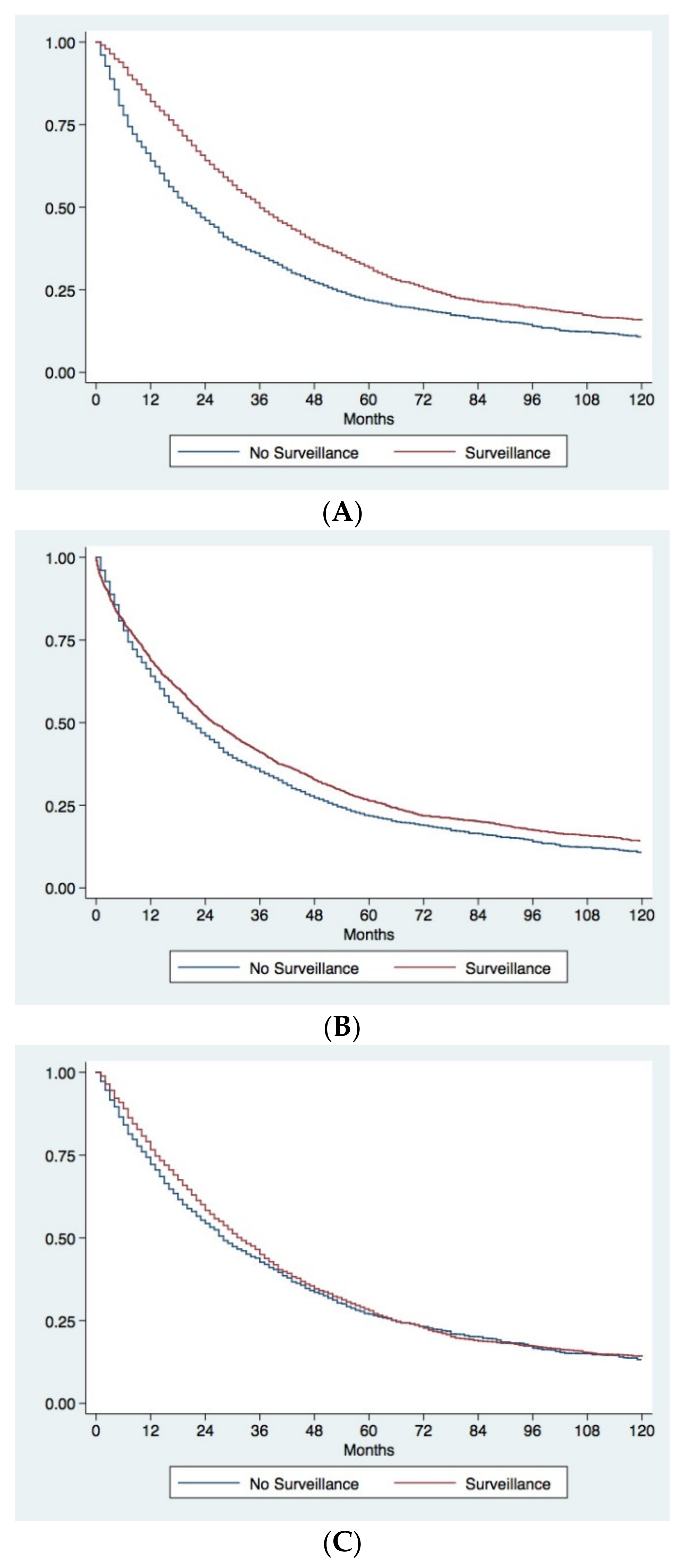
3.3. Survival Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fact Sheets by Population—Globocan—IARC n.d. Available online: https://gco.iarc.fr/today/fact-sheets-populations (accessed on 16 October 2020).
- Mittal, S.; El-Serag, H.B. Epidemiology of hepatocellular carcinoma: Consider the population. J. Clin. Gastroenterol. 2013, 47 S2–S6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucci, L.; Garuti, F.; Lenzi, B.; Pecorelli, A.; Farinati, F.; Giannini, E.G.; Granito, A.; Ciccarese, F.; Rapaccini, G.L.; Di Marco, M.; et al. The evolutionary scenario of hepatocellular carcinoma in Italy: An update. Liver Int. 2017, 37, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Italian Association of Cancer Registries (AIRTUM). Available online: https://www.registri-tumori.it/cms/pubblicazioni/i-numeri-del-cancro-italia-2019 (accessed on 16 October 2020).
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.L.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villa, E.; Critelli, R.; Lei, B.; Marzocchi, G.; Camma, C.; Giannelli, G.; Pontisso, P.; Cabibbo, G.; Enea, M.; Colopi, S.; et al. Neoangiogenesis-related genes are hallmarks of fast-growing hepatocellular carcinomas and worst survival. Results from a prospective study. Gut 2016, 65, 861–869. [Google Scholar] [CrossRef]
- Villa, E.; Moles, A.; Ferretti, I.; Buttafoco, P.; Grottola, A.; Del Buono, M.; De Santis, M.; Manenti, F. Natural history of inoperable hepatocellular carcinoma: Estrogen receptors’ status in the tumor is the strongest prognostic factor for survival. Hepatology 2000, 32, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, N.; Oka, M.; Yamada-okabe, H.; Nishida, M.; Maeda, Y.; Mori, N.; Takao, T. Oligonucleotide microarray for prediction of early intrahepatic recurrence of hepatocellular carcinoma after curative resection. Lancet 2003, 361, 923–929. [Google Scholar] [CrossRef]
- Hoshida, Y.; Villanueva, A.; Kobayashi, M.; Peix, J.; Chiang, D.Y.; Camargo, A.; Gupta, S.; Moore, J.; Wrobel, M.J.; Lerner, J.; et al. Gene Expression in Fixed Tissues and Outcome in Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 1995–2004. [Google Scholar] [CrossRef] [Green Version]
- Giannini, E.G.; Farinati, F.; Ciccarese, F.; Pecorelli, A.; Rapaccini, G.L.; Di Marco, M.; Benvegnù, L.; Caturelli, E.; Zoli, M.; Borzio, F.; et al. Prognosis of untreated hepatocellular carcinoma. Hepatology 2015, 61, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Garnelo, M.; Tan, A.; Her, Z.; Yeong, J.; Lim, C.J.; Chen, J.; Lim, K.H.; Weber, A.; Chow, P.; Chung, A.; et al. Interaction between tumour-infiltrating B cells and T cells controls the progression of hepatocellular carcinoma. Gut 2017, 66, 342–351. [Google Scholar] [CrossRef] [Green Version]
- Vitale, A.; Farinati, F.; Pawlik, T.M.; Frigo, A.C.; Giannini, E.G.; Napoli, L.; Ciccarese, F.; Rapaccini, G.L.; Di Marco, M.; Caturelli, E.; et al. The concept of therapeutic hierarchy for patients with hepatocellular carcinoma: A multicenter cohort study. Liver Int. 2019, 39, 1478–1489. [Google Scholar] [CrossRef]
- Zheng, J.; Kuk, D.; Gönen, M.; Balachandran, V.P.; Kingham, T.P.; Allen, P.J.; D’Angelica, M.I.; Jarnagin, W.R.; DeMatteo, R.P. Actual Ten-Year Survivors after Resection of Hepatocellular Carcinoma. Ann. Surg. Oncol. 2017, 24, 1358–1366. [Google Scholar] [CrossRef]
- Kim, K.H.; Choi, Y.-K. Long-term survival after resection of hepatocellular carcinoma. Korean J. Hepato-Biliary-Pancreat. Surg. 2012, 16, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-T.; Wang, C.-C.; Lu, L.-G.; Zhang, W.-D.; Zhang, F.-J.; Shi, F.; Li, C.-X. Hepatocellular carcinoma: Clinical study of long-term survival and choice of treatment modalities. World J. Gastroenterol. 2013, 19, 3649–3657. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Zhang, B.; Xu, Y.; Wang, W.; Shen, Y.; Zhang, A.; Xu, Z. Prospective study of early detection for primary liver cancer. J. Cancer Res. Clin. Oncol. 1997, 123, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.H.; Yang, B.H.; Tang, Z.Y. Randomized controlled trial of screening for hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2004, 130, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Trevisani, F.; De Notariis, S.; Rapaccini, G.; Farinati, F.; Benvegnù, L.; Zoli, M.; Grazi, G.L.; Del Poggio, P.; Di Nolfo, M.A.; Bernardi, M. Semiannual and annual surveillance of cirrhotic patients for hepatocellular carcinoma: Effects on cancer stage and patient survival (Italian experience). Am. J. Gastroenterol. 2002, 97, 734–744. [Google Scholar] [CrossRef]
- Trevisani, F.; Santi, V.; Gramenzi, A.; Di Nolfo, M.A.; Del Poggio, P.; Benvegnu, L.; Rapaccini, G.; Farinati, F.; Zoli, M.; Borzio, F.; et al. Surveillance for early diagnosis of hepatocellular carcinoma: Is it effective in intermediate/advanced cirrhosis? Am. J. Gastroenterol. 2007, 102, 2448–2457. [Google Scholar] [CrossRef]
- Santi, V.; Trevisani, F.; Gramenzi, A.; Grignaschi, A.; Mirici-Cappa, F.; Del Poggio, P.; Di Nolfo, M.A.; Benvegnu, L.; Farinati, F.; Zoli, M.; et al. Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. J. Hepatol. 2010, 53, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Thompson Coon, J.; Rogers, G.; Hewson, P.; Wright, D.; Anderson, R.; Jackson, S.; Ryder, S.; Cramp, M.; Stein, K. Surveillance of cirrhosis for hepatocellular carcinoma: A cost-utility analysis. Br. J. Cancer 2008, 98, 1166–1175. [Google Scholar] [CrossRef] [Green Version]
- Santagostino, E.; Colombo, M.; Rivi, M.; Rumi, M.G.; Rocino, A.; Linari, S.; Mannucci, P.M. A 6-month versus a 12-month surveillance for hepatocellular carcinoma in 559 hemophiliacs infected with the hepatitis C virus. Blood 2003, 102, 78–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannini, E.G.; Cucchetti, A.; Erroi, V.; Garuti, F.; Odaldi, F.; Trevisani, F. Surveillance for early diagnosis of hepatocellular carcinoma: How best to do it? World J. Gastroenterol. 2013, 19, 8808–8821. [Google Scholar] [CrossRef]
- Singal, A.; Volk, M.L.; Waljee, A.; Salgia, R.; Higgins, P.; Rogers, M.A.M.; Marrero, J.A. Meta-analysis: Surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment. Pharmacol. Ther. 2009, 30, 37–47. [Google Scholar] [CrossRef]
- Singal, A.G.; Pillai, A.; Tiro, J. Early Detection, Curative Treatment, and Survival Rates for Hepatocellular Carcinoma Surveillance in Patients with Cirrhosis: A Meta-analysis. PLoS Med. 2014, 11, e1001624. [Google Scholar] [CrossRef] [PubMed]
- Cucchetti, A.; Trevisani, F.; Pecorelli, A.; Erroi, V.; Farinati, F.; Ciccarese, F.; Rapaccini, G.L.; Di Marco, M.; Caturelli, E.; Giannini, E.G.; et al. Estimation of lead-time bias and its impact on the outcome of surveillance for the early diagnosis of hepatocellular carcinoma. J. Hepatol. 2014, 61, 333–341. [Google Scholar] [CrossRef]
- Roayaie, S.; Jibara, G.; Tabrizian, P.; Park, J.-W.; Yang, J.; Yan, L.; Schwartz, M.; Han, G.; Izzo, F.; Chen, M.; et al. The role of hepatic resection in the treatment of hepatocellular cancer. Hepatology 2015, 62, 440–451. [Google Scholar] [CrossRef]
- Farinati, F.; Vitale, A.; Spolverato, G.; Pawlik, T.M.; Huo, T.; Lee, Y.-H.; Frigo, A.C.; Giacomin, A.; Giannini, E.G.; Ciccarese, F.; et al. Development and Validation of a New Prognostic System for Patients with Hepatocellular Carcinoma. PLoS Med. 2016, 13, e1002006. [Google Scholar] [CrossRef]
- Borzio, M.; Dionigi, E.; Rossini, A.; Marignani, M.; Sacco, R.; De Sio, I.; Bertolini, E.; Francica, G.; Giacomin, A.; Parisi, G.; et al. External validation of the ITA.LI.CA prognostic system for patients with hepatocellular carcinoma: A multicenter cohort study. Hepatology 2018, 67, 2215–2225. [Google Scholar] [CrossRef] [PubMed]
- UCLA Institute for Digital Research & Education, Statistical Consulting. Available online: https://stats.idre.ucla.edu/stata/webbooks/logistic/chapter3/lesson-3-logistic-regression-diagnostics (accessed on 15 January 2021).
- Nattino, G.; Lemeshow, S.; Phillips, G.; Finazzi, S.; Bertolini, G. Assessing the Calibration of Dichotomous Outcome Models with the Calibration Belt. Stata J. Promot. Commun. Stat. Stata 2017, 17, 1003–1014. [Google Scholar] [CrossRef] [Green Version]
- McCaffrey, D.F.; Griffin, B.A.; Almirall, D.; Slaughter, M.E.; Ramchand, R.; Burgette, L.F. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat. Med. 2013, 32, 3388–3414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baraldi, A.N.; Enders, C.K. An introduction to modern missing data analyses. J. Sch. Psychol. 2010, 48, 5–37. [Google Scholar] [CrossRef]
- Llovet, J.M.; Bustamante, J.; Castells, A.; Vilana, R.; Ayuso, M.d.C.; Sala, M.; Brú, C.; Rodés, J.; Bruix, J. Natural history of untreated nonsurgical hepatocellular carcinoma: Rationale for the design and evaluation of therapeutic trials. Hepatology 1999, 29, 62–67. [Google Scholar] [CrossRef]
- Cabibbo, G.; Enea, M.; Attanasio, M.; Bruix, J.; Craxì, A.; Cammà, C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology 2010, 51, 1274–1283. [Google Scholar] [CrossRef] [Green Version]
- Farinati, F.; Vanin, V.; Giacomin, A.; Pozzan, C.; Cillo, U.; Vitale, A.; Di Nolfo, A.M.; Del Poggio, P.; Benvegnu’, L.; Rapaccini, G.; et al. BCLC stage B hepatocellular carcinoma and transcatheter arterial chemoembolization: A 20-year survey by the Italian Liver Cancer group. Liver Int. 2015, 35, 223–231. [Google Scholar] [CrossRef]
- Cillo, U.; Vitale, A.; Volk, M.L.; Frigo, A.C.; Grigoletto, F.; Brolese, A.; Zanus, G.; D’Amico, F.; Farinati, F.; Burra, P.; et al. The survival benefit of liver transplantation in hepatocellular carcinoma patients. Dig. Liver Dis. 2010, 42, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Vitale, A.; Burra, P.; Frigo, A.C.; Trevisani, F.; Farinati, F.; Spolverato, G.; Volk, M.; Giannini, E.G.; Ciccarese, F.; Piscaglia, F.; et al. Survival benefit of liver resection for patients with hepatocellular carcinoma across different Barcelona Clinic Liver Cancer stages: A multicentre study. J. Hepatol. 2015, 62, 617–624. [Google Scholar] [CrossRef]
- Tan, D.; Yopp, A.; Beg, M.S.; Gopal, P.; Singal, A.G. Meta analysis: Underutilization and disparities of treatment among patients with hepatocellular carcinoma in the united states. Aliment. Pharmacol. Ther. 2013, 38, 703–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparchez, Z.; Mocan, T.; Radu, P.; Mocan, L.P.; Sparchez, M.; Leucuta, D.C.; Al Hajjar, N. Prognostic factors after percutaneous radiofrequency ablation in the treatment of hepatocellular carcinoma. Impact of incomplete ablation on recurrence and overall survival rates. J. Gastrointest. Liver Dis. 2018, 27, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Ryu, T.; Takami, Y.; Wada, Y.; Hara, T.; Sasaki, S.; Saitsu, H. Actual 10-Year Survival After Surgical Microwave Ablation for Hepatocellular Carcinoma: A Single-Center Experience in Japan. Ann. Surg. Oncol. 2019, 26, 4126–4133. [Google Scholar] [CrossRef]
- Kadalayil, L.; Benini, R.; Pallan, L.; O’Beirne, J.; Marelli, L.; Yu, D.; Hackshaw, A.; Fox, R.; Johnson, P.; Burroughs, A.K.; et al. A Simple Prognostic Scoring System for Patients Receiving Transarterial Embolisation for Hepatocellular Cancer. Ann. Oncol. 2013, 24, 2565–2570. [Google Scholar] [CrossRef]
- Kim, B.K.; Kim, S.U.; Kim, K.A.; Chung, Y.E.; Kim, M.J.; Park, M.S.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Kim, M.D.; et al. Complete response at first chemoembolization is still the most robust predictor for favorable outcome in hepatocellular carcinoma. J. Hepatol. 2015, 62, 1304–1310. [Google Scholar] [CrossRef]
- Lu, L.C.; Shao, Y.Y.; Chan, S.Y.; Hsu, C.H.; Cheng, A.L. Clinical characteristics of advanced hepatocellular carcinoma patients with prolonged survival in the era of anti-angiogenic targeted-therapy. Anticancer Res. 2014, 34, 1047–1052. [Google Scholar] [PubMed]
- Lombardi, G.; Zustovich, F.; Farinati, F.; Cillo, U.; Vitale, A.; Zanus, G.; Donach, M.; Farina, M.; Zovato, S.; Pastorelli, D. Pegylated liposomal doxorubicin and gemcitabine in patients with advanced hepatocellular carcinoma: Results of a phase 2 study. Cancer 2011, 117, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Davila, J.A.; Henderson, L.; Kramer, J.R.; Kanwal, F.; Richardson, P.A.; Duan, Z.; El-Serag, H.B. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann. Intern. Med. 2011, 154, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Yopp, A.; Skinner, C.S.; Packer, M.; Lee, W.M.; Tiro, J.A. Utilization of hepatocellular carcinoma surveillance among American patients: A systematic review. J. Gen. Intern. Med. 2012, 27, 861–867. [Google Scholar] [CrossRef] [Green Version]
- Garuti, F.; Neri, A.; Avanzato, F.; Gramenzi, A.; Rampoldi, D.; Rucci, P.; Farinati, F.; Giannini, E.G.; Piscaglia, F.; Rapaccini, G.L.; et al. The changing scenario of hepatocellular carcinoma in Italy: An update. Liver Int. 2020. [Google Scholar] [CrossRef]
- Boonstra, K.; Weersma, R.K.; van Erpecum, K.J.; Rauws, E.A.; Spanier, B.W.M.; Poen, A.C.; van Nieuwkerk, K.M.; Drenth, J.P.; Witteman, B.J.; Tuynman, H.A.; et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology 2013, 58, 2045–2055. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [Green Version]
- Vitale, A.; Trevisani, F.; Farinati, F.; Cillo, U. Treatment of hepatocellular carcinoma in the Precision Medicine era: From treatment stage migration to therapeutic hierarchy. Hepatology 2020, 72, 2206–2218. [Google Scholar] [CrossRef]
- Park, J.W.; Chen, M.; Colombo, M.; Roberts, L.R.; Schwartz, M.; Chen, P.J.; Kudo, M.; Johnson, P.; Wagner, S.; Orsini, L.S.; et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: The BRIDGE Study. Liver Int. 2015, 35, 2155–2166. [Google Scholar] [CrossRef] [Green Version]
- Giannini, E.G.; Bucci, L.; Garuti, F.; Brunacci, M.; Lenzi, B.; Valente, M.; Caturelli, E.; Cabibbo, G.; Piscaglia, F.; Virdone, R.; et al. Patients with advanced hepatocellular carcinoma need a personalized management: A lesson from clinical practice. Hepatology 2018, 67, 1784–1796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sangiovanni, A.; Triolo, M.; Iavarone, M.; Forzenigo, L.V.; Nicolini, A.; Rossi, G.; La Mura, V.; Colombo, M.; Lampertico, P. Multimodality treatment of hepatocellular carcinoma: How field practice complies with international recommendations. Liver Int. 2018, 38, 1624–1634. [Google Scholar] [CrossRef] [PubMed]
- Cucchetti, A.; Garuti, F.; Pinna, A.D.; Trevisani, F. Length time bias in surveillance for hepatocellular carcinoma and how to avoid it. Hepatol. Res. 2016, 46, 1275–1280. [Google Scholar] [CrossRef]
- Kanwal, F.; Singal, A.G. Surveillance for Hepatocellular Carcinoma: Current Best Practice and Future Direction. Gastroenterology 2019, 157, 54–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Variable | Cases—LS n = 1028 | Controls—SS n = 2721 | p† | |
|---|---|---|---|---|
| Gender - males | 791 (76.9) | 2067 (76.0) | 0.55 | |
| Age (years) | 69 (62–74) | 69 (62–75) | 0.04 | |
| BMI (kg/m2) | ||||
| ≤25 | 661 (64.3) | 1977 (72.7) | <0.0001 | |
| 25–30 | 264 (25.7) | 532 (19.5) | ||
| >30 | 103 (10.0) | 212 (7.8) | ||
| T2DM | 339 (33.0) | 891 (32.7) | 0.91 | |
| Cirrhosis | 943 (91.7) | 2586 (95.0) | 0.0002 | |
| Viral etiology | 712 (69.3) | 1899 (69.8) | 0.75 | |
| MAFLD | 146 (14.2) | 327 (12.0) | 0.08 | |
| CSPH | 740 (72.0) | 2263 (83.2) | <0.0001 | |
| Child-Pugh class | ||||
| A | 893 (86.9) | 1850 (68.0) | <0.0001 | |
| B | 135 (13.1) | 871 (32.0) | ||
| MELD | 9 (7–10) | 10 (8–12) | <0.0001 | |
| Surveillance | 698 (67.9) | 1516 (55.7) | <0.0001 | |
| ECOG-PS 0 | 893 (86.9) | 1880 (69.1) | <0.0001 | |
| Multifocality | 257 (25.0) | 1476 (54.2) | <0.0001 | |
| Number of nodules | 1 (1–2) | 2 (1–4) | <0.0001 | |
| Diameter (cm) | 2.7 (2.0–3.7) | 3.5 (2.3–5.3) | <0.0001 | |
| MVI | 35 (3.4) | 432 (15.9) | <0.0001 | |
| EHS | 8 (0.8) | 128 (4.7) | <0.0001 | |
| AFP ≤ 200 ng/mL | 816 (79.4) | 1802 (66.2) | <0.0001 | |
| ITA.LI.CA staging system | ||||
| A | 431 (42.0) | 582 (21.4) | <0.0001 | |
| B1 | 198 (19.3) | 701 (25.8) | ||
| B2 | 59 (5.7) | 290 (10.6) | ||
| B3 | 29 (2.8) | 233 (8.6) | ||
| C | 27 (2.6) | 310 (11.4) | ||
| D | 19 (1.8) | 273 (10.0) | ||
| Main treatment | ||||
| LR | 301 (29.3) | 310 (11.4) | <0.0001 | |
| ABL | 487 (47.4) | 757 (27.8) | ||
| IAT | 138 (13.4) | 743 (27.3) | ||
| SOR | 11 (1.1) | 166 (6.1) | ||
| Other | 91 (8.8) | 745 (27.4) | ||
| Management in “Low-volume” Institutions | 338 (32.9) | 550 (20.2) | <0.0001 | |
| Variable | Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | p | aOR (95% CI) | p | ||
| Surveillance | No | Ref | - | Ref | - |
| Yes | 1.681 (1.445–1.956) | <0.0001 | 1.413 (1.195–1.671) | <0.0001 | |
| Gender | Female | Ref | - | ||
| Male | 0.947 (0.799–1.122) | 0.53 | |||
| Age † | 0.993 (0.986–0.999) | 0.04 | 0.989 (0.982–0.997) | 0.008 | |
| BMI (kg/m2) | ≤25 | Ref | - | ||
| >25 | 1.475 (1.266-.719) | <0.0001 | |||
| T2DM | No | Ref | - | ||
| Yes | 1.011 (0.867–1.177) | 0.89 | |||
| Cirrhosis | No | Ref | - | ||
| Yes | 0.579 (0.437–0.767) | <0.0001 | |||
| Viral Etiology | No | Ref | - | ||
| Yes | 0.975 (0.835–1.140) | 0.75 | |||
| MAFLD | No | Ref | - | Ref | - |
| Yes | 1.212 (0.983–1.495) | 0.07 | 1.299 (1.032–1.636) | 0.03 | |
| CSPH | No | Ref | - | Ref | - |
| Yes | 0.520 (0.439–0.616) | <0.0001 | 0.705 (0.582–0.854) | 0.0003 | |
| Child-Pugh | A | Ref | - | ||
| B | 0.321 (0.263–0.391) | <0.0001 | |||
| MELD † | 0.840 (0.816–0865) | <0.0001 | 0.877 (0.850–0.905) | <0.0001 | |
| ECOG-PS | 0 | Ref | - | ||
| ≥1 | 0.338 (0.277–0.412) | <0.0001 | |||
| Multifocality | No | Ref | - | ||
| Yes | 0.281 (0.240–0.330) | <0.0001 | |||
| Diameter (cm) | ≤5 | Ref | - | ||
| >5 | 0.325 (0.261–0.405) | <0.0001 | |||
| MVI | No | Ref | - | ||
| Yes | 0.187 (0.131–0.266) | <0.0001 | |||
| EHS | No | Ref | - | ||
| Yes | 0.159 (0.077–0.326) | <0.0001 | |||
| AFP (ng/mL) | ≤200 | Ref | - | ||
| >200 | 0.509 (0.429–0.604) | <0.0001 | |||
| ITA.LI.CA stage | 0–A | Ref | - | ||
| B–D | 0.241 (0.207–0.281) | <0.0001 | |||
| Treatment | Palliative | Ref | - | Ref | - |
| Curative | 4.810 (4.083–5.667) | <0.0001 | 3.924 (3.312–4.650) | <0.0001 | |
| ITA.LI.CA Institution | HV | Ref | - | Ref | - |
| LV | 1.934 (1.647–2.270) | <0.0001 | 1.741 (1.463–2.070) | <0.0001 | |
| Variable | Before IPW | After IPW | |||||
|---|---|---|---|---|---|---|---|
| Surveillance (n = 2214) | No Surveillance (n = 1535) | p† | Surveillance (n = 2215) | No Surveillance (n = 1531) | p† | ||
| Gender—males | 1621 (73.2) | 1237 (80.6) | <0.0001 | 1676 (75.7) | 1158 (75.7) | 0.97 | |
| Age—≤70 years | 1250 (56.5) | 859 (56.0) | 0.76 | 1228 (55.5) | 853 (55.7) | 0.95 | |
| BMI >25 kg/m2 | 621 (28.0) | 490 (31.9) | 0.01 | 627 (28.3) | 451 (29.4) | 0.46 | |
| T2DM | 681 (30.8) | 549 (35.8) | 0.002 | 708 (32.0) | 505 (33.0) | 0.52 | |
| Cirrhosis | 2140 (96.7) | 1389 (90.5) | <0.0001 | 2089 (94.3) | 1443 (94.2) | 0.94 | |
| Viral etiology | 1723 (77.8) | 888 (57.8) | <0.0001 | 1544 (69.7) | 1062 (69.4) | 0.83 | |
| MAFLD | 199 (9.0) | 274 (17.8) | <0.0001 | 260 (11.7) | 194 (12.6) | 0.39 | |
| CSPH | 1833 (82.8) | 1170 (76.2) | <0.0001 | 1792 (80.9) | 1235 (80.7) | 0.90 | |
| Child-Pugh A | 1699 (76.7) | 1044 (68.0) | <0.0001 | 1606 (72.5) | 1106 (72.2) | 0.82 | |
| MELD >10 | 845 (38.2) | 591 (38.5) | 0.84 | 848 (38.3) | 584 (38.1) | 0.97 | |
| ECOG-PS 0 | 1799 (81.3) | 974 (63.5) | <0.0001 | 1641 (74.1) | 1129 (73.8) | 0.82 | |
| Multifocality | 852 (38.5) | 881 (57.4) | <0.0001 | 1025 (46.3) | 708 (46.2) | 1.00 | |
| Diameter >5 cm | 207 (9.4) | 597 (38.9) | <0.0001 | 475 (21.4) | 331 (21.6) | 0.90 | |
| MVI | 155 (7.0) | 312 (20.3) | <0.0001 | 290 (13.1) | 197 (12.9) | 0.88 | |
| EHS | 35 (1.6) | 101 (6.6) | <0.0001 | 86 (3.9) | 56 (3.7) | 0.93 | |
| AFP ≤ 200 ng/mL | 1628 (73.5) | 990 (64.5) | <0.0001 | 1559 (70.4) | 1073 (70.1) | 0.83 | |
| ITA.LI.CA stage | |||||||
| 0–A | 1184 (53.5) | 426 (27.8) | <0.0001 | 944 (42.6) | 652 (42.5) | 0.95 | |
| B–D | 1030 (46.5) | 1109 (72.2) | 1271 (57.4) | 880 (57.5) | |||
| Treatment | |||||||
| LR + ABL | 1299 (58.7) | 602 (39.2) | <0.0001 | 1120 (50.6) | 771 (50.4) | 0.89 | |
| IAT + SOR + Other | 915 (41.3) | 933 (60.8) | 1094 (49.4) | 760 (49.6) | |||
| “Low-volume” Institutions | 543 (24.5) | 345 (22.5) | 0.15 | 537 (24.2) | 374 (24.4) | 0.88 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelizzaro, F.; Vitale, A.; Sartori, A.; Vieno, A.; Penzo, B.; Russo, F.P.; Frigo, A.C.; Giannini, E.G.; Piccinnu, M.; Rapaccini, G.L.; et al. Surveillance as Determinant of Long-Term Survival in Non-Transplanted Hepatocellular Carcinoma Patients. Cancers 2021, 13, 897. https://doi.org/10.3390/cancers13040897
Pelizzaro F, Vitale A, Sartori A, Vieno A, Penzo B, Russo FP, Frigo AC, Giannini EG, Piccinnu M, Rapaccini GL, et al. Surveillance as Determinant of Long-Term Survival in Non-Transplanted Hepatocellular Carcinoma Patients. Cancers. 2021; 13(4):897. https://doi.org/10.3390/cancers13040897
Chicago/Turabian StylePelizzaro, Filippo, Alessandro Vitale, Anna Sartori, Andrea Vieno, Barbara Penzo, Francesco Paolo Russo, Anna Chiara Frigo, Edoardo G Giannini, Manuela Piccinnu, Gian Ludovico Rapaccini, and et al. 2021. "Surveillance as Determinant of Long-Term Survival in Non-Transplanted Hepatocellular Carcinoma Patients" Cancers 13, no. 4: 897. https://doi.org/10.3390/cancers13040897






