Genome Instability in Multiple Myeloma: Facts and Factors
Abstract
:Simple Summary
Abstract
1. Clinical Manifestation of Multiple Myeloma and Recent Research Approaches
1.1. Clinical Characteristics of Pre-MM and MM: Heterogeneity and Clonal Evolution of Cancer Cells
1.2. Current Treatment Algorithms of MM: Mechanisms of Action of Anti-MM Agents
2. Predisposition to MM
3. Mutational Landscape of MM and Its Precursors
4. Mutational Signatures in MM Genomes
5. Structural Variations in the MM Genomes
5.1. Driving Genomic Rearrangements in MM
5.2. Mechanisms of Genomic Instability at 14q32 and 8q24 Regions
5.3. Complex Chromosomal Rearrangements in MM and Their Mechanisms
6. Telomere Maintenance Pathways and MM Risk
7. Conclusions and Further Perspectives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rajkumar, S.V. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am. J. Hematol. 2020, 95, 548–567. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V. Updated Diagnostic Criteria and Staging System for Multiple Myeloma. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, e418–e423. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Kyle, R. Monoclonal gammopathy of undetermined significance. Natural history in 241 cases. Am. J. Med. 1978, 64, 814–826. [Google Scholar] [CrossRef]
- Dhodapkar, M.V. MGUS to myeloma: A mysterious gammopathy of underexplored significance. Blood 2016, 128, 2599–2606. [Google Scholar] [CrossRef]
- Dispenzieri, A.; Katzmann, J.A.; Kyle, R.A.; Larson, D.R.; Melton, L.J.; Colby, C.L.; Therneau, T.M.; Clark, R.; Kumar, S.K.; Bradwell, A.; et al. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: A retrospective population-based cohort study. Lancet 2010, 375, 1721–1728. [Google Scholar] [CrossRef]
- Kyle, R.A.; Therneau, T.M.; Rajkumar, S.V.; Larson, D.R.; Plevak, M.F.; Offord, J.R.; Dispenzieri, A.; Katzmann, J.A.; Melton, L.J. Prevalence of Monoclonal Gammopathy of Undetermined Significance. N. Engl. J. Med. 2006, 354, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Chudasama, R.; Barth, P. Risk Stratification of Precursors to Multiple Myeloma in 2020. Rhode Isl. Med. J. 2020, 103, 46–47. [Google Scholar]
- Kyle, R.A.; Therneau, T.M.; Rajkumar, S.V.; Offord, J.R.; Larson, D.R.; Plevak, M.F.; Melton, L.J. A Long-Term Study of Prognosis in Monoclonal Gammopathy of Undetermined Significance. N. Engl. J. Med. 2002, 346, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Kyle, R.A.; Larson, D.R.; Therneau, T.M.; Dispenzieri, A.; Kumar, S.; Cerhan, J.R.; Rajkumar, S.V. Long-Term Follow-up of Monoclonal Gammopathy of Undetermined Significance. N. Engl. J. Med. 2018, 378, 241–249. [Google Scholar] [CrossRef]
- Landgren, O.; Kyle, R.A.; Pfeiffer, R.M.; Katzmann, J.A.; Caporaso, N.E.; Hayes, R.B.; Dispenzieri, A.; Kumar, S.; Clark, R.J.; Baris, D.; et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: A prospective study. Blood 2009, 113, 5412–5417. [Google Scholar] [CrossRef] [PubMed]
- Kyle, R.; Greipp, P. Smoldering multiple myeloma. N. Engl. J. Med. 1980, 302, 1347–1349. [Google Scholar] [CrossRef]
- Kyle, R.A.; Remstein, E.D.; Therneau, T.M.; Dispenzieri, A.; Kurtin, P.J.; Hodnefield, J.M.; Larson, D.R.; Plevak, M.F.; Jelinek, D.F.; Fonseca, R.; et al. Clinical Course and Prognosis of Smoldering (Asymptomatic) Multiple Myeloma. N. Engl. J. Med. 2007, 356, 2582–2590. [Google Scholar] [CrossRef]
- Weiss, B.M.; Abadie, J.; Verma, P.; Howard, R.S.; Kuehl, W.M. A monoclonal gammopathy precedes multiple myeloma in most patients. Blood 2009, 113, 5418–5422. [Google Scholar] [CrossRef]
- Lonial, S.; Dhodapkar, M.V.; Rajkumar, S.V. Smoldering myeloma and the art of war. J. Clin. Oncol. 2020, 38, 2363–2365. [Google Scholar] [CrossRef] [PubMed]
- Kyle, R.A.; Dispenzieri, A.; Kumar, S.; Larson, D.; Therneau, T.; Vincent Rajkumar, S. IgM monoclonal gammopathy of undetermined significance (MGUS) and smoldering Waldenström’s macroglobulinemia (SWM). Clin. Lymphoma Myeloma Leuk. 2011, 11, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Girard, L.P.; Soekojo, C.Y.; Ooi, M.; Poon, L.M.; Chng, W.J.; de Mel, S. Immunoglobulin M paraproteinaemias. Cancers 2020, 12, 1688. [Google Scholar] [CrossRef]
- Kyle, R.A.; Gertz, M.A.; Witzig, T.E.; Lust, J.A.; Lacy, M.Q.; Dispenzieri, A.; Fonseca, R.; Rajkumar, S.V.; Offord, J.R.; Larson, D.R.; et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin. Proc. 2003, 78, 21–33. [Google Scholar] [CrossRef]
- Chawla, S.S.; Kumar, S.K.; Dispenzieri, A.; Greenberg, A.J.; Larson, D.R.; Kyle, R.A.; Lacy, M.Q.; Gertz, M.A.; Rajkumar, S.V. Clinical course and prognosis of non-secretory multiple myeloma. Eur. J. Haematol. 2015, 95, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.K.; Fink, J.L.; Grady, J.P.; Morgan, G.J.; Mullighan, C.G.; To, L.B.; Hewett, D.R.; Zannettino, A.C.W. Subclonal evolution in disease progression from MGUS/SMM to multiple myeloma is characterised by clonal stability. Leukemia 2018, 33, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Rasche, L.; Chavan, S.S.; Stephens, O.W.; Patel, P.; Tytarenko, R.; Ashby, C.; Bauer, M.; Stein, C.; Deshpande, S.; Wardell, C.; et al. Spatial genomic heterogeneity in multiple myeloma revealed by multi-region sequencing. Nat. Commun. 2017, 8, 268. [Google Scholar] [CrossRef] [PubMed]
- Keats, J.J.; Chesi, M.; Egan, J.B.; Garbitt, V.M.; Palmer, S.E.; Braggio, E.; Van Wier, S.; Blackburn, P.R.; Baker, A.S.; Dispenzieri, A.; et al. Clonal competition with alternating dominance in multiple myeloma. Blood 2012, 120, 1067–1076. [Google Scholar] [CrossRef]
- Walker, B.A.; Wardell, C.P.; Melchor, L.; Brioli, A.; Johnson, D.C.; Kaiser, M.F.; Mirabella, F.; Lopez-Corral, L.; Humphray, S.; Murray, L.; et al. Intraclonal heterogeneity is a critical early event in the development of myeloma and precedes the development of clinical symptoms. Leukemia 2014, 28, 384–390. [Google Scholar] [CrossRef]
- Kortüm, K.M.; Langer, C.; Monge, J.; Bruins, L.; Zhu, Y.X.; Shi, C.X.; Jedlowski, P.; Egan, J.B.; Ojha, J.; Bullinger, L.; et al. Longitudinal analysis of 25 sequential sample-pairs using a custom multiple myeloma mutation sequencing panel (M3P). Ann. Hematol. 2015, 94, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Weinhold, N.; Ashby, C.; Rasche, L.; Chavan, S.S.; Stein, C.; Stephens, O.W.; Tytarenko, R.; Bauer, M.A.; Meissner, T.; Deshpande, S.; et al. Clonal selection and double-hit events involving tumor suppressor genes underlie relapse in myeloma. Blood 2016, 128, 1735–1744. [Google Scholar] [CrossRef]
- Egan, J.B.; Shi, C.X.; Tembe, W.; Christoforides, A.; Kurdoglu, A.; Sinari, S.; Middha, S.; Asmann, Y.; Schmidt, J.; Braggio, E.; et al. Whole-genome sequencing of multiple myeloma from diagnosis to plasma cell leukemia reveals genomic initiating events, evolution, and clonal tides. Blood 2012, 120, 1060–1066. [Google Scholar] [CrossRef]
- Melchor, L.; Brioli, A.; Wardell, C.P.; Murison, A.; Potter, N.E.; Kaiser, M.F.; Fryer, R.A.; Johnson, D.C.; Begum, D.B.; Wilson, S.H.; et al. Single-cell genetic analysis reveals the composition of initiating clones and phylogenetic patterns of branching and parallel evolution in myeloma. Leukemia 2014, 28, 1705–1715. [Google Scholar] [CrossRef] [PubMed]
- Lohr, J.G.; Stojanov, P.; Carter, S.L.; Cruz-Gordillo, P.; Lawrence, M.S.; Auclair, D.; Sougnez, C.; Knoechel, B.; Gould, J.; Saksena, G.; et al. Widespread genetic heterogeneity in multiple myeloma: Implications for targeted therapy. Cancer Cell 2014, 25, 91–101. [Google Scholar] [CrossRef]
- Bolli, N.; Avet-Loiseau, H.; Wedge, D.C.; Van Loo, P.; Alexandrov, L.B.; Martincorena, I.; Dawson, K.J.; Iorio, F.; Nik-Zainal, S.; Bignell, G.R.; et al. Heterogeneity of genomic evolution and mutational profiles in multiple myeloma. Nat. Commun. 2014, 5, 1–13. [Google Scholar] [CrossRef]
- Ledergor, G.; Weiner, A.; Zada, M.; Wang, S.Y.; Cohen, Y.C.; Gatt, M.E.; Snir, N.; Magen, H.; Koren-Michowitz, M.; Herzog-Tzarfati, K.; et al. Single cell dissection of plasma cell heterogeneity in symptomatic and asymptomatic myeloma. Nat. Med. 2018, 24, 1867–1876. [Google Scholar] [CrossRef]
- Bochtler, T.; Merz, M.; Hielscher, T.; Granzow, M.; Hoffmann, K.; Krämer, A.; Raab, M.S.; Hillengass, J.; Seckinger, A.; Kimmich, C.; et al. Cytogenetic intraclonal heterogeneity of plasma cell dyscrasia in AL amyloidosis as compared with multiple myeloma. Blood Adv. 2018, 2, 2607–2618. [Google Scholar] [CrossRef] [PubMed]
- Paíno, T.; Paiva, B.; Sayagués, J.M.; Mota, I.; Carvalheiro, T.; Corchete, L.A.; Aires-Mejía, I.; Pérez, J.J.; Sanchez, M.L.; Barcena, P.; et al. Phenotypic identification of subclones in multiple myeloma with different chemoresistant, cytogenetic and clonogenic potential. Leukemia 2015, 29, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Morgan, G.J.; Walker, B.A.; Davies, F.E. The genetic architecture of multiple myeloma. Nat. Rev. Cancer 2012, 12, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.A.; Wardell, C.P.; Melchor, L.; Hulkki, S.; Potter, N.E.; Johnson, D.; Fenwick, K.; Kozarewa, I.; Gonzalez, D.; Lord, C.; et al. Intraclonal heterogeneity and distinct molecular mechanisms characterize the development of t(4;14) and t(11;14) myeloma. Blood 2012, 120, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Nowell, P.C. The clonal evolution of tumor cell populations. Science 1976, 194, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Greaves, M.; Maley, C.C. Clonal evolution in cancer. Nature 2012, 481, 306–313. [Google Scholar] [CrossRef]
- Pon, J.R.; Marra, M.A. Driver and passenger mutations in cancer. Annu. Rev. Pathol. Mech. Dis. 2015, 10, 25–50. [Google Scholar] [CrossRef] [PubMed]
- Baylin, S.B.; Jones, P.A. Epigenetic determinants of cancer. Cold Spring Harb. Perspect. Biol. 2016, 8, a019505. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, S.; Swanton, C.; Taylor, B.S.; Costello, J.F. Treatment-Induced Mutagenesis and Selective Pressures Sculpt Cancer Evolution. Cold Spring Harb. Perspect. Med. 2017, 7, a026617. [Google Scholar] [CrossRef]
- Chatterjee, A.; Rodger, E.J.; Eccles, M.R. Epigenetic drivers of tumourigenesis and cancer metastasis. Semin. Cancer Biol. 2018, 51, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.; Gao, R.; Navin, N. Tumor evolution: Linear, branching, neutral or punctuated? Biochim. Biophys. Acta 2017, 1867, 151. [Google Scholar] [CrossRef]
- Furukawa, Y.; Kikuchi, J. Molecular basis of clonal evolution in multiple myeloma. Int. J. Hematol. 2020, 111, 496–511. [Google Scholar] [CrossRef] [PubMed]
- Bahlis, N.J. Darwinian evolution and tiding clones in multiple myeloma. Blood 2012, 120, 927–928. [Google Scholar] [CrossRef]
- Magrangeas, F.; Avet-Loiseau, H.; Gouraud, W.; Lodé, L.; Decaux, O.; Godmer, P.; Garderet, L.; Voillat, L.; Facon, T.; Stoppa, A.M.; et al. Minor clone provides a reservoir for relapse in multiple myeloma. Leukemia 2013, 27, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R.; Weinhold, N.; Ashby, C.; Walker, B.A.; Wardell, C.; Pawlyn, C.; Rasche, L.; Melchor, L.; Cairns, D.A.; Gregory, W.M.; et al. Clonal evolution in myeloma: The impact of maintenance lenalidomide and depth of response on the genetics and sub-clonal structure of relapsed disease in uniformly treated newly diagnosed patients. Haematologica 2019, 104, 1440–1450. [Google Scholar] [CrossRef] [PubMed]
- Diamond, B.; Yellapantula, V.; Rustad, E.H.; Maclachlan, K.H.; Mayerhoefer, M.; Kaiser, M.; Morgan, G.; Landgren, O.; Maura, F. Positive selection as the unifying force for clonal evolution in multiple myeloma. Leukemia 2021, 35, 1511–1515. [Google Scholar] [CrossRef]
- Bolli, N.; Maura, F.; Minvielle, S.; Gloznik, D.; Szalat, R.; Fullam, A.; Martincorena, I.; Dawson, K.J.; Samur, M.K.; Zamora, J.; et al. Genomic patterns of progression in smoldering multiple myeloma. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bustoros, M.; Sklavenitis-Pistofidis, R.; Park, J.; Redd, R.; Zhitomirsky, B.; Dunford, A.J.; Salem, K.; Tai, Y.T.; Anand, S.; Mouhieddine, T.H.; et al. Genomic profiling of smoldering multiple myeloma identifies patients at a high risk of disease progression. J. Clin. Oncol. 2020, 38, 2380–2389. [Google Scholar] [CrossRef]
- Johnson, D.C.; Lenive, O.; Mitchell, J.; Jackson, G.; Owen, R.; Drayson, M.; Cook, G.; Jones, J.R.; Pawlyn, C.; Davies, F.E.; et al. Neutral tumor evolution in myeloma is associated with poor prognosis. Blood 2017, 130, 1639–1643. [Google Scholar] [CrossRef]
- Merz, M.; Hielscher, T.; Schult, D.; Mai, E.K.; Raab, M.S.; Hillengass, J.; Seckinger, A.; Hose, D.; Granzow, M.; Jauch, A.; et al. Cytogenetic subclone formation and evolution in progressive smoldering multiple myeloma. Leukemia 2020, 34, 1192–1196. [Google Scholar] [CrossRef]
- Boyle, E.M.; Deshpande, S.; Tytarenko, R.; Ashby, C.; Wang, Y.; Bauer, M.A.; Johnson, S.K.; Wardell, C.P.; Thanendrarajan, S.; Zangari, M.; et al. The molecular make up of smoldering myeloma highlights the evolutionary pathways leading to multiple myeloma. Nat. Commun. 2021, 12, 293. [Google Scholar] [CrossRef]
- Mikulasova, A.; Wardell, C.P.; Murison, A.; Boyle, E.M.; Jackson, G.H.; Smetana, J.; Kufova, Z.; Pour, L.; Sandecka, V.; Almasi, M.; et al. The spectrum of somatic mutations in monoclonal gammopathy of undetermined significance indicates a less complex genomic landscape than that in multiple myeloma. Haematologica 2017, 102, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Corre, J.; Cleynen, A.; Robiou du Pont, S.; Buisson, L.; Bolli, N.; Attal, M.; Munshi, N.; Avet-Loiseau, H. Multiple myeloma clonal evolution in homogeneously treated patients. Leukemia 2018, 32, 2636–2647. [Google Scholar] [CrossRef] [PubMed]
- Ziccheddu, B.; Biancon, G.; Bagnoli, F.; De Philippis, C.; Maura, F.; Rustad, E.H.; Dugo, M.; Devecchi, A.; De Cecco, L.; Sensi, M.; et al. Integrative analysis of the genomic and transcriptomic landscape of double-refractory multiple myeloma. Blood Adv. 2020, 4, 830–844. [Google Scholar] [CrossRef] [PubMed]
- López-Corral, L.; Gutiérrez, N.C.; Vidriales, M.B.; Mateos, M.V.; Rasillo, A.; García-Sanz, R.; Paiva, B.; San Miguel, J.F. The progression from MGUS to smoldering myeloma and eventually to multiple myeloma involves a clonal expansion of genetically abnormal plasma cells. Clin. Cancer Res. 2011, 17, 1692–1700. [Google Scholar] [CrossRef]
- Mailankody, S.; Kazandjian, D.; Korde, N.; Roschewski, M.; Manasanch, E.; Bhutani, M.; Tageja, N.; Kwok, M.; Zhang, Y.; Zingone, A.; et al. Baseline mutational patterns and sustained MRD negativity in patients with high-risk smoldering myeloma. Blood Adv. 2017, 1, 1911–1918. [Google Scholar] [CrossRef] [PubMed]
- Maura, F.; Bolli, N.; Rustad, E.H.; Hultcrantz, M.; Munshi, N.; Landgren, O. Moving from Cancer Burden to Cancer Genomics for Smoldering Myeloma: A Review. JAMA Oncol. 2020, 6, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Samur, M.K.; Samur, A.A.; Fulciniti, M.; Szalat, R.; Han, T.; Shammas, M.; Richardson, P.; Magrangeas, F.; Minvielle, S.; Corre, J.; et al. Genome-wide somatic alterations in multiple myeloma reveal a superior outcome group. J. Clin. Oncol. 2020, 38, 3107–3118. [Google Scholar] [CrossRef]
- Samur, M.K.; Roncador, M.; Aktas-Samur, A.; Fulciniti, M.; Bazarbachi, A.H.; Szalat, R.; Shammas, M.A.; Sperling, A.S.; Richardson, P.G.; Magrangeas, F.; et al. High-Dose Melphalan Significantly Increases Mutational Burden in Multiple Myeloma Cells at Relapse: Results from a Randomized Study in Multiple Myeloma. Blood 2020, 136, 4–5. [Google Scholar] [CrossRef]
- Hoang, P.H.; Cornish, A.J.; Sherborne, A.L.; Chubb, D.; Kimber, S.; Jackson, G.; Morgan, G.J.; Cook, G.; Kinnersley, B.; Kaiser, M.; et al. An enhanced genetic model of relapsed IGH-translocated multiple myeloma evolutionary dynamics. Blood Cancer J. 2020, 10, 101. [Google Scholar] [CrossRef]
- Maura, F.; Bolli, N.; Angelopoulos, N.; Dawson, K.J.; Leongamornlert, D.; Martincorena, I.; Mitchell, T.J.; Fullam, A.; Gonzalez, S.; Szalat, R.; et al. Genomic landscape and chronological reconstruction of driver events in multiple myeloma. Nat. Commun. 2019, 10, 3835. [Google Scholar] [CrossRef]
- Oben, B.; Froyen, G.; Maclachlan, K.H.; Leongamornlert, D.; Abascal, F.; Zheng-Lin, B.; Yellapantula, V.; Derkach, A.; Geerdens, E.; Diamond, B.T.; et al. Whole-genome sequencing reveals progressive versus stable myeloma precursor conditions as two distinct entities. Nat. Commun. 2021, 12, 1861. [Google Scholar] [CrossRef] [PubMed]
- Rustad, E.H.; Yellapantula, V.; Leongamornlert, D.; Bolli, N.; Ledergor, G.; Nadeu, F.; Angelopoulos, N.; Dawson, K.J.; Mitchell, T.J.; Osborne, R.J.; et al. Timing the initiation of multiple myeloma. Nat. Commun. 2020, 11, 1917. [Google Scholar] [CrossRef] [PubMed]
- Poh, C.; Keegan, T.; Rosenberg, A.S. Second primary malignancies in multiple myeloma: A review. Blood Rev. 2021, 46, 100757. [Google Scholar] [CrossRef]
- Musto, P.; Anderson, K.C.; Attal, M.; Richardson, P.G.; Badros, A.; Hou, J.; Comenzo, R.; Du, J.; Durie, B.G.M.; San Miguel, J.; et al. Second primary malignancies in multiple myeloma: An overview and IMWG consensus. Ann. Oncol. 2017, 28, 228–245. [Google Scholar] [CrossRef]
- Maclachlan, K.; Diamond, B.; Maura, F.; Hillengass, J.; Turesson, I.; Landgren, C.O.; Kazandjian, D. Second malignancies in multiple myeloma; emerging patterns and future directions. Best Pract. Res. Clin. Haematol. 2020, 33, 101144. [Google Scholar] [CrossRef]
- Engelhardt, M.; Ihorst, G.; Landgren, O.; Pantic, M.; Reinhardt, H.; Waldschmidt, J.; May, A.M.; Schumacher, M.; Kleber, M.; Wäsch, R. Large registry analysis to accurately define second malignancy rates and risks in a well-characterized cohort of 744 consecutive multiple myeloma patients followed-up for 25 years. Haematologica 2015, 100, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, R.; Jagannath, S.; Flick, J.T.; Vesole, D.H.; Sawyer, J.; Barlogie, B.; Tricot, G. Preceding standard therapy is the likely cause of MDS after autotransplants for multiple myeloma. Br. J. Haematol. 1996, 95, 349–353. [Google Scholar] [CrossRef]
- Kyle, R.A.; Pierre, R.V.; Bayrd, E.D. Multiple Myeloma and Acute Myelomonocytic Leukemia. N. Engl. J. Med. 1970, 283, 1121–1125. [Google Scholar] [CrossRef]
- Pemmaraju, N.; Shah, D.; Kantarjian, H.; Orlowski, R.Z.; Nogueras González, G.M.; Baladandayuthapani, V.; Jain, N.; Wagner, V.; Garcia-Manero, G.; Shah, J.; et al. Characteristics and outcomes of patients with multiple myeloma who develop therapy-related myelodysplastic syndrome, chronic myelomonocytic leukemia, or acute myeloid leukemia. Clin. Lymphoma Myeloma Leuk. 2015, 15, 110–114. [Google Scholar] [CrossRef]
- Palumbo, A.; Bringhen, S.; Kumar, S.K.; Lupparelli, G.; Usmani, S.; Waage, A.; Larocca, A.; van der Holt, B.; Musto, P.; Offidani, M.; et al. Second primary malignancies with lenalidomide therapy for newly diagnosed myeloma: A meta-analysis of individual patient data. Lancet Oncol. 2014, 15, 333–342. [Google Scholar] [CrossRef]
- Finnish Leukaemia Group. Acute leukaemia and other secondary neoplasms in patients treated with conventional chemotherapy for multiple myeloma: A Finnish Leukaemia Group study. Eur. J. Haematol. 2000, 65, 123–127. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Sawyer, J.; Rosenthal, A.; Cottler-Fox, M.; Epstein, J.; Yaccoby, S.; Sexton, R.; Hoering, A.; Singh, Z.; Heuck, C.J.; et al. Risk factors for MDS and acute leukemia following total therapy 2 and 3 for multiple myeloma. Blood 2013, 121, 4753–4757. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Richardson, P.G.; Brandenburg, N.; Yu, Z.; Weber, D.M.; Niesvizky, R.; Morgan, G.J. A review of second primary malignancy in patients with relapsed or refractory multiple myeloma treated with lenalidomide. Blood 2012, 119, 2764–2767. [Google Scholar] [CrossRef]
- Mailankody, S.; Pfeiffer, R.M.; Kristinsson, S.Y.; Korde, N.; Bjorkholm, M.; Goldin, L.R.; Turesson, I.; Landgren, O. Risk of acute myeloid leukemia and myelodysplastic syndromes after multiple myeloma and its precursor disease (MGUS). Blood 2011, 118, 4086–4092. [Google Scholar] [CrossRef]
- Roeker, L.E.; Larson, D.R.; Kyle, R.A.; Kumar, S.; Dispenzieri, A.; Rajkumar, S.V. Risk of acute leukemia and myelodysplastic syndromes in patients with monoclonal gammopathy of undetermined significance (MGUS): A population-based study of 17 315 patients. Leukemia 2013, 27, 1391–1393. [Google Scholar] [CrossRef]
- Turesson, I.; Kovalchik, S.A.; Pfeiffer, R.M.; Kristinsson, S.Y.; Goldin, L.R.; Drayson, M.T.; Landgren, O. Monoclonal gammopathy of undetermined significance and risk of lymphoid and myeloid malignancies: 728 cases followed up to 30 years in Sweden. Blood 2014, 123, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Manier, S.; Salem, K.Z.; Park, J.; Landau, D.A.; Getz, G.; Ghobrial, I.M. Genomic complexity of multiple myeloma and its clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 100–113. [Google Scholar] [CrossRef]
- Maura, F.; Rustad, E.H.; Boyle, E.M.; Morgan, G.J. Reconstructing the evolutionary history of multiple myeloma. Best Pract. Res. Clin. Haematol. 2020, 33, 101145. [Google Scholar] [CrossRef] [PubMed]
- Bolli, N.; Genuardi, E.; Ziccheddu, B.; Martello, M.; Oliva, S.; Terragna, C. Next-Generation Sequencing for Clinical Management of Multiple Myeloma: Ready for Prime Time? Front. Oncol. 2020, 10, 189. [Google Scholar] [CrossRef]
- Chapman, M.A.; Lawrence, M.S.; Keats, J.J.; Cibulskis, K.; Sougnez, C.; Schinzel, A.C.; Harview, C.L.; Brunet, J.P.; Ahmann, G.J.; Adli, M.; et al. Initial genome sequencing and analysis of multiple myeloma. Nature 2011, 471, 467–472. [Google Scholar] [CrossRef]
- Walker, B.A.; Boyle, E.M.; Wardell, C.P.; Murison, A.; Begum, D.B.; Dahir, N.M.; Proszek, P.Z.; Johnson, D.C.; Kaiser, M.F.; Melchor, L.; et al. Mutational Spectrum, Copy Number Changes, and Outcome: Results of a Sequencing Study of Patients With Newly Diagnosed Myeloma. J. Clin. Oncol. 2015, 33, 3911–3920. [Google Scholar] [CrossRef]
- Rustad, E.H.; Yellapantula, V.; Glodzik, D.; Gundem, G.; Leongamornlert, D.A.; Campbell, P.J.; Papaemmanuil, E.; Landgren, O.; Maura, F. Revealing the Impact of Recurrent and Rare Structural Variations in Multiple Myeloma. Blood 2019, 134, 576. [Google Scholar] [CrossRef]
- Keats, J.J.; Craig, D.W.; Liang, W.; Venkata, Y.; Kurdoglu, A.; Aldrich, J.; Auclair, D.; Allen, K.; Harrison, B.; Jewell, S.; et al. Interim Analysis Of The MMRF CoMMpass Trial, a Longitudinal Study In Multiple Myeloma Relating Clinical Outcomes To Genomic and Immunophenotypic Profiles. Blood 2013, 122, 532. [Google Scholar] [CrossRef]
- Walker, B.A.; Samur, K.M.; Mavrommatis, K.; Ashby, C.; Wardell, P.C.; Ortiz, M.; Towfic, F.; Stein, K.C.; Bauer, A.M. The Multiple Myeloma Genome Project: Development of a Molecular Segmentation Strategy for the Clinical Classification of Multiple Myeloma. Blood 2016, 128, 196. [Google Scholar] [CrossRef]
- Walker, B.A.; Mavrommatis, K.; Wardell, C.P.; Ashby, T.C.; Bauer, M.; Davies, F.E.; Rosenthal, A.; Wang, H.; Qu, P.; Hoering, A.; et al. Identification of novel mutational drivers reveals oncogene dependencies in multiple myeloma. Blood 2018, 132, 587–597. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Kumar, S. Multiple myeloma current treatment algorithms. Blood Cancer J. 2020, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Moreau, P.; Terpos, E.; Mateos, M.-V.; Zweegman, S.; Cook, G.; Delforge, M.; Hájek, R.; Schjesvold, F.; Cavo, M.; et al. Multiple Myeloma: EHA-ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-up. HemaSphere 2021, 5, e528. [Google Scholar] [CrossRef] [PubMed]
- Mohty, M.; Knauf, W.; Romanus, D.; Corman, S.; Verleger, K.; Kwon, Y.; Cherepanov, D.; Cambron-Mellott, M.J.; Vikis, H.G.; Gonzalez, F.; et al. Real-world treatment patterns and outcomes in non-transplant newly diagnosed multiple Myeloma in France, Germany, Italy, and the United Kingdom. Eur. J. Haematol. 2020, 105, 308–325. [Google Scholar] [CrossRef]
- Mateos, M.V.; Ludwig, H.; Bazarbachi, A.; Beksac, M.; Bladé, J.; Boccadoro, M.; Cavo, M.; Delforge, M.; Dimopoulos, M.A.; Facon, T.; et al. Insights on Multiple Myeloma Treatment Strategies. HemaSphere 2019, 3, e163. [Google Scholar] [CrossRef]
- Bobin, A.; Liuu, E.; Moya, N.; Gruchet, C.; Sabirou, F.; Lévy, A.; Gardeney, H.; Nsiala, L.; Cailly, L.; Guidez, S.; et al. Multiple myeloma: An overview of the current and novel therapeutic approaches in 2020. Cancers 2020, 12, 2885. [Google Scholar] [CrossRef]
- Muchtar, E.; Dingli, D.; Kumar, S.; Buadi, F.K.; Dispenzieri, A.; Hayman, S.R.; Wolf, R.C.; Gastineau, D.A.; Chakraborty, R.; Hogan, W.J.; et al. Autologous stem cell transplant for multiple myeloma patients 70 years or older. Bone Marrow Transplant. 2016, 51, 1449–1455. [Google Scholar] [CrossRef]
- Fiala, M.A.; King, J.; Feinberg, D.; Goldsmith, S.R.; Schroeder, M.A.; Ghobadi, A.; Stockerl-Goldstein, K.E.; Vij, R.; Wildes, T.M. Autologous stem cell transplant for patients with multiple myeloma between ages 75 and 78. Bone Marrow Transplant. 2021, 56, 2016–2018. [Google Scholar] [CrossRef] [PubMed]
- Vaxman, I.; Visram, A.; Kumar, S.; Dispenzieri, A.; Buadi, F.; Dingli, D.; Lacy, M.; Muchtar, E.; Kapoor, P.; Hogan, W.; et al. Autologous stem cell transplantation for multiple myeloma patients aged ≥ 75 treated with novel agents. Bone Marrow Transplant. 2021, 56, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; McKiernan, P.; Siegel, D.S.D.; Vesole, D.H.; Rowley, S.D.; Andrews, T.; Ortega, A.; Skarbnik, A.P.; Biran, N.; Richter, J.R.; et al. Autologous stem cell transplantation in multiple myeloma patients over age 75. J. Clin. Oncol. 2018, 36, 8025. [Google Scholar] [CrossRef]
- Josepht, N.S.; Gupta, V.A.; Wyman, S.; Graiser, M.; Kaufman, J.L.; Almaula, D.; Andrews, J.; Hofmeister, C.; Dhodapkar, M.; Heffner, L.T.; et al. Benefits of autologous stem cell transplant for elderly myeloma patients in the last quarter of life. Transplant. Cell. Ther. 2021, in press. [Google Scholar]
- Palumbo, A.; Cavallo, F.; Gay, F.; Di Raimondo, F.; Ben Yehuda, D.; Petrucci, M.T.; Pezzatti, S.; Caravita, T.; Cerrato, C.; Ribakovsky, E.; et al. Autologous Transplantation and Maintenance Therapy in Multiple Myeloma. N. Engl. J. Med. 2014, 371, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Attal, M.; Harousseau, J.-L.; Stoppa, A.-M.; Sotto, J.-J.; Fuzibet, J.-G.; Rossi, J.-F.; Casassus, P.; Maisonneuve, H.; Facon, T.; Ifrah, N.; et al. A Prospective, Randomized Trial of Autologous Bone Marrow Transplantation and Chemotherapy in Multiple Myeloma. N. Engl. J. Med. 1996, 335, 91–97. [Google Scholar] [CrossRef]
- Ntanasis-Stathopoulos, I.; Gavriatopoulou, M.; Kastritis, E.; Terpos, E.; Dimopoulos, M.A. Multiple myeloma: Role of autologous transplantation. Cancer Treat. Rev. 2020, 82, 101929. [Google Scholar] [CrossRef]
- Cavo, M.; Gay, F.; Beksac, M.; Pantani, L.; Petrucci, M.T.; Dimopoulos, M.A.; Dozza, L.; van der Holt, B.; Zweegman, S.; Oliva, S.; et al. Autologous haematopoietic stem-cell transplantation versus bortezomib–melphalan–prednisone, with or without bortezomib–lenalidomide–dexamethasone consolidation therapy, and lenalidomide maintenance for newly diagnosed multiple myeloma (EMN02/HO95): A mult. Lancet Haematol. 2020, 7, e456–e468. [Google Scholar] [CrossRef]
- Stadtmauer, E.A.; Pasquini, M.C.; Blackwell, B.; Hari, P.; Bashey, A.; Devine, S.; Efebera, Y.; Ganguly, S.; Gasparetto, C.; Geller, N.; et al. Autologous transplantation, consolidation, and maintenance therapy in multiple myeloma: Results of the BMT CTN 0702 trial. J. Clin. Oncol. 2019, 37, 589–597. [Google Scholar] [CrossRef]
- Gonsalves, W.I.; Buadi, F.K.; Ailawadhi, S.; Bergsagel, P.L.; Chanan Khan, A.A.; Dingli, D.; Dispenzieri, A.; Fonseca, R.; Hayman, S.R.; Kapoor, P.; et al. Utilization of hematopoietic stem cell transplantation for the treatment of multiple myeloma: A Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus statement. Bone Marrow Transplant. 2019, 54, 353–367. [Google Scholar] [CrossRef]
- Legarda, M.A.; Cejalvo, M.J.; de la Rubia, J. Recent advances in the treatment of patients with multiple myeloma. Cancers 2020, 12, 3576. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Buadi, F.K.; Vincent Rajkumar, S. Pros and cons of frontline autologous transplant in multiple myeloma: The debate over timing. Blood 2019, 133, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, A.J.; Rajkumar, S.V.; Vachon, C.M. Familial monoclonal gammopathy of undetermined significance and multiple myeloma: Epidemiology, risk factors, and biological characteristics. Blood 2012, 119, 5359–5366. [Google Scholar] [CrossRef]
- Alexanian, R.; Haut, A.; Khan, A.U.; Lane, M.; McKelvey, E.M.; Migliore, P.J.; Stuckey, W.J., Jr.; Wilson, H.E. Treatment for Multiple Myeloma: Combination Chemotherapy With Different Melphalan Dose Regimens. JAMA 1969, 208, 1680–1685. [Google Scholar] [CrossRef]
- Bensinger, W.I.; Becker, P.S.; Gooley, T.A.; Chauncey, T.R.; Maloney, D.G.; Gopal, A.K.; Green, D.J.; Press, O.W.; Lill, M.; Ifthikharuddin, J.J.; et al. A randomized study of melphalan 200 mg/m2 vs 280 mg/m2 as a preparative regimen for patients with multiple myeloma undergoing auto-SCT. Bone Marrow Transplant. 2016, 51, 67–71. [Google Scholar] [CrossRef]
- Palumbo, A.; Bringhen, S.; Bruno, B.; Falcone, A.P.; Liberati, A.M.; Grasso, M.; Ria, R.; Pisani, F.; Cangialosi, C.; Caravita, T.; et al. Melphalan 200 mg/m2 versus melphalan 100 mg/m2 in newly diagnosed myeloma patients: A prospective, multicenter phase 3 study. Blood 2010, 115, 1873–1879. [Google Scholar] [CrossRef]
- Gay, F.; Oliva, S.; Petrucci, M.T.; Conticello, C.; Catalano, L.; Corradini, P.; Siniscalchi, A.; Magarotto, V.; Pour, L.; Carella, A.; et al. Chemotherapy plus lenalidomide versus autologous transplantation, followed by lenalidomide plus prednisone versus lenalidomide maintenance, in patients with multiple myeloma: A randomised, multicentre, phase 3 trial. Lancet Oncol. 2015, 16, 1617–1629. [Google Scholar] [CrossRef]
- Kazandjian, D.; Mo, C.C.; Landgren, O.; Richardson, P.G. The role of high-dose melphalan with autologous stem-cell transplant in multiple myeloma: Is it time for a paradigm shift? Br. J. Haematol. 2020, 191, 692–703. [Google Scholar] [CrossRef]
- Derudas, D.; Capraro, F.; Martinelli, G.; Cerchione, C. How i manage frontline transplant-ineligible multiple myeloma. Hematol. Rep. 2020, 12 (Suppl. 1), 8956. [Google Scholar] [CrossRef]
- Facon, T.; Kumar, S.; Plesner, T.; Orlowski, R.Z.; Moreau, P.; Bahlis, N.; Basu, S.; Nahi, H.; Hulin, C.; Quach, H.; et al. Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma. N. Engl. J. Med. 2019, 380, 2104–2115. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Jakubowiak, A.J.; McCarthy, P.L.; Orlowski, R.Z.; Attal, M.; Bladé, J.; Goldschmidt, H.; Weisel, K.C.; Ramasamy, K.; Zweegman, S.; et al. Developments in continuous therapy and maintenance treatment approaches for patients with newly diagnosed multiple myeloma. Blood Cancer J. 2020, 10, 1–19. [Google Scholar] [CrossRef]
- Cook, G.; Ashcroft, A.J.; Cairns, D.A.; Williams, C.D.; Brown, J.M.; Cavenagh, J.D.; Snowden, J.A.; Parrish, C.; Yong, K.; Cavet, J.; et al. The effect of salvage autologous stem-cell transplantation on overall survival in patients with relapsed multiple myeloma (final results from BSBMT/UKMF Myeloma X Relapse [Intensive]): A randomised, open-label, phase 3 trial. Lancet Haematol. 2016, 3, e340–e351. [Google Scholar] [CrossRef]
- Cavo, M.; Tosi, P.; Zamagni, E.; Cellini, C.; Tacchetti, P.; Patriarca, F.; Di Raimondo, F.; Volpe, E.; Ronconi, S.; Cangini, D.; et al. Prospective, randomized study of single compared with double autologous stem-cell transplantation for multiple myeloma: Bologna 96 clinical study. J. Clin. Oncol. 2007, 25, 2434–2441. [Google Scholar] [CrossRef]
- Kropff, M.; Vogel, M.; Bisping, G.; Schlag, R.; Weide, R.; Knauf, W.; Fiechtner, H.; Kojouharoff, G.; Kremers, S.; Berdel, W.E. Bortezomib and low-dose dexamethasone with or without continuous low-dose oral cyclophosphamide for primary refractory or relapsed multiple myeloma: A randomized phase III study. Ann. Hematol. 2017, 96, 1857–1866. [Google Scholar] [CrossRef]
- Reeder, C.B.; Reece, D.E.; Kukreti, V.; Mikhael, J.R.; Chen, C.; Trudel, S.; Laumann, K.; Vohra, H.; Fonseca, R.; Bergsagel, P.L.; et al. Long-term survival with cyclophosphamide, bortezomib and dexamethasone induction therapy in patients with newly diagnosed multiple myeloma. Br. J. Haematol. 2014, 167, 563–565. [Google Scholar] [CrossRef]
- Garderet, L.; Kuhnowski, F.; Berge, B.; Roussel, M.; Escoffre-Barbe, M.; Lafon, I.; Facon, T.; Leleu, X.; Karlin, L.; Perrot, A.; et al. Pomalidomide, cyclophosphamide, and dexamethasone for relapsed multiple myeloma. Blood 2018, 132, 2555–2563. [Google Scholar] [CrossRef] [PubMed]
- Morgan, G.J.; Davies, F.E.; Gregory, W.M.; Russell, N.H.; Bell, S.E.; Szubert, A.J.; Coy, N.N.; Cook, G.; Feyler, S.; Byrne, J.L.; et al. Cyclophosphamide, thalidomide, and dexamethasone (CTD) as initial therapy for patients with multiple myeloma unsuitable for autologous transplantation. Blood 2011, 118, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Flinn, I.; Richardson, P.G.; Hari, P.; Callander, N.; Noga, S.J.; Stewart, A.K.; Turturro, F.; Rifkin, R.; Wolf, J.; et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood 2012, 119, 4375–4382. [Google Scholar] [CrossRef] [PubMed]
- Yimer, H.; Melear, J.; Faber, E.; Bensinger, W.I.; Burke, J.M.; Narang, M.; Stevens, D.; Gunawardena, S.; Lutska, Y.; Qi, K.; et al. Daratumumab, bortezomib, cyclophosphamide and dexamethasone in newly diagnosed and relapsed multiple myeloma: LYRA study. Br. J. Haematol. 2019, 185, 492–502. [Google Scholar] [CrossRef]
- Jimenez Zepeda, V.H.; Duggan, P.; Neri, P.E.; Bahlis, N.J. Cyclophosphamide, Bortezomib and Dexamethasone (CyBORD) Is a Feasible and Active Regimen for Non-Transplant Eligible Multiple Myeloma Patients. Blood 2014, 124, 5751. [Google Scholar] [CrossRef]
- Reeder, C.B.; Reece, D.E.; Kukreti, V.; Chen, C.; Trudel, S.; Hentz, J.; Noble, B.; Pirooz, N.A.; Spong, J.E.; Piza, J.G.; et al. Cyclophosphamide, bortezomib and dexamethasone induction for newly diagnosed multiple myeloma: High response rates in a phase II clinical trial. Leukemia 2009, 23, 1337–1341. [Google Scholar] [CrossRef]
- Areethamsirikul, N.; Masih-Khan, E.; Chu, C.M.; Jimenez-Zepeda, V.; Reece, D.E.; Trudel, S.; Kukreti, V.; Tiedemann, R.; Chen, C. CyBorD induction therapy in clinical practice. Bone Marrow Transplant. 2015, 50, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.L.; Reeder, C.B.; Kumar, S.; Lacy, M.; Reece, D.E.; Laumann, K.M.; Mikhael, J.; Fonseca, R.; Rajkumar, V.; Stewart, A.K. A comparison of lenalidomide/dexamethasone (RD) versus cyclophosphamide/lenalidomide/dexamethasone (CRD) versus cyclophosphamide/bortezomib/dexamethasone (CyborD) in newly diagnosed multiple myeloma (MM). J. Clin. Oncol. 2010, 28, 8131. [Google Scholar] [CrossRef]
- Pinto, V.; Bergantim, R.; Caires, H.R.; Seca, H.; Guimarães, J.E.; Vasconcelos, M.H. Multiple myeloma: Available therapies and causes of drug resistance. Cancers 2020, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Chari, A.; Vogl, D.T.; Gavriatopoulou, M.; Nooka, A.K.; Yee, A.J.; Huff, C.A.; Moreau, P.; Dingli, D.; Cole, C.; Lonial, S.; et al. Oral Selinexor–Dexamethasone for Triple-Class Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 381, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.K.; Chari, A.; Abdallah, A.O.; Callander, N.; Lendvai, N.; Sborov, D.; et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): A two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020, 21, 207–221. [Google Scholar] [CrossRef]
- Shah, J.J.; Kaufman, J.L.; Zonder, J.A.; Cohen, A.D.; Bensinger, W.I.; Hilder, B.W.; Rush, S.A.; Walker, D.H.; Tunquist, B.J.; Litwiler, K.S.; et al. A Phase 1 and 2 study of Filanesib alone and in combination with low-dose dexamethasone in relapsed/refractory multiple myeloma. Cancer 2017, 123, 4617–4630. [Google Scholar] [CrossRef]
- Mikkilineni, L.; Kochenderfer, J.N. CAR T cell therapies for patients with multiple myeloma. Nat. Rev. Clin. Oncol. 2021, 18, 71–84. [Google Scholar] [CrossRef]
- van de Donk, N.W.C.J.; Usmani, S.Z.; Yong, K. CAR T-cell therapy for multiple myeloma: State of the art and prospects. Lancet Haematol. 2021, 8, e446–e461. [Google Scholar] [CrossRef]
- Offidani, M.; Corvatta, L.; Morè, S.; Olivieri, A. Novel experimental drugs for treatment of multiple myeloma. J. Exp. Pharmacol. 2021, 13, 245–264. [Google Scholar] [CrossRef] [PubMed]
- Lassiter, G.; Bergeron, C.; Guedry, R.; Cucarola, J.; Kaye, A.M.; Cornett, E.M.; Kaye, A.D.; Varrassi, G.; Viswanath, O.; Urits, I. Belantamab mafodotin to treat multiple myeloma: A comprehensive review of disease, drug efficacy and side effects. Curr. Oncol. 2021, 28, 63. [Google Scholar] [CrossRef]
- Raje, N.; Berdeja, J.; Lin, Y.; Siegel, D.; Jagannath, S.; Madduri, D.; Liedtke, M.; Rosenblatt, J.; Maus, M.V.; Turka, A.; et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 380, 1726–1737. [Google Scholar] [CrossRef] [PubMed]
- Gourzones, C.; Bret, C.; Moreaux, J. Treatment May Be Harmful: Mechanisms/Prediction/Prevention of Drug-Induced DNA Damage and Repair in Multiple Myeloma. Front. Genet. 2019, 10, 861. [Google Scholar] [CrossRef]
- Esma, F.; Salvini, M.; Troia, R.; Boccadoro, M.; Larocca, A.; Pautasso, C. Melphalan hydrochloride for the treatment of multiple myeloma. Expert Opin. Pharmacother. 2017, 18, 1127–1136. [Google Scholar] [CrossRef]
- Osborne, M.R.; Lawley, P.D. Alkylation of DNA by melphalan with special reference to adenine derivatives and adenine-guanine cross-linking. Chem. Biol. Interact. 1993, 89, 49–60. [Google Scholar] [CrossRef]
- Osborne, M.R.; Lawley, P.D. The reaction of melphalan with deoxyguanosine and deoxyguanylic acid. Chem. Biol. Interact. 1992, 84, 189–198. [Google Scholar] [CrossRef]
- Musto, P.; D’Auria, F. Melphalan: Old and new uses of a still master drug for multiple myeloma. Expert Opin. Investig. Drugs 2007, 16, 1467–1487. [Google Scholar] [CrossRef]
- Schjesvold, F.; Oriol, A. Current and novel alkylators in multiple myeloma. Cancers 2021, 13, 2465. [Google Scholar] [CrossRef]
- Mehta, J.R.; Przybylski, M.; Ludlum, D.B. Alkylation of Guanosine and Deoxyguanosine by Phosphoramide Mustard. Cancer Res. 1980, 40, 4183–4186. [Google Scholar]
- Maccubbin, A.E.; C’aballes, L.; Gurtoo, H.L. A Cyclophosphamide/DNA Phosphoester Adduct Formed in Vitro and in Vivo. Cancer Res. 1991, 51, 886–892. [Google Scholar]
- Benson, A.J.; Martin, C.N.; Garner, R.C. N-(2-hydroxyethyl)-N-[2-(7-guaninyl)ethyl]amine, the putative major DNA adduct of cyclophosphamide in vitro and in vivo in the rat. Biochem. Pharmacol. 1988, 37, 2979–2985. [Google Scholar] [CrossRef]
- Swan, D.; Gurney, M.; Krawczyk, J.; Ryan, A.E.; O’Dwyer, M. Beyond DNA Damage: Exploring the Immunomodulatory Effects of Cyclophosphamide in Multiple Myeloma. HemaSphere 2020, 4, e350. [Google Scholar] [CrossRef]
- Landau, H.J.; Yellapantula, V.; Diamond, B.T.; Rustad, E.H.; Maclachlan, K.H.; Gundem, G.; Medina-Martinez, J.; Ossa, J.A.; Levine, M.F.; Zhou, Y.; et al. Accelerated single cell seeding in relapsed multiple myeloma. Nat. Commun. 2020, 11, 3617. [Google Scholar] [CrossRef] [PubMed]
- Maura, F.; Weinhold, N.; Diamond, B.; Kazandjian, D.; Rasche, L.; Morgan, G.; Landgren, O. The mutagenic impact of melphalan in multiple myeloma. Leukemia 2021, 35, 2145–2150. [Google Scholar] [CrossRef]
- Gonzalez, F.; Trujillo, J.M.; Alexanian, R. Acute leukemia in multiple myeloma. Ann. Intern. Med. 1977, 86, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Law, I.P.; Blom, J. Second malignancies in patients with multiple myeloma. Oncology 1977, 34, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Bergsagel, D.E.; Bailey, A.J.; Langley, G.R.; MacDonald, R.N.; White, D.F.; Miller, A.B. The Chemotherapy of Plasma-Cell Myeloma and the Incidence of Acute Leukemia. N. Engl. J. Med. 1979, 301, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Rosner, F.; Grünwald, H. Multiple myeloma terminating in acute leukemia. Report of 12 cases and review of the literature. Am. J. Med. 1974, 57, 927–939. [Google Scholar] [CrossRef]
- Richardson, P.G.; Bringhen, S.; Voorhees, P.; Plesner, T.; Mellqvist, U.H.; Reeves, B.; Paba-Prada, C.; Zubair, H.; Byrne, C.; Chauhan, D.; et al. Melflufen plus dexamethasone in relapsed and refractory multiple myeloma (O-12-M1): A multicentre, international, open-label, phase 1–2 study. Lancet Haematol. 2020, 7, e395–e407. [Google Scholar] [CrossRef]
- Wickström, M.; Nygren, P.; Larsson, R.; Harmenberg, J.; Lindberg, J.; Sjöberg, P.; Jerling, M.; Lehmann, F.; Richardson, P.; Anderson, K.; et al. Melflufen-a peptidase-potentiated alkylating agent in clinical trials. Oncotarget 2017, 8, 66641–66655. [Google Scholar] [CrossRef]
- Mateos, M.V.; González-Calle, V. Is there a role for new drugs with alkylating properties in multiple myeloma? Lancet Haematol. 2020, 7, e357–e359. [Google Scholar] [CrossRef]
- Soll, J.M.; Sobol, R.W.; Mosammaparast, N. Regulation of DNA Alkylation Damage Repair: Lessons and Therapeutic Opportunities. Trends Biochem. Sci. 2017, 42, 206–218. [Google Scholar] [CrossRef]
- Friedberg, E.C.; Walker, G.C.; Siede, W.; Wood, R.D.; Schultz, R.A.; Ellenberger, T. DNA Repair and Mutagenesis, 2nd ed.; ASM Press: Washington, DC, USA, 2005. [Google Scholar]
- Datta, A.; Brosh, R.M. Holding all the cards—how fanconi anemia proteins deal with replication stress and preserve genomic stability. Genes 2019, 10, 170. [Google Scholar] [CrossRef] [PubMed]
- Ceccaldi, R.; Sarangi, P.; D’Andrea, A.D. The Fanconi anaemia pathway: New players and new functions. Nat. Rev. Mol. Cell Biol. 2016, 17, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Renaudin, X.; Rosselli, F. The FANC/BRCA Pathway Releases Replication Blockades by Eliminating DNA Interstrand Cross-Links. Genes 2020, 11, 585. [Google Scholar] [CrossRef] [PubMed]
- Lyakhovich, A.; Surralles, J. Disruption of the Fanconi anemia/BRCA pathway in sporadic cancer. Cancer Lett. 2006, 232, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Van Der Sluis, P.C.; Boulware, D.; Hazlehurst, L.A.; Dalton, W.S. The FA/BRCA pathway is involved in melphalan-induced DNA interstrand cross-link repair and accounts for melphalan resistance in multiple myeloma cells. Blood 2005, 106, 698–705. [Google Scholar] [CrossRef]
- Tessoulin, B.; Moreau-Aubry, A.; Descamps, G.; Gomez-Bougie, P.; Maïga, S.; Gaignard, A.; Chiron, D.; Ménoret, E.; Le Gouill, S.; Moreau, P.; et al. Whole-exon sequencing of human myeloma cell lines shows mutations related to myeloma patients at relapse with major hits in the DNA regulation and repair pathways. J. Hematol. Oncol. 2018, 11, 137. [Google Scholar] [CrossRef]
- Turner, J.G.; Cui, Y.; Bauer, A.A.; Dawson, J.L.; Gomez, J.A.; Kim, J.; Cubitt, C.L.; Nishihori, T.; Dalton, W.S.; Sullivan, D.M. Melphalan and Exportin 1 Inhibitors Exert Synergistic Antitumor Effects in Preclinical Models of Human Multiple Myeloma. Cancer Res. 2020, 80, 5344–5354. [Google Scholar] [CrossRef] [PubMed]
- Herrero, A.B.; SanMiguel, J.; Gutierrez, N.C. Deregulation of DNA double-strand break repair in multiple myeloma: Implications for genome stability. PLoS ONE 2015, 10, e0121581. [Google Scholar] [CrossRef]
- Herrero, A.B.; Gutiérrez, N.C. Targeting ongoing DNA damage in multiple myeloma: Effects of DNA damage response inhibitors on plasma cell survival. Front. Oncol. 2017, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Shammas, M.A.; Reis, R.J.S.; Koley, H.; Batchu, R.B.; Li, C.; Munshi, N.C. Dysfunctional homologous recombination mediates genomic instability and progression in myeloma. Blood 2009, 113, 2290–2297. [Google Scholar] [CrossRef]
- Spanswick, V.J.; Lowe, H.L.; Newton, C.; Bingham, J.P.; Bagnobianchi, A.; Kiakos, K.; Craddock, C.; Ledermann, J.A.; Hochhauser, D.; Hartley, J.A. Evidence for different mechanisms of “unhooking” for melphalan and cisplatin-induced DNA interstrand cross-links in vitro and in clinical acquired resistant tumour samples. BMC Cancer 2012, 12, 436. [Google Scholar] [CrossRef]
- Tageja, N.; Nagi, J. Bendamustine: Something old, something new. Cancer Chemother. Pharmacol. 2010, 66, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, N.; Kikuchi, J.; Yamauchi, T.; Koyama, D.; Wada, T.; Uesawa, M.; Akutsu, M.; Mori, S.; Nakamura, Y.; Ueda, T.; et al. Purine analog-like properties of bendamustine underlie rapid activation of DNA damage response and synergistic effects with pyrimidine analogues in lymphoid malignancies. PLoS ONE 2014, 9, e90675. [Google Scholar]
- Cives, M.; Ciavarella, S.; Rizzo, F.M.; De Matteo, M.; Dammacco, F.; Silvestris, F. Bendamustine overcomes resistance to melphalan in myeloma cell lines by inducing cell death through mitotic catastrophe. Cell. Signal. 2013, 25, 1108–1117. [Google Scholar] [CrossRef]
- Gomez-Arteaga, A.; Mark, T.M.; Guarneri, D.; Christos, P.J.; Gergis, U.; Greenberg, J.D.; Hsu, J.; Mayer, S.A.; Niesvizky, R.; Pearse, R.N.; et al. High-dose bendamustine and melphalan conditioning for autologous stem cell transplantation for patients with multiple myeloma. Bone Marrow Transplant. 2019, 54, 2027–2038. [Google Scholar] [CrossRef]
- Gentile, M.; Vigna, E.; Recchia, A.G.; Morabito, L.; Mendicino, F.; Giagnuolo, G.; Morabito, F. Bendamustine in multiple myeloma. Eur. J. Haematol. 2015, 95, 377–388. [Google Scholar] [CrossRef]
- Mateos, M.V.; Orio, A.; Rosiño, L.; Arriba, F.D.; Puig, N.; Martín, J.; Martínez-López, J.; Echeveste, M.A.; Sarrá, J.; Ocio, E.; et al. Bendamustine, bortezomib and prednisone for the treatment of patients with newly diagnosed multiple myeloma: Results of a prospective phase 2 Spanish/PETHEMA trial. Haematologica 2015, 100, 1096–1102. [Google Scholar] [CrossRef]
- Rodon, P.; Hulin, C.; Pegourie, B.; Tiab, M.; Anglaret, B.; Benboubker, L.; Jardel, H.; Decaux, O.; Kolb, B.; Roussel, M.; et al. Phase ii study of bendamustine, bortezomib and dexamethasone as Second-Line treatment for elderly patients with multiple myeloma: The intergroupe francophone du myelome 2009-01 trial. Haematologica 2015, 100, e56–e59. [Google Scholar] [CrossRef]
- Knauf, W.; Dingeldein, G.; Schlag, R.; Welslau, M.; Moehler, T.; Terzer, T.; Walter, S.; Habermehl, C.; Kunz, C.; Goldschmidt, H.; et al. First-line therapy with bendamustine/prednisone/bortezomib—A GMMG trial for non-transplant eligible symptomatic multiple myeloma patients. Eur. J. Haematol. 2020, 105, 116–125. [Google Scholar] [CrossRef]
- Pönisch, W.; Mitrou, P.S.; Merkle, K.; Herold, M.; Assmann, M.; Wilhelm, G.; Dachselt, K.; Richter, P.; Schirmer, V.; Schulze, A.; et al. Treatment of Bendamustine and Prednisone in patients with newly diagnosed multiple myeloma results in superior complete response rate, prolonged time to treatment failure and improved quality of life compared to treatment with Melphalan and Prednisone—A randomized phase III study of the East German Study Group of Hematology and Oncology (OSHO). J. Cancer Res. Clin. Oncol. 2006, 132, 205–212. [Google Scholar] [PubMed]
- Mark, T.M.; Reid, W.; Niesvizky, R.; Gergis, U.; Pearse, R.; Mayer, S.; Greenberg, J.; Coleman, M.; Van Besien, K.; Shore, T. A phase 1 study of bendamustine and melphalan conditioning for autologous stem cell transplantation in multiple myeloma. Biol. Blood Marrow Transplant. 2013, 19, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Asatsuma-Okumura, T.; Ito, T.; Handa, H. Molecular mechanisms of cereblon-based drugs. Pharmacol. Ther. 2019, 202, 132–139. [Google Scholar] [CrossRef]
- Moreau, P.; Hulin, C.; Macro, M.; Caillot, D.; Chaleteix, C.; Roussel, M.; Garderet, L.; Royer, B.; Brechignac, S.; Tiab, M.; et al. VTD is superior to VCD prior to intensive therapy in multiple myeloma: Results of the prospective IFM2013-04 trial. Blood 2016, 127, 2569–2574. [Google Scholar] [CrossRef]
- Richardson, P.G.; Weller, E.; Lonial, S.; Jakubowiak, A.J.; Jagannath, S.; Raje, N.S.; Avigan, D.E.; Xie, W.; Ghobrial, I.M.; Schlossman, R.L.; et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood 2010, 116, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Miguel, J.S.; Weisel, K.; Moreau, P.; Lacy, M.; Song, K.; Delforge, M.; Karlin, L.; Goldschmidt, H.; Banos, A.; Oriol, A.; et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): A randomised, open-label, phase 3 trial. Lancet Oncol. 2013, 14, 1055–1066. [Google Scholar] [CrossRef]
- Siegel, D.S.; Schiller, G.J.; Song, K.W.; Agajanian, R.; Stockerl-Goldstein, K.; Kaya, H.; Sebag, M.; Samaras, C.; Malek, E.; Talamo, G.; et al. Pomalidomide plus low-dose dexamethasone in relapsed refractory multiple myeloma after lenalidomide treatment failure. Br. J. Haematol. 2020, 188, 501–510. [Google Scholar] [CrossRef]
- Terpos, E.; Repousis, P.; Lalayanni, C.; Hatjiharissi, E.; Assimakopoulou, T.; Vassilopoulos, G.; Pouli, A.; Spanoudakis, E.; Michalis, E.; Pangalis, G.; et al. Pomalidomide Plus Low-Dose Dexamethasone in Relapsed/Refractory Multiple Myeloma Patients: Results of the Real-World “POWERFUL” Study. J. Clin. Med. 2021, 10, 1509. [Google Scholar] [CrossRef]
- Barosi, G.; Gale, R.P. Is lenalidomide the standard-of-care after an autotransplant for plasma cell myeloma? Leukemia 2019, 33, 588–596. [Google Scholar] [CrossRef]
- Attal, M.; Lauwers-Cances, V.; Marit, G.; Caillot, D.; Moreau, P.; Facon, T.; Stoppa, A.M.; Hulin, C.; Benboubker, L.; Garderet, L.; et al. Lenalidomide Maintenance after Stem-Cell Transplantation for Multiple Myeloma. N. Engl. J. Med. 2012, 366, 1782–1791. [Google Scholar] [CrossRef] [PubMed]
- Jackson, G.H.; Davies, F.E.; Pawlyn, C.; Cairns, D.A.; Striha, A.; Collett, C.; Hockaday, A.; Jones, J.R.; Kishore, B.; Garg, M.; et al. Lenalidomide maintenance versus observation for patients with newly diagnosed multiple myeloma (Myeloma XI): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2019, 20, 57–73. [Google Scholar] [CrossRef]
- McCarthy, P.L.; Holstein, S.A.; Petrucci, M.T.; Richardson, P.G.; Hulin, C.; Tosi, P.; Bringhen, S.; Musto, P.; Anderson, K.C.; Caillot, D.; et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: A meta-analysis. J. Clin. Oncol. 2017, 35, 3279–3289. [Google Scholar] [CrossRef]
- Palumbo, A.; Hajek, R.; Delforge, M.; Kropff, M.; Petrucci, M.T.; Catalano, J.; Gisslinger, H.; Wiktor-Jędrzejczak, W.; Zodelava, M.; Weisel, K.; et al. Continuous Lenalidomide Treatment for Newly Diagnosed Multiple Myeloma. N. Engl. J. Med. 2012, 366, 1759–1769. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, P.L.; Owzar, K.; Hofmeister, C.C.; Hurd, D.D.; Hassoun, H.; Richardson, P.G.; Giralt, S.; Stadtmauer, E.A.; Weisdorf, D.J.; Vij, R.; et al. Lenalidomide after Stem-Cell Transplantation for Multiple Myeloma. N. Engl. J. Med. 2012, 366, 1770–1781. [Google Scholar] [CrossRef]
- Ito, T.; Ando, H.; Suzuki, T.; Ogura, T.; Hotta, K.; Imamura, Y.; Yamaguchi, Y.; Handa, H. Identification of a primary target of thalidomide teratogenicity. Science 2010, 327, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Girona, A.; Mendy, D.; Ito, T.; Miller, K.; Gandhi, A.K.; Kang, J.; Karasawa, S.; Carmel, G.; Jackson, P.; Abbasian, M.; et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia 2012, 26, 2326–2335. [Google Scholar] [CrossRef]
- Fischer, E.S.; Böhm, K.; Lydeard, J.R.; Yang, H.; Stadler, M.B.; Cavadini, S.; Nagel, J.; Serluca, F.; Acker, V.; Lingaraju, G.M.; et al. Structure of the DDB1-CRBN E3 ubiquitin ligase in complex with thalidomide. Nature 2014, 512, 49–53. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Braggio, E.; Shi, C.X.; Bruins, L.A.; Schmidt, J.E.; Van Wier, S.; Chang, X.B.; Bjorklund, C.C.; Fonseca, R.; Bergsagel, P.L.; et al. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood 2011, 118, 4771–4779. [Google Scholar] [CrossRef]
- Abruzzese, M.P.; Bilotta, M.T.; Fionda, C.; Zingoni, A.; Soriani, A.; Petrucci, M.T.; Ricciardi, M.R.; Molfetta, R.; Paolini, R.; Santoni, A.; et al. The homeobox transcription factor MEIS2 is a regulator of cancer cell survival and IMiDs activity in Multiple Myeloma: Modulation by Bromodomain and Extra-Terminal (BET) protein inhibitors. Cell Death Dis. 2019, 10, 324. [Google Scholar] [CrossRef]
- Krönke, J.; Udeshi, N.D.; Narla, A.; Grauman, P.; Hurst, S.N.; McConkey, M.; Svinkina, T.; Heckl, D.; Comer, E.; Li, X.; et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 2014, 343, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Middleton, R.E.; Sun, H.; Naniong, M.V.; Ott, C.J.; Mitsiades, C.S.; Wong, K.K.; Bradner, J.E.; Kaelin, W.G. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of ikaros proteins. Science 2014, 343, 305–309. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Braggio, E.; Shi, C.X.; Kortuem, K.M.; Bruins, L.A.; Schmidt, J.E.; Chang, X.B.; Langlais, P.; Luo, M.; Jedlowski, P.; et al. Identification of cereblon-binding proteins and relationship with response and survival after IMiDs in multiple myeloma. Blood 2014, 124, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Agnarelli, A.; Chevassut, T.; Mancini, E.J. IRF4 in multiple myeloma—Biology, disease and therapeutic target. Leuk. Res. 2018, 72, 52–58. [Google Scholar] [CrossRef]
- Jovanović, K.K.; Roche-Lestienne, C.; Ghobrial, I.M.; Facon, T.; Quesnel, B.; Manier, S. Targeting MYC in multiple myeloma. Leukemia 2018, 32, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, A.L.; Tolga Emre, N.C.; Romesser, P.B.; Staudt, L.M. IRF4: Immunity. Malignancy! Therapy? Clin. Cancer Res. 2009, 15, 2954–2961. [Google Scholar] [CrossRef]
- Klein, U.; Casola, S.; Cattoretti, G.; Shen, Q.; Lia, M.; Mo, T.; Ludwig, T.; Rajewsky, K.; Dalla-Favera, R. Transcription factor IRF4 controls plasma cell differentiation and class-switch recombination. Nat. Immunol. 2006, 7, 773–782. [Google Scholar] [CrossRef]
- Iida, S.; Rao, P.H.; Butler, M.; Corradini, P.; Boccadoro, M.; Klein, B.; Chaganti, R.S.K.; Dalla-Favera, R. Deregulation of MUM1/IRF4 by chromosomal translocation in multiple myeloma. Nat. Genet. 1997, 17, 230. [Google Scholar] [CrossRef]
- Yoshida, S.; Nakazawa, N.; Iida, S.; Hayami, Y.; Sato, S.; Wakita, A.; Shimizu, S.; Taniwaki, M.; Ueda, R. Detection of MUM1/IRF4-IgH fusion in multiple myeloma. Leukemia 1999, 13, 1812–1816. [Google Scholar] [CrossRef]
- Wang, L.; Yao, Z.Q.; Moorman, J.P.; Xu, Y.; Ning, S. Gene expression profiling identifies IRF4-Associated molecular signatures in hematological malignancies. PLoS ONE 2014, 9, e106788. [Google Scholar] [CrossRef] [PubMed]
- Misiewicz-Krzeminska, I.; de Ramón, C.; Corchete, L.A.; Krzeminski, P.; Rojas, E.A.; Isidro, I.; García-Sanz, R.; Martínez-López, J.; Oriol, A.; Bladé, J.; et al. Quantitative expression of Ikaros, IRF4, and PSMD10 proteins predicts survival in VRD-treated patients with multiple myeloma. Blood Adv. 2020, 4, 6023–6033. [Google Scholar] [CrossRef]
- Moreira, A.L.; Sampaio, E.P.; Zmuidzinas, A.; Frindt, P.; Smith, K.A.; Kaplan, G. Thalidomide exerts its inhibitory action on tumor necrosis factor α by enhancing mrna degradation. J. Exp. Med. 1993, 177, 1675–1680. [Google Scholar] [CrossRef] [PubMed]
- Payvandi, F.; Wu, L.; Haley, M.; Schafer, P.H.; Zhang, L.H.; Chen, R.S.; Muller, G.W.; Stirling, D.I. Immunomodulatory drugs inhibit expression of cyclooxygenase-2 from TNF-α, IL-1β, and LPS-stimulated human PBMC in a partially IL-10-dependent manner. Cell. Immunol. 2004, 230, 81–88. [Google Scholar] [CrossRef]
- Hideshima, T.; Chauhan, D.; Richardson, P.; Mitsiades, C.; Mitsiades, N.; Hayashi, T.; Munshi, N.; Dang, L.; Castro, A.; Palombella, V.; et al. NF-κB as a therapeutic target in multiple myeloma. J. Biol. Chem. 2002, 277, 16639–16647. [Google Scholar] [CrossRef] [PubMed]
- Keifer, J.A.; Guttridge, D.C.; Ashburner, B.P.; Baldwin, A.S. Inhibition of NF-κB Activity by Thalidomide through Suppression of IκB Kinase Activity. J. Biol. Chem. 2001, 276, 22382–22387. [Google Scholar] [CrossRef]
- Tachita, T.; Kinoshita, S.; Ri, M.; Aoki, S.; Asano, A.; Kanamori, T.; Yoshida, T.; Totani, H.; Ito, A.; Kusumoto, S.; et al. Expression, mutation, and methylation of cereblon-pathway genes at pre- and post-lenalidomide treatment in multiple myeloma. Cancer Sci. 2020, 111, 1333–1343. [Google Scholar] [CrossRef]
- Donovan, K.A.; An, J.; Nowak, R.P.; Yuan, J.C.; Fink, E.C.; Berry, B.C.; Ebert, B.L.; Fischer, E.S. Thalidomide promotes degradation of SALL4, a transcription factor implicated in Duane radial ray syndrome. Elife 2018, 7, e38430. [Google Scholar] [CrossRef]
- Aguiar, P.M.; de Mendonça Lima, T.; Colleoni, G.W.B.; Storpirtis, S. Efficacy and safety of bortezomib, thalidomide, and lenalidomide in multiple myeloma: An overview of systematic reviews with meta-analyses. Crit. Rev. Oncol. Hematol. 2017, 113, 195–212. [Google Scholar] [CrossRef]
- Nutt, S.L.; Hodgkin, P.D.; Tarlinton, D.M.; Corcoran, L.M. The generation of antibody-secreting plasma cells. Nat. Rev. Immunol. 2015, 15, 160–171. [Google Scholar] [CrossRef]
- Wang, H.; Morse, H.C.; Bolland, S. Transcriptional Control of Mature B Cell Fates. Trends Immunol. 2020, 41, 601–613. [Google Scholar] [CrossRef]
- Laidlaw, B.J.; Cyster, J.G. Transcriptional regulation of memory B cell differentiation. Nat. Rev. Immunol. 2021, 21, 209–220. [Google Scholar] [CrossRef]
- Ma, S.; Turetsky, A.; Trinh, L.; Lu, R. IFN Regulatory Factor 4 and 8 Promote Ig Light Chain κ Locus Activation in Pre-B Cell Development. J. Immunol. 2006, 177, 7898–7904. [Google Scholar] [CrossRef] [PubMed]
- Tellier, J.; Nutt, S.L. Plasma cells: The programming of an antibody-secreting machine. Eur. J. Immunol. 2019, 49, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, A.L.; Lin, K.I.; Kuo, T.C.; Yu, X.; Hurt, E.M.; Rosenwald, A.; Giltnane, J.M.; Yang, L.; Zhao, H.; Calame, K.; et al. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity 2002, 17, 51–62. [Google Scholar] [CrossRef]
- Fricker, L.D. Proteasome Inhibitor Drugs. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 457–476. [Google Scholar] [CrossRef]
- Ito, S. Proteasome inhibitors for the treatment of multiple myeloma. Cancers 2020, 12, 265. [Google Scholar] [CrossRef] [PubMed]
- Hershko, A. The ubiquitin system for protein degradation and some of its roles in the control of the cell division cycle. Cell Death Differ. 2005, 12, 1191–1197. [Google Scholar] [CrossRef]
- Adams, J.; Palombella, V.J.; Sausville, E.A.; Johnson, J.; Destree, A.; Lazarus, D.D.; Maas, J.; Pien, C.S.; Prakash, S.; Elliott, P.J. Proteasome inhibitors: A novel class of potent and effective antitumor agents. Cancer Res. 1999, 59, 2615–2622. [Google Scholar]
- Drexler, H.C.A. Activation of the cell death program by inhibition of proteasome function. Proc. Natl. Acad. Sci. USA 1997, 94, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, S.; Laubach, J.P.; Hideshima, T.; Chauhan, D.; Anderson, K.C.; Richardson, P.G. The proteasome and proteasome inhibitors in multiple myeloma. Cancer Metastasis Rev. 2017, 36, 561–584. [Google Scholar] [CrossRef] [PubMed]
- Manasanch, E.E.; Orlowski, R.Z. Proteasome inhibitors in cancer therapy. Nat. Rev. Clin. Oncol. 2017, 14, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Obeng, E.A.; Carlson, L.M.; Gutman, D.M.; Harrington, W.J.; Lee, K.P.; Boise, L.H. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood 2006, 107, 4907–4916. [Google Scholar] [CrossRef]
- Meister, S.; Schubert, U.; Neubert, K.; Herrmann, K.; Burger, R.; Gramatzki, M.; Hahn, S.; Schreiber, S.; Wilhelm, S.; Herrmann, M.; et al. Extensive immunoglobulin production sensitizes myeloma cells for proteasome inhibition. Cancer Res. 2007, 67, 1783–1792. [Google Scholar] [CrossRef]
- Cavo, M.; Tacchetti, P.; Patriarca, F.; Petrucci, M.T.; Pantani, L.; Galli, M.; Di Raimondo, F.; Crippa, C.; Zamagni, E.; Palumbo, A.; et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: A randomised phase 3. Lancet 2010, 376, 2075–2085. [Google Scholar] [CrossRef]
- Barlogie, B.; Anaissie, E.; Van Rhee, F.; Haessler, J.; Hollmig, K.; Pineda-Roman, M.; Cottler-Fox, M.; Mohiuddin, A.; Alsayed, Y.; Tricot, G.; et al. Incorporating bortezomib into upfront treatment for multiple myeloma: Early results of total therapy 3. Br. J. Haematol. 2007, 138, 176–185. [Google Scholar] [CrossRef]
- Richardson, P.G.; Xie, W.; Jagannath, S.; Jakubowiak, A.; Lonial, S.; Raje, N.S.; Alsina, M.; Ghobrial, I.M.; Schlossman, R.L.; Munshi, N.C.; et al. A phase 2 trial of lenalidomide, bortezomib, and dexamethasone in patients with relapsed and relapsed/refractory myeloma. Blood 2014, 123, 1461–1469. [Google Scholar] [CrossRef]
- Sonneveld, P.; Schmidt-Wolf, I.G.H.; Van Der Holt, B.; El Jarari, L.; Bertsch, U.; Salwender, H.; Zweegman, S.; Vellenga, E.; Broyl, A.; Blau, I.W.; et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: Results of the randomized phase III HOVON-65/ GMMG-HD4 trial. J. Clin. Oncol. 2012, 30, 2946–2955. [Google Scholar] [CrossRef]
- Robak, P.; Robak, T. Bortezomib for the Treatment of Hematologic Malignancies: 15 Years Later. Drugs RD 2019, 19, 73–92. [Google Scholar] [CrossRef]
- Richardson, P.G.; Sonneveld, P.; Schuster, M.; Irwin, D.; Stadtmauer, E.; Facon, T.; Harousseau, J.L.; Ben-Yehuda, D.; Lonial, S.; Goldschmidt, H.; et al. Extended follow-up of a phase 3 trial in relapsed multiple myeloma: Final time-to-event results of the APEX trial. Blood 2007, 110, 3557–3560. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.G.; Barlogie, B.; Berenson, J.; Singhal, S.; Jagannath, S.; Irwin, D.; Rajkumar, S.V.; Srkalovic, G.; Alsina, M.; Alexanian, R.; et al. A Phase 2 Study of Bortezomib in Relapsed, Refractory Myeloma. N. Engl. J. Med. 2003, 348, 2609–2617. [Google Scholar] [CrossRef] [PubMed]
- Durie, B.G.M.; Hoering, A.; Abidi, M.H.; Rajkumar, S.V.; Epstein, J.; Kahanic, S.P.; Thakuri, M.; Reu, F.; Reynolds, C.M.; Sexton, R.; et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): A randomised, open-label, phase 3 trial. Lancet 2017, 389, 519–527. [Google Scholar] [CrossRef]
- San Miguel, J.F.; Schlag, R.; Khuageva, N.K.; Dimopoulos, M.A.; Shpilberg, O.; Kropff, M.; Spicka, I.; Petrucci, M.T.; Palumbo, A.; Samoilova, O.S.; et al. Bortezomib plus Melphalan and Prednisone for Initial Treatment of Multiple Myeloma. N. Engl. J. Med. 2008, 359, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Attal, M.; Lauwers-Cances, V.; Hulin, C.; Facon, T.; Caillot, D.; Escoffre, M.; Arnulf, B.; MACRO, M.; Belhadj, K.; Garderet, L.; et al. Autologous Transplantation for Multiple Myeloma in the Era of New Drugs: A Phase III Study of the Intergroupe Francophone Du Myelome (IFM/DFCI 2009 Trial). Blood 2015, 126, 391. [Google Scholar] [CrossRef]
- Goldschmidt, H.; Lokhorst, H.M.; Mai, E.K.; Van Der Holt, B.; Blau, I.W.; Zweegman, S.; Weisel, K.C.; Vellenga, E.; Pfreundschuh, M.; Kersten, M.J.; et al. Bortezomib before and after high-dose therapy in myeloma: Long-term results from the phase III HOVON-65/GMMG-HD4 trial. Leukemia 2018, 32, 383–390. [Google Scholar] [CrossRef]
- Richardson, P.G.; Sonneveld, P.; Schuster, M.W.; Irwin, D.; Stadtmauer, E.A.; Facon, T.; Harousseau, J.-L.; Ben-Yehuda, D.; Lonial, S.; Goldschmidt, H.; et al. Bortezomib or High-Dose Dexamethasone for Relapsed Multiple Myeloma. N. Engl. J. Med. 2005, 352, 2487–2498. [Google Scholar] [CrossRef]
- Stewart, A.K.; Rajkumar, S.V.; Dimopoulos, M.A.; Masszi, T.; Špička, I.; Oriol, A.; Hájek, R.; Rosiñol, L.; Siegel, D.S.; Mihaylov, G.G.; et al. Carfilzomib, Lenalidomide, and Dexamethasone for Relapsed Multiple Myeloma. N. Engl. J. Med. 2015, 372, 142–152. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Moreau, P.; Palumbo, A.; Joshua, D.; Pour, L.; Hájek, R.; Facon, T.; Ludwig, H.; Oriol, A.; Goldschmidt, H.; et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): And randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016, 17, 27–38. [Google Scholar] [CrossRef]
- Moreau, P.; Masszi, T.; Grzasko, N.; Bahlis, N.J.; Hansson, M.; Pour, L.; Sandhu, I.; Ganly, P.; Baker, B.W.; Jackson, S.R.; et al. Oral Ixazomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 374, 1621–1634. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Gay, F.; Schjesvold, F.; Beksac, M.; Hajek, R.; Weisel, K.C.; Goldschmidt, H.; Maisnar, V.; Moreau, P.; Min, C.K.; et al. Oral ixazomib maintenance following autologous stem cell transplantation (TOURMALINE-MM3): A double-blind, randomised, placebo-controlled phase 3 trial. Lancet 2019, 393, 253–264. [Google Scholar] [CrossRef]
- Hari, P.; Mateos, M.-V.; Abonour, R.; Knop, S.; Bensinger, W.; Ludwig, H.; Song, K.; Hajek, R.; Moreau, P.; Siegel, D.S.; et al. Efficacy and safety of carfilzomib regimens in multiple myeloma patients relapsing after autologous stem cell transplant: ASPIRE and ENDEAVOR outcomes. Leukemia 2017, 31, 2630–2641. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Goldschmidt, H.; Niesvizky, R.; Joshua, D.; Chng, W.J.; Oriol, A.; Orlowski, R.Z.; Ludwig, H.; Facon, T.; Hajek, R.; et al. Carfilzomib or bortezomib in relapsed or refractory multiple myeloma (ENDEAVOR): An interim overall survival analysis of an open-label, randomised, phase 3 trial. Lancet Oncol. 2017, 18, 1327–1337. [Google Scholar] [CrossRef]
- Yarde, D.N.; Oliveira, V.; Mathews, L.; Wang, X.; Villagra, A.; Boulware, D.; Shain, K.H.; Hazlehurst, L.A.; Alsina, M.; Chen, D.T.; et al. Targeting the Fanconi anemia/BRCA pathway circumvents drug resistance in multiple myeloma. Cancer Res. 2009, 69, 9367–9375. [Google Scholar] [CrossRef] [PubMed]
- Neri, P.; Ren, L.; Gratton, K.; Stebner, E.; Johnson, J.; Klimowicz, A.; Duggan, P.; Tassone, P.; Mansoor, A.; Stewart, D.A.; et al. Bortezomib-induced “BRCAness” sensitizes multiple myeloma cells to PARP inhibitors. Blood 2011, 118, 6368–6379. [Google Scholar] [CrossRef]
- Jacquemont, C.; Taniguchi, T. Proteasome function is required for DNA damage response and fanconi anemia pathway activation. Cancer Res. 2007, 67, 7395–7405. [Google Scholar] [CrossRef]
- Murakawa, Y.; Sonoda, E.; Barber, L.J.; Zeng, W.; Yokomori, K.; Kimura, H.; Niimi, A.; Lehmann, A.; Guang, Y.Z.; Hochegger, H.; et al. Inhibitors of the proteasome suppress homologous DNA recombination in mammalian cells. Cancer Res. 2007, 67, 8536–8543. [Google Scholar] [CrossRef]
- Vrábel, D.; Pour, L.; Ševčíková, S. The impact of NF-κB signaling on pathogenesis and current treatment strategies in multiple myeloma. Blood Rev. 2019, 34, 56–66. [Google Scholar] [CrossRef]
- Hideshima, T.; Ikeda, H.; Chauhan, D.; Okawa, Y.; Raje, N.; Podar, K.; Mitsiades, C.; Munshi, N.C.; Richardson, P.G.; Carrasco, R.D.; et al. Bortezomib induces canonical nuclear factor-κB activation in multiple myeloma cells. Blood 2009, 114, 1046–1052. [Google Scholar] [CrossRef]
- Li, C.; Chen, S.; Yue, P.; Deng, X.; Lonial, S.; Khuri, F.R.; Sun, S.Y. Proteasome inhibitor PS-341 (Bortezomib) induces calpain-dependent IκBα degradation. J. Biol. Chem. 2010, 285, 16096–16104. [Google Scholar] [CrossRef]
- Xie, Z.; Bi, C.; Chooi, J.Y.; Chan, Z.L.; Mustafa, N.; Chng, W.J. MMSET regulates expression of IRF4 in t(4;14) myeloma and its silencing potentiates the effect of bortezomib. Leukemia 2015, 29, 2347–2354. [Google Scholar] [CrossRef]
- Marango, J.; Shimoyama, M.; Nishio, H.; Meyer, J.A.; Min, D.J.; Sirulnik, A.; Martinez-Martinez, Y.; Chesi, M.; Bergsagel, P.L.; Zhou, M.M.; et al. The MMSET protein is a histone methyltransferase with characteristics of a transcriptional corepressor. Blood 2008, 111, 3145–3154. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Chng, W.J. MMSET: Role and therapeutic opportunities in multiple myeloma. Biomed. Res. Int. 2014, 2014, 636514. [Google Scholar] [CrossRef]
- Barwick, B.G.; Gupta, V.A.; Vertino, P.M.; Boise, L.H. Cell of origin and genetic alterations in the pathogenesis of multiple myeloma. Front. Immunol. 2019, 10, 1121. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Hirano, M.; Kobayashi, M.; Futami, M.; Tojo, A. HDAC Inhibitors Exert Anti-Myeloma Effects through Multiple Modes of Action. Cancers 2019, 11, 475. [Google Scholar] [CrossRef] [PubMed]
- Hideshima, T.; Bradner, J.E.; Wong, J.; Chauhan, D.; Richardson, P.; Schreiber, S.L.; Anderson, K.C. Small-molecule inhibition of proteasome and aggresome function induces synergistic antitumor activity in multiple myeloma. Proc. Natl. Acad. Sci. USA 2005, 102, 8567–8572. [Google Scholar] [CrossRef]
- Pei, X.Y.; Dai, Y.; Grant, S. Synergistic induction of oxidative injury and apoptosis in human multiple myeloma cells by the proteasome inhibitor bortezomib and histone deacetylase inhibitors. Clin. Cancer Res. 2004, 10, 3839–3852. [Google Scholar] [CrossRef]
- Laubach, J.P.; Schjesvold, F.; Mariz, M.; Dimopoulos, M.A.; Lech-Maranda, E.; Spicka, I.; Hungria, V.T.M.; Shelekhova, T.; Abdo, A.; Jacobasch, L.; et al. Efficacy and safety of oral panobinostat plus subcutaneous bortezomib and oral dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma (PANORAMA 3): An open-label, randomised, phase 2 study. Lancet Oncol. 2021, 22, 142–154. [Google Scholar] [CrossRef]
- San-Miguel, J.F.; Hungria, V.T.M.; Yoon, S.S.; Beksac, M.; Dimopoulos, M.A.; Elghandour, A.; Jedrzejczak, W.W.; Günther, A.; Nakorn, T.N.; Siritanaratkul, N.; et al. Overall survival of patients with relapsed multiple myeloma treated with panobinostat or placebo plus bortezomib and dexamethasone (the PANORAMA 1 trial): A randomised, placebo-controlled, phase 3 trial. Lancet Haematol. 2016, 3, e506–e515. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Z.; Liu, J. Role of HDACs in normal and malignant hematopoiesis. Mol. Cancer 2020, 19, 5. [Google Scholar] [CrossRef]
- Boyault, C.; Zhang, Y.; Fritah, S.; Caron, C.; Gilquin, B.; So, H.K.; Garrido, C.; Yao, T.P.; Vourc’h, C.; Matthias, P.; et al. HDAC6 controls major cell response pathways to cytotoxic accumulation of protein aggregates. Genes Dev. 2007, 21, 2172–2181. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Ma, D.; Cheng, B.; Fang, Q.; Kuang, X.; Yu, K.; Wang, W.; Hu, B.; Wang, J. Crucial role of HO-1/IRF4-dependent apoptosis induced by panobinostat and lenalidomide in multiple myeloma. Exp. Cell Res. 2018, 363, 196–207. [Google Scholar] [CrossRef]
- Bat-Erdene, A.; Miki, H.; Oda, A.; Nakamura, S.; Teramachi, J.; Amachi, R.; Tenshin, H.; Hiasa, M.; Iwasa, M.; Harada, T.; et al. Synergistic targeting of Sp1, a critical transcription factor for myeloma cell growth and survival, by panobinostat and proteasome inhibitors. Oncotarget 2016, 7, 79064–79075. [Google Scholar] [CrossRef] [PubMed]
- Ntanasis-Stathopoulos, I.; Gavriatopoulou, M.; Terpos, E. Antibody therapies for multiple myeloma. Expert Opin. Biol. Ther. 2020, 20, 295–303. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; San-Miguel, J.; Belch, A.; White, D.; Benboubker, L.; Cook, G.; Leiba, M.; Morton, J.; Joy Ho, P.; Kim, K.; et al. Daratumumab plus lenalidomide and dexamethasone versus lenalidomide and dexamethasone in relapsed or refractory multiple myeloma: Updated analysis of POLLUX. Haematologica 2018, 103, 2088–2096. [Google Scholar] [CrossRef] [PubMed]
- Spencer, A.; Lentzsch, S.; Weisel, K.; Avet-Loiseau, H.; Mark, T.M.; Spicka, I.; Masszi, T.; Lauri, B.; Levin, M.D.; Bosi, A.; et al. Daratumumab plus bortezomib and dexamethasone versus bortezomib and dexamethasone in relapsed or refractory multiple myeloma: Updated analysis of CASTOR. Haematologica 2018, 103, 2079–2087. [Google Scholar] [CrossRef] [PubMed]
- Attal, M.; Richardson, P.G.; Rajkumar, S.V.; San-Miguel, J.; Beksac, M.; Spicka, I.; Leleu, X.; Schjesvold, F.; Moreau, P.; Dimopoulos, M.A.; et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): A randomised, multicentre, open-label, phase 3 study. Lancet 2019, 394, 2096–2107. [Google Scholar] [CrossRef]
- Van De Donk, N.W.C.J.; Richardson, P.G.; Malavasi, F. CD38 antibodies in multiple myeloma: Back to the future. Blood 2018, 131, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Van De Donk, N.W.C.J.; Usmani, S.Z. CD38 antibodies in multiple myeloma: Mechanisms of action and modes of resistance. Front. Immunol. 2018, 9, 2134. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, A.J.; Perrone, R.; Grozio, A.; Verdin, E. NAD+ metabolism and its roles in cellular processes during ageing. Nat. Rev. Mol. Cell Biol. 2020, 22, 119–141. [Google Scholar] [CrossRef]
- D’agostino, M.; Innorcia, S.; Boccadoro, M.; Bringhen, S. Monoclonal antibodies to treat multiple myeloma: A dream come true. Int. J. Mol. Sci. 2020, 21, 8192. [Google Scholar] [CrossRef] [PubMed]
- Dima, D.; Dower, J.; Comenzo, R.L.; Varga, C. Evaluating daratumumab in the treatment of multiple myeloma: Safety, efficacy and place in therapy. Cancer Manag. Res. 2020, 12, 7891–7903. [Google Scholar] [CrossRef]
- Kennedy, B.E.; Sadek, M.; Elnenaei, M.O.; Reiman, A.; Gujar, S.A. Targeting NAD+ Synthesis to Potentiate CD38-Based Immunotherapy of Multiple Myeloma. Trends Cancer 2020, 6, 9–12. [Google Scholar] [CrossRef]
- Baum, N.; Fliegert, R.; Bauche, A.; Hambach, J.; Menzel, S.; Haag, F.; Bannas, P.; Koch-Nolte, F. Daratumumab and nanobody-based heavy chain antibodies inhibit the adpr cyclase but not the nad+ hydrolase activity of cd38-expressing multiple myeloma cells. Cancers 2021, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Katsyuba, E.; Romani, M.; Hofer, D.; Auwerx, J. NAD+ homeostasis in health and disease. Nat. Metab. 2020, 2, 9–31. [Google Scholar] [CrossRef] [PubMed]
- Saville, K.M.; Clark, J.; Wilk, A.; Rogers, G.D.; Andrews, J.F.; Koczor, C.A.; Sobol, R.W. NAD+-mediated regulation of mammalian base excision repair. DNA Repair (Amst) 2020, 93, 102930. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.S.; Cohen, A.D.; Pazina, T. Mechanisms of NK cell activation and clinical activity of the therapeutic SLAMF7 antibody, elotuzumab in multiple myeloma. Front. Immunol. 2018, 9, 2551. [Google Scholar] [CrossRef] [PubMed]
- Lonial, S.; Dimopoulos, M.; Palumbo, A.; White, D.; Grosicki, S.; Spicka, I.; Walter-Croneck, A.; Moreau, P.; Mateos, M.-V.; Magen, H.; et al. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2015, 373, 621–631. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Lonial, S.; White, D.; Moreau, P.; Weisel, K.; San-Miguel, J.; Shpilberg, O.; Grosicki, S.; Špička, I.; Walter-Croneck, A.; et al. Elotuzumab, lenalidomide, and dexamethasone in RRMM: Final overall survival results from the phase 3 randomized ELOQUENT-2 study. Blood Cancer J. 2020, 10, 91. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Dytfeld, D.; Grosicki, S.; Moreau, P.; Takezako, N.; Hori, M.; Leleu, X.; LeBlanc, R.; Suzuki, K.; Raab, M.S.; et al. Elotuzumab plus Pomalidomide and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2018, 379, 1811–1822. [Google Scholar] [CrossRef]
- Suzuki, A.; Kakugawa, S.; Miyoshi, M.; Hori, M.; Suzuki, K.; Furukawa, Y.; Ohta, K. Soluble SLAMF7 is a predictive biomarker for elotuzumab therapy. Leukemia 2020, 34, 3088–3090. [Google Scholar] [CrossRef] [PubMed]
- Trudel, S.; Moreau, P.; Touzeau, C. Update on elotuzumab for the treatment of relapsed/refractory multiple myeloma: Patients’ selection and perspective. Onco. Targets. Ther. 2019, 12, 5813–5822. [Google Scholar] [CrossRef]
- Guo, H.; Cruz-Munoz, M.-E.; Wu, N.; Robbins, M.; Veillette, A. Immune Cell Inhibition by SLAMF7 Is Mediated by a Mechanism Requiring Src Kinases, CD45, and SHIP-1 That Is Defective in Multiple Myeloma Cells. Mol. Cell. Biol. 2015, 35, 41–51. [Google Scholar] [CrossRef]
- Balasa, B.; Yun, R.; Belmar, N.A.; Fox, M.; Chao, D.T.; Robbins, M.D.; Starling, G.C.; Rice, A.G. Elotuzumab enhances natural killer cell activation and myeloma cell killing through interleukin-2 and TNF-α pathways. Cancer Immunol. Immunother. 2015, 64, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Gunaratne, J.; Cheong, L.L.; Liu, S.C.; Koh, T.L.; Huang, G.; Blackstock, W.P.; Chng, W.J. Plasma membrane proteomics identifies biomarkers associated with MMSET overexpression in t(4;14) multiple myeloma. Oncotarget 2013, 4, 1008–1018. [Google Scholar] [CrossRef]
- Gabellini, C.; Trisciuoglio, D.; Bufalo, D. Del Non-canonical roles of Bcl-2 and Bcl-xL proteins: Relevance of BH4 domain. Carcinogenesis 2017, 38, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Jamil, S.; Stoica, C.; Hackett, T.L.; Duronio, V. MCL-1 localizes to sites of DNA damage and regulates DNA damage response. Cell Cycle 2010, 9, 2915–2927. [Google Scholar] [CrossRef]
- Mattoo, A.R.; Pandita, R.K.; Chakraborty, S.; Charaka, V.; Mujoo, K.; Hunt, C.R.; Pandita, T.K. MCL-1 Depletion Impairs DNA Double-Strand Break Repair and Reinitiation of Stalled DNA Replication Forks. Mol. Cell. Biol. 2017, 37, e00535-16. [Google Scholar] [CrossRef]
- Gong, J.N.; Khong, T.; Segal, D.; Yao, Y.; Riffkin, C.D.; Garnier, J.M.; Lin Khaw, S.; Lessene, G.; Spencer, A.; Herold, M.J.; et al. Hierarchy for targeting prosurvival BCL2 family proteins in multiple myeloma: Pivotal role of MCL1. Blood 2016, 128, 1834–1844. [Google Scholar] [CrossRef]
- Tron, A.E.; Belmonte, M.A.; Adam, A.; Aquila, B.M.; Boise, L.H.; Chiarparin, E.; Cidado, J.; Embrey, K.J.; Gangl, E.; Gibbons, F.D.; et al. Discovery of Mcl-1-specific inhibitor AZD5991 and preclinical activity in multiple myeloma and acute myeloid leukemia. Nat. Commun. 2018, 9, 5341. [Google Scholar] [CrossRef]
- Kotschy, A.; Szlavik, Z.; Murray, J.; Davidson, J.; Maragno, A.L.; Le Toumelin-Braizat, G.; Chanrion, M.; Kelly, G.L.; Gong, J.N.; Moujalled, D.M.; et al. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature 2016, 538, 477–482. [Google Scholar] [CrossRef]
- Touzeau, C.; Maciag, P.; Amiot, M.; Moreau, P. Targeting Bcl-2 for the treatment of multiple myeloma. Leukemia 2018, 32, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- Paner, A.; Patel, P.; Dhakal, B. The evolving role of translocation t(11;14) in the biology, prognosis, and management of multiple myeloma. Blood Rev. 2020, 41, 100643. [Google Scholar] [CrossRef] [PubMed]
- Punnoose, E.A.; Leverson, J.D.; Peale, F.; Boghaert, E.R.; Belmont, L.D.; Tan, N.; Young, A.; Mitten, M.; Ingalla, E.; Darbonne, W.C.; et al. Expression profile of BCL-2, BCL-XL, and MCL-1 predicts pharmacological response to the BCL-2 selective antagonist venetoclax in multiple myeloma models. Mol. Cancer Ther. 2016, 15, 1132–1144. [Google Scholar] [CrossRef]
- Chauhan, D.; Auclair, D.; Robinson, E.K.; Hideshima, T.; Li, G.; Podar, K.; Gupta, D.; Richardson, P.; Schlossma, R.L.; Krett, N.; et al. Identification of genes regulated by Dexamethasone in multiple myeloma cells using oligonucleotide arrays. Oncogene 2002, 21, 1346–1358. [Google Scholar] [CrossRef]
- Burington, B.; Barlogie, B.; Zhan, F.; Crowley, J.; Shaughnessy, J.D. Tumor cell gene expression changes following short-term in vivo exposure to single agent chemotherapeutics are related to survival in multiple myeloma. Clin. Cancer Res. 2008, 14, 4821. [Google Scholar] [CrossRef]
- Cannon-Albright, L.A.; Thomas, A.; Goldgar, D.E.; Gholami, K.; Rowe, K.; Jacobsen, M.; McWhorter, W.P.; Skolnick, M.H. Familiality of Cancer in Utah. Cancer Res. 1994, 54, 2378–2385. [Google Scholar] [PubMed]
- Landgren, O.; Linet, M.S.; McMaster, M.L.; Gridley, G.; Hemminki, K.; Goldin, L.R. Familial characteristics of autoimmune and hematologic disorders in 8,406 multiple myeloma patients: A population-based case-control study. Int. J. Cancer 2006, 118, 3095–3098. [Google Scholar] [CrossRef]
- Albright, F.; Teerlink, C.; Werner, T.L.; Cannon-Albright, L.A. Significant evidence for a heritable contribution to cancer predisposition: A review of cancer familiality by site. BMC Cancer 2012, 12, 138. [Google Scholar] [CrossRef]
- Schinasi, L.H.; Brown, E.E.; Camp, N.J.; Wang, S.S.; Hofmann, J.N.; Chiu, B.C.; Miligi, L.; Beane Freeman, L.E.; de Sanjose, S.; Bernstein, L.; et al. Multiple myeloma and family history of lymphohaematopoietic cancers: Results from the International Multiple Myeloma Consortium. Br. J. Haematol. 2016, 175, 87–101. [Google Scholar] [CrossRef]
- Landgren, O.; Kristinsson, S.Y.; Goldin, L.R.; Caporaso, N.E.; Blimark, C.; Mellqvist, U.H.; Wahlin, A.; Bjorkholm, M.; Turesson, I. Risk of plasma cell and lymphoproliferative disorders among 14 621 first-degree relatives of 4458 patients with monoclonal gammopathy of undetermined significance in Sweden. Blood 2009, 114, 791–795. [Google Scholar] [CrossRef]
- Greenberg, A.J.; Vincent Rajkumar, S.; Larson, D.R.; Dispenzieri, A.; Therneau, T.M.; Colby, C.L.; Phelps, T.K.; Kumar, S.K.; Katzmann, J.A.; Kyle, R.A.; et al. Increased prevalence of light chain monoclonal gammopathy of undetermined significance (LC-MGUS) in first-degree relatives of individuals with multiple myeloma. Br. J. Haematol. 2012, 157, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Vachon, C.M.; Kyle, R.A.; Therneau, T.M.; Foreman, B.J.; Larson, D.R.; Colby, C.L.; Phelps, T.K.; Dispenzieri, A.; Kumar, S.K.; Katzmann, J.A.; et al. Increased risk of monoclonal gammopathy in first-degree relatives of patients with multiple myeloma or monoclonal gammopathy of undetermined significance. Blood 2009, 114, 785–790. [Google Scholar] [CrossRef]
- Wei, X.; Calvo-Vidal, M.N.; Chen, S.; Wu, G.; Revuelta, M.V.; Sun, J.; Zhang, J.; Walsh, M.F.; Nichols, K.E.; Joseph, V.; et al. Germline lysine-specific demethylase 1 (lsd1/kdm1a) mutations confer susceptibility to multiple myeloma. Cancer Res. 2018, 78, 2747–2759. [Google Scholar] [CrossRef] [PubMed]
- Saleque, S.; Kim, J.; Rooke, H.M.; Orkin, S.H. Epigenetic Regulation of Hematopoietic Differentiation by Gfi-1 and Gfi-1b Is Mediated by the Cofactors CoREST and LSD1. Mol. Cell 2007, 27, 562–572. [Google Scholar] [CrossRef]
- De Smedt, E.; Lui, H.; Maes, K.; De Veirman, K.; Menu, E.; Vanderkerken, K.; De Bruyne, E. The epigenome in multiple myeloma: Impact on tumor cell plasticity and drug response. Front. Oncol. 2018, 8, 566. [Google Scholar] [CrossRef]
- Shi, Y.J.; Matson, C.; Lan, F.; Iwase, S.; Baba, T.; Shi, Y. Regulation of LSD1 histone demethylase activity by its associated factors. Mol. Cell 2005, 19, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Huang, W.; Qiu, R.; Liu, R.; Zeng, Y.; Gao, J.; Zheng, Y.; Hou, Y.; Wang, S.; Yu, W.; et al. LSD1 coordinates with the SIN3A/HDAC complex and maintains sensitivity to chemotherapy in breast cancer. J. Mol. Cell Biol. 2018, 10, 285–301. [Google Scholar] [CrossRef]
- Gu, F.; Lin, Y.; Wang, Z.; Wu, X.; Ye, Z.; Wang, Y.; Lan, H. Biological roles of LSD1 beyond its demethylase activity. Cell. Mol. Life Sci. 2020, 77, 3341–3350. [Google Scholar] [CrossRef]
- Hyun, K.; Jeon, J.; Park, K.; Kim, J. Writing, erasing and reading histone lysine methylations. Exp. Mol. Med. 2017, 49, 324. [Google Scholar] [CrossRef]
- Nanou, A.; Toumpeki, C.; Lavigne, M.D.; Lazou, V.; Demmers, J.; Paparountas, T.; Thanos, D.; Katsantoni, E. The dual role of LSD1 and HDAC3 in STAT5-dependent transcription is determined by protein interactions, binding affinities, motifs and genomic positions. Nucleic Acids Res. 2017, 45, 142–154. [Google Scholar] [CrossRef]
- Karakaidos, P.; Verigos, J.; Magklara, A. Lsd1/kdm1a, a gate-keeper of cancer stemness and a promising therapeutic target. Cancers 2019, 11, 1821. [Google Scholar] [CrossRef] [PubMed]
- Majello, B.; Gorini, F.; Saccà, C.D.; Amente, S. Expanding the role of the histone lysine-specific demethylase lsd1 in cancer. Cancers 2019, 11, 324. [Google Scholar] [CrossRef]
- Hatzi, K.; Geng, H.; Doane, A.S.; Meydan, C.; LaRiviere, R.; Cardenas, M.; Duy, C.; Shen, H.; Vidal, M.N.C.; Baslan, T.; et al. Histone demethylase LSD1 is required for germinal center formation and BCL6-driven lymphomagenesis. Nat. Immunol. 2019, 20, 86–96. [Google Scholar] [CrossRef]
- Roco, J.A.; Mesin, L.; Binder, S.C.; Nefzger, C.; Gonzalez-Figueroa, P.; Canete, P.F.; Ellyard, J.; Shen, Q.; Robert, P.A.; Cappello, J.; et al. Class-Switch Recombination Occurs Infrequently in Germinal Centers. Immunity 2019, 51, 337–350.e7. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Sengupta, R.; Espejo, A.B.; Lee, M.G.; Dorsey, J.A.; Richter, M.; Opravil, S.; Shiekhattar, R.; Bedford, M.T.; Jenuwein, T.; et al. p53 is regulated by the lysine demethylase LSD1. Nature 2007, 449, 105–108. [Google Scholar] [CrossRef]
- Escoubet-Lozach, L.; Lin, I.L.; Jensen-Pergakes, K.; Brady, H.A.; Gandhi, A.K.; Schafer, P.H.; Muller, G.W.; Worland, P.J.; Chan, K.W.H.; Verhelle, D. Pomalidomide and lenalidomide induce p21WAF-1 expression in both lymphoma and multiple myeloma through a LSD1-mediated epigenetic mechanism. Cancer Res. 2009, 69, 7347–7356. [Google Scholar] [CrossRef]
- Waller, R.G.; Darlington, T.M.; Wei, X.; Madsen, M.J.; Thomas, A.; Curtin, K.; Coon, H.; Rajamanickam, V.; Musinsky, J.; Jayabalan, D.; et al. Novel pedigree analysis implicates DNA repair and chromatin remodeling in multiple myeloma risk. PLOS Genet. 2018, 14, e1007111. [Google Scholar] [CrossRef]
- Masliah-Planchon, J.; Bièche, I.; Guinebretière, J.-M.; Bourdeaut, F.; Delattre, O. SWI/SNF Chromatin Remodeling and Human Malignancies. Annu. Rev. Pathol. Mech. Dis. 2015, 10, 145–171. [Google Scholar] [CrossRef]
- Mathur, R. ARID1A loss in cancer: Towards a mechanistic understanding. Pharmacol. Ther. 2018, 190, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Imai, K. Microsatellite instability: An update. Arch. Toxicol. 2015, 89, 899–921. [Google Scholar] [CrossRef]
- Dykhuizen, E.C.; Hargreaves, D.C.; Miller, E.L.; Cui, K.; Korshunov, A.; Kool, M.; Pfister, S.; Cho, Y.J.; Zhao, K.; Crabtree, G.R. BAF complexes facilitate decatenation of DNA by topoisomerase IIα. Nature 2013, 497, 624–627. [Google Scholar] [CrossRef]
- Trizzino, M.; Barbieri, E.; Petracovici, A.; Wu, S.; Welsh, S.A.; Owens, T.A.; Licciulli, S.; Zhang, R.; Gardini, A. The Tumor Suppressor ARID1A Controls Global Transcription via Pausing of RNA Polymerase II. Cell Rep. 2018, 23, 3933–3945. [Google Scholar] [CrossRef] [PubMed]
- Perez-Oliva, A.B.; Lachaud, C.; Szyniarowski, P.; Muñoz, I.; Macartney, T.; Hickson, I.; Rouse, J.; Alessi, D.R. USP 45 deubiquitylase controls ERCC 1– XPF endonuclease-mediated DNA damage responses. EMBO J. 2015, 34, 326–343. [Google Scholar] [CrossRef]
- Schrader, C.E.; Vardo, J.; Linehan, E.; Twarog, M.Z.; Niedernhofer, L.J.; Hoeijmakers, J.H.J.; Stavnezer, J. Deletion of the nucleotide excision repair gene Ercc1 reduces immunoglobulin class switching and alters mutations near switch recombination junctions. J. Exp. Med. 2004, 200, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Weißbach, S.; Langer, C.; Puppe, B.; Nedeva, T.; Bach, E.; Kull, M.; Bargou, R.; Einsele, H.; Rosenwald, A.; Knop, S.; et al. The molecular spectrum and clinical impact of DIS3 mutations in multiple myeloma. Br. J. Haematol. 2015, 169, 57–70. [Google Scholar] [CrossRef]
- Bolli, N.; Biancon, G.; Moarii, M.; Gimondi, S.; Li, Y.; de Philippis, C.; Maura, F.; Sathiaseelan, V.; Tai, Y.T.Y.T.; Mudie, L.; et al. Analysis of the genomic landscape of multiple myeloma highlights novel prognostic markers and disease subgroups. Leukemia 2018, 32, 2604–2616. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Heredia, Y.; Sánchez-Vega, B.; Onecha, E.; Barrio, S.; Alonso, R.; Martínez-ávila, J.C.; Cuenca, I.; Agirre, X.; Braggio, E.; Hernández, M.T.; et al. Mutational screening of newly diagnosed multiple myeloma patients by deep targeted sequencing. Haematologica 2018, 103, e544–e548. [Google Scholar] [CrossRef]
- Pertesi, M.; Vallée, M.; Wei, X.; Revuelta, M.V.; Galia, P.; Demangel, D.; Oliver, J.; Foll, M.; Chen, S.; Perrial, E.; et al. Exome sequencing identifies germline variants in DIS3 in familial multiple myeloma. Leukemia 2019, 33, 2324–2330. [Google Scholar] [CrossRef]
- Laffleur, B.; Basu, U.; Lim, J. RNA Exosome and Non-coding RNA-Coupled Mechanisms in AID-Mediated Genomic Alterations. J. Mol. Biol. 2017, 429, 3230–3241. [Google Scholar] [CrossRef]
- Basu, U.; Meng, F.L.; Keim, C.; Grinstein, V.; Pefanis, E.; Eccleston, J.; Zhang, T.; Myers, D.; Wasserman, C.R.; Wesemann, D.R.; et al. The RNA exosome targets the AID cytidine deaminase to both strands of transcribed duplex DNA substrates. Cell 2011, 144, 353–363. [Google Scholar] [CrossRef]
- Bolli, N.; Barcella, M.; Salvi, E.; D’Avila, F.; Vendramin, A.; De Philippis, C.; Munshi, N.C.; Avet-Loiseau, H.; Campbell, P.J.; Mussetti, A.; et al. Next-generation sequencing of a family with a high penetrance of monoclonal gammopathies for the identification of candidate risk alleles. Cancer 2017, 123, 3701–3708. [Google Scholar] [CrossRef]
- Dilworth, D.; Liu, L.; Stewart, A.K.; Berenson, J.R.; Lassam, N.; Hogg, D. Germline CDKN2A mutation implicated in predisposition to multiple myeloma. Blood 2000, 95, 1869–1871. [Google Scholar] [CrossRef]
- Shah, V.; Boyd, K.D.; Houlston, R.S.; Kaiser, M.F. Constitutional mutation in CDKN2A is associated with long term survivorship in multiple myeloma: A case report. BMC Cancer 2017, 17, 718. [Google Scholar] [CrossRef]
- Rayess, H.; Wang, M.B.; Srivatsan, E.S. Cellular senescence and tumor suppressor gene p16. Int. J. Cancer 2012, 130, 1715–1725. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Sharpless, N.E. Senescence in Health and Disease. Cell 2017, 169, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J. The INK4a/ARF network in tumour suppression. Nat. Rev. Mol. Cell Biol. 2001, 2, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Tessoulin, B.; Eveillard, M.; Lok, A.; Chiron, D.; Moreau, P.; Amiot, M.; Moreau-Aubry, A.; Le Gouill, S.; Pellat-Deceunynck, C. p53 dysregulation in B-cell malignancies: More than a single gene in the pathway to hell. Blood Rev. 2017, 31, 251–259. [Google Scholar] [CrossRef]
- Stanganelli, C.; Arbelbide, J.; Fantl, D.B.; Corrado, C.; Slavutsky, I. DNA methylation analysis of tumor suppressor genes in monoclonal gammopathy of undetermined significance. Ann. Hematol. 2010, 89, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.H.L.; Chung, Y.F.; Lo, K.W.; Wickham, N.W.R.; Lee, J.C.K.; Huang, D.P. Frequent hypermethylation of p16 and p15 genes in multiple myeloma. Blood 1997, 89, 2500–2506. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, T.; Chikatsu, N.; Takahashi, S.; Fujita, A.; Uchimaru, K.; Asano, S.; Fujita, T.; Motokura, T. Expression of p16(INK4A) and p14(ARF) in hematological malignancies. Leukemia 1999, 13, 1760–1769. [Google Scholar] [CrossRef]
- Chubb, D.; Weinhold, N.; Broderick, P.; Chen, B.; Johnson, D.C.; Försti, A.; Vijayakrishnan, J.; Migliorini, G.; Dobbins, S.E.; Holroyd, A.; et al. Common variation at 3q26.2, 6p21.33, 17p11.2 and 22q13.1 influences multiple myeloma risk. Nat. Genet. 2013, 45, 1221–1225. [Google Scholar] [CrossRef]
- Mitchell, J.S.; Li, N.; Weinhold, N.; Försti, A.; Ali, M.; Van Duin, M.; Thorleifsson, G.; Johnson, D.C.; Chen, B.; Halvarsson, B.M.; et al. Genome-wide association study identifies multiple susceptibility loci for multiple myeloma. Nat. Commun. 2016, 7, 12050. [Google Scholar] [CrossRef]
- Went, M.; Sud, A.; Försti, A.; Halvarsson, B.M.; Weinhold, N.; Kimber, S.; van Duin, M.; Thorleifsson, G.; Holroyd, A.; Johnson, D.C.; et al. Identification of multiple risk loci and regulatory mechanisms influencing susceptibility to multiple myeloma. Nat. Commun. 2018, 9, 3707. [Google Scholar] [CrossRef] [PubMed]
- Pertesi, M.; Went, M.; Hansson, M.; Hemminki, K.; Houlston, R.S.; Nilsson, B. Genetic predisposition for multiple myeloma. Leukemia 2020, 34, 697–708. [Google Scholar] [CrossRef]
- Janz, S.; Zhan, F.; Sun, F.; Cheng, Y.; Pisano, M.; Yang, Y.; Goldschmidt, H.; Hari, P. Germline risk contribution to genomic instability in multiple myeloma. Front. Genet. 2019, 10, 424. [Google Scholar] [CrossRef]
- Broderick, P.; Chubb, D.; Johnson, D.C.; Weinhold, N.; Försti, A.; Lloyd, A.; Olver, B.; Ma, Y.P.; Dobbins, S.E.; Walker, B.A.; et al. Common variation at 3p22.1 and 7p15.3 influences multiple myeloma risk. Nat. Genet. 2012, 44, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Weinhold, N.; Johnson, D.C.; Chubb, D.; Chen, B.; Försti, A.; Hosking, F.J.; Broderick, P.; Ma, Y.P.; Dobbins, S.E.; Hose, D.; et al. The CCND1 c.870G>A polymorphism is a risk factor for t(11;14)(q13;q32) multiple myeloma. Nat. Genet. 2013, 45, 522–525. [Google Scholar] [CrossRef]
- Martino, A.; Campa, D.; Jamroziak, K.; Reis, R.M.; Sainz, J.; Buda, G.; García-Sanz, R.; Lesueur, F.; Marques, H.; Moreno, V.; et al. Impact of polymorphic variation at 7p15.3, 3p22.1 and 2p23.3 loci on risk of multiple myeloma. Br. J. Haematol. 2012, 158, 805–809. [Google Scholar] [CrossRef]
- Erickson, S.W.; Raj, V.R.; Stephens, O.W.; Dhakal, I.; Chavan, S.S.; Sanathkumar, N.; Coleman, E.A.; Lee, J.Y.; Goodwin, J.A.; Apewokin, S.; et al. Genome-wide scan identifies variant in 2q12.3 associated with risk for multiple myeloma. Blood 2014, 124, 2001–2003. [Google Scholar] [CrossRef] [PubMed]
- Weinhold, N.; Johnson, D.C.; Rawstron, A.C.; Försti, A.; Doughty, C.; Vijayakrishnan, J.; Broderick, P.; Dahir, N.B.; Begum, D.B.; Hosking, F.J.; et al. Inherited genetic susceptibility to monoclonal gammopathy of unknown significance. Blood 2014, 123, 2513–2517. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, H.; Campo, C.; Weinhold, N.; da Silva Filho, M.I.; Pour, L.; Gregora, E.; Vodicka, P.; Vodickova, L.; Hoffmann, P.; Nöthen, M.M.; et al. Genomewide association study on monoclonal gammopathy of unknown significance (MGUS). Eur. J. Haematol. 2017, 99, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Went, M.; Kinnersley, B.; Sud, A.; Johnson, D.C.; Weinhold, N.; Försti, A.; van Duin, M.; Orlando, G.; Mitchell, J.S.; Kuiper, R.; et al. Transcriptome-wide association study of multiple myeloma identifies candidate susceptibility genes. Hum. Genomics 2019, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Clay-Gilmour, A.I.; Hildebrandt, M.A.T.; Brown, E.E.; Hofmann, J.N.; Spinelli, J.J.; Giles, G.G.; Cozen, W.; Bhatti, P.; Wu, X.; Waller, R.G.; et al. Coinherited genetics of multiple myeloma and its precursor, monoclonal gammopathy of undetermined significance. Blood Adv. 2020, 4, 2789–2797. [Google Scholar] [CrossRef] [PubMed]
- Piunti, A.; Shilatifard, A. Epigenetic balance of gene expression by polycomb and compass families. Science 2016, 352. [Google Scholar] [CrossRef]
- Barbour, H.; Daou, S.; Hendzel, M.; Affar, E.B. Polycomb group-mediated histone H2A monoubiquitination in epigenome regulation and nuclear processes. Nat. Commun. 2020, 11, 5947. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.L.; Gil, J.; Hernando, E.; Teruya-Feldstein, J.; Narita, M.; Martínez, D.; Visakorpi, T.; Mu, D.; Cordon-Cardo, C.; Peters, G.; et al. Role of the chromobox protein CBX7 in lymphomagenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 5389–5394. [Google Scholar] [CrossRef]
- Hu, D.; Shilatifard, A. Epigenetics of hematopoiesis and hematological malignancies. Genes Dev. 2016, 30, 2021–2041. [Google Scholar] [CrossRef]
- Di Carlo, V.; Mocavini, I.; Di Croce, L. Polycomb complexes in normal and malignant hematopoiesis. J. Cell Biol. 2019, 218, 55–69. [Google Scholar] [CrossRef]
- Ginjala, V.; Nacerddine, K.; Kulkarni, A.; Oza, J.; Hill, S.J.; Yao, M.; Citterio, E.; van Lohuizen, M.; Ganesan, S. BMI1 Is Recruited to DNA Breaks and Contributes to DNA Damage-Induced H2A Ubiquitination and Repair. Mol. Cell. Biol. 2011, 31, 1972–1982. [Google Scholar] [CrossRef]
- Ismail, H.; Andrin, C.; McDonald, D.; Hendzel, M.J. BMI1-mediated histone ubiquitylation promotes DNA double-strand break repair. J. Cell Biol. 2010, 191, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Jagani, Z.; Wiederschain, D.; Loo, A.; He, D.; Mosher, R.; Fordjour, P.; Monahan, J.; Morrissey, M.; Yao, Y.M.; Lengauer, C.; et al. The polycomb group protein Bmi-1 is essential for the growth of multiple myeloma cells. Cancer Res. 2010, 70, 5528–5538. [Google Scholar] [CrossRef]
- Wu, S.Q.; Xu, Z.Z.; Niu, W.Y.; Huang, H.B.; Zhan, R. shRNA-mediated Bmi-1 silencing sensitizes multiple myeloma cells to bortezomib. Int. J. Mol. Med. 2014, 34, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Laugesen, A.; Højfeldt, J.W.; Helin, K. Role of the polycomb repressive complex 2 (PRC2) in transcriptional regulation and cancer. Cold Spring Harb. Perspect. Med. 2016, 6, a026575. [Google Scholar] [CrossRef] [PubMed]
- Bracken, A.P.; Kleine-Kohlbrecher, D.; Dietrich, N.; Pasini, D.; Gargiulo, G.; Beekman, C.; Theilgaard-Mönch, K.; Minucci, S.; Porse, B.T.; Marine, J.C.; et al. The Polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells. Genes Dev. 2007, 21, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Agherbi, H.; Gaussmann-Wenger, A.; Verthuy, C.; Chasson, L.; Serrano, M.; Djabali, M. Polycomb mediated epigenetic silencing and replication timing at the INK4a/ARF locus during senescence. PLoS ONE 2009, 4, e5622. [Google Scholar] [CrossRef]
- Rand, K.A.; Song, C.; Dean, E.; Serie, D.J.; Curtin, K.; Sheng, X.; Hu, D.; Huff, C.A.; Bernal-Mizrachi, L.; Tomasson, H.M.; et al. A meta-analysis of multiple myeloma risk regions in African and European ancestry populations identifies putatively functional loci. Cancer Epidemiol. Biomarkers Prev. 2016, 25, 1609–1618. [Google Scholar] [CrossRef]
- Weinhold, N.; Meissner, T.; Johnson, D.C.; Seckinger, A.; Moreaux, J.; Försti, A.; Chen, B.; Nickel, J.; Chubb, D.; Rawstron, A.C.; et al. The 7p15.3 (rs4487645) association for multiple myeloma shows strong allele-specific regulation of the myc-interacting gene cdca7l in malignant plasma cells. Haematologica 2015, 100, e110–e113. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Johnson, D.C.; Weinhold, N.; Studd, J.B.; Orlando, G.; Mirabella, F.; Mitchell, J.S.; Meissner, T.; Kaiser, M.; Goldschmidt, H.; et al. Multiple myeloma risk variant at 7p15.3 creates an IRF4-binding site and interferes with CDCA7L expression. Nat. Commun. 2016, 7, 13656. [Google Scholar] [CrossRef]
- Ji, Q.K.; Ma, J.W.; Liu, R.H.; Li, X.S.; Shen, F.Z.; Huang, L.Y.; Hui, L.; Ma, Y.J.; Jin, B.Z. CDCA7L promotes glioma proliferation by targeting CCND1 and predicts an unfavorable prognosis. Mol. Med. Rep. 2019, 20, 1149–1156. [Google Scholar] [CrossRef]
- Hop, P.J.; Luijk, R.; Daxinger, L.; Iterson, M.V.; Dekkers, K.F.; Jansen, R.; Meurs, J.B.J.V.; Hoen, P.A.C.T.; Ikram, M.A.; Greevenbroek, M.M.J.V.; et al. Genome-wide identification of genes regulating DNA methylation using genetic anchors for causal inference. Genome Biol. 2020, 21, 220. [Google Scholar] [CrossRef]
- Karki, K.; Harishchandra, S.; Safe, S. Bortezomib targets SP transcription factors in cancer cells s. Mol. Pharmacol. 2018, 94, 1187–1196. [Google Scholar] [CrossRef]
- Winkelmann, R.; Sandrock, L.; Kirberg, J.; Jäck, H.M.; Schuh, W. KLF2- a negative regulator of pre-B cell clonal expansion and B cell activation. PLoS ONE 2014, 9, e97953. [Google Scholar]
- Salama, Y.; Heida, A.H.; Yokoyama, K.; Takahashi, S.; Hattori, K.; Heissig, B. The EGFL7-ITGB3-KLF2 axis enhances survival of multiple myeloma in preclinical models. Blood Adv. 2020, 4, 1021–1037. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Ajore, R.; Wihlborg, A.K.; Niroula, A.; Swaminathan, B.; Johnsson, E.; Stephens, O.W.; Morgan, G.; Meissner, T.; Turesson, I.; et al. The multiple myeloma risk allele at 5q15 lowers ELL2 expression and increases ribosomal gene expression. Nat. Commun. 2018, 9, 1649. [Google Scholar] [CrossRef]
- Swaminathan, B.; Thorleifsson, G.; Jöud, M.; Ali, M.; Johnsson, E.; Ajore, R.; Sulem, P.; Halvarsson, B.M.; Eyjolfsson, G.; Haraldsdottir, V.; et al. Variants in ELL2 influencing immunoglobulin levels associate with multiple myeloma. Nat. Commun. 2015, 6, 12. [Google Scholar] [CrossRef]
- Martincic, K.; Alkan, S.A.; Cheatle, A.; Borghesi, L.; Milcarek, C. Transcription elongation factor ELL2 directs immunoglobulin secretion in plasma cells by stimulating altered RNA processing. Nat. Immunol. 2009, 10, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Campa, D.; Martino, A.; Macauda, A.; Dudziński, M.; Suska, A.; Druzd-Sitek, A.; Raab, M.S.; Moreno, V.; Huhn, S.; Butrym, A.; et al. Genetic polymorphisms in genes of class switch recombination and multiple myeloma risk and survival: An IMMEnSE study. Leuk. Lymphoma 2019, 60, 1803–1811. [Google Scholar] [CrossRef]
- Roddam, P.L.; Rollinson, S.; O’Driscoll, M.; Jeggo, P.A.; Jack, A.; Morgan, G.J. Genetic variants of NHEJ DNA ligase IV can affect the risk of developing multiple myeloma, a tumour characterised by aberrant class switch recombination. J. Med. Genet. 2002, 39, 900–905. [Google Scholar] [CrossRef]
- Hayden, P.J.; Tewari, P.; Morris, D.W.; Staines, A.; Crowley, D.; Nieters, A.; Becker, N.; De sanjosé, S.; Foretova, L.; Maynadié, M.; et al. Variation in DNA repair genes XRCC3, XRCC4, XRCC5 and susceptibility to myeloma. Hum. Mol. Genet. 2007, 16, 3117–3127. [Google Scholar] [CrossRef]
- Elia, A.E.H.; Wang, D.C.; Willis, N.A.; Boardman, A.P.; Hajdu, I.; Adeyemi, R.O.; Lowry, E.; Gygi, S.P.; Scully, R.; Elledge, S.J. RFWD3-Dependent Ubiquitination of RPA Regulates Repair at Stalled Replication Forks. Mol. Cell 2015, 60, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Inano, S.; Sato, K.; Katsuki, Y.; Kobayashi, W.; Tanaka, H.; Nakajima, K.; Nakada, S.; Miyoshi, H.; Knies, K.; Takaori-Kondo, A.; et al. RFWD3-Mediated Ubiquitination Promotes Timely Removal of Both RPA and RAD51 from DNA Damage Sites to Facilitate Homologous Recombination. Mol. Cell 2017, 66, 622–634.e8. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Pathania, S. RPA, RFWD3 and BRCA2 at stalled forks: A balancing act. Mol. Cell. Oncol. 2020, 7, 1801089. [Google Scholar] [CrossRef]
- Gallina, I.; Hendriks, I.A.; Hoffmann, S.; Larsen, N.B.; Johansen, J.; Colding-Christensen, C.S.; Schubert, L.; Sellés-Baiget, S.; Fábián, Z.; Kühbacher, U.; et al. The ubiquitin ligase RFWD3 is required for translesion DNA synthesis. Mol. Cell 2021, 81, 442–458.e9. [Google Scholar] [CrossRef]
- Feeney, L.; Muñoz, I.M.; Lachaud, C.; Toth, R.; Appleton, P.L.; Schindler, D.; Rouse, J. RPA-Mediated Recruitment of the E3 Ligase RFWD3 Is Vital for Interstrand Crosslink Repair and Human Health. Mol. Cell 2017, 66, 610–621.e4. [Google Scholar] [CrossRef]
- Salzer, U.; Jennings, S.; Grimbacher, B. To switch or not to switch—The opposing roles of TACI in terminal B cell differentiation. Eur. J. Immunol. 2007, 37, 17–20. [Google Scholar] [CrossRef]
- Wang, T.; Liu, N.S.; Seet, L.F.; Hong, W. The emerging role of VHS domain-containing Tom1, Tom1L1 and Tom1L2 in membrane trafficking. Traffic 2010, 11, 1119–1128. [Google Scholar] [CrossRef]
- Pengo, N.; Scolari, M.; Oliva, L.; Milan, E.; Mainoldi, F.; Raimondi, A.; Fagioli, C.; Merlini, A.; Mariani, E.; Pasqualetto, E.; et al. Plasma cells require autophagy for sustainable immunoglobulin production. Nat. Immunol. 2013, 14, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Cenci, S. Autophagy, a new determinant of plasma cell differentiation and antibody responses. Mol. Immunol. 2014, 62, 289–295. [Google Scholar] [CrossRef]
- Greenberg, A.J.; Lee, A.M.; Serie, D.J.; McDonnell, S.K.; Cerhan, J.R.; Liebow, M.; Larson, D.R.; Colby, C.L.; Norman, A.D.; Kyle, R.A.; et al. Single-nucleotide polymorphism rs1052501 associated with monoclonal gammopathy of undetermined significance and multiple myeloma. Leukemia 2013, 27, 515–516. [Google Scholar] [CrossRef]
- Preuss, F.; Chatterjee, D.; Mathea, S.; Shrestha, S.; St-Germain, J.; Saha, M.; Kannan, N.; Raught, B.; Rottapel, R.; Knapp, S. Nucleotide Binding, Evolutionary Insights, and Interaction Partners of the Pseudokinase Unc-51-like Kinase 4. Structure 2020, 28, 1184–1196.e6. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Rajendran, V.; Sethumadhavan, R.; Purohit, R. CEP proteins: The knights of centrosome dynasty. Protoplasma 2013, 250, 965–983. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.J.R.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Hoang, P.H.; Cornish, A.J.; Dobbins, S.E.; Kaiser, M.; Houlston, R.S. Mutational processes contributing to the development of multiple myeloma. Blood Cancer J. 2019, 9, 60. [Google Scholar] [CrossRef]
- Lawrence, M.S.; Stojanov, P.; Polak, P.; Kryukov, G.V.; Cibulskis, K.; Sivachenko, A.; Carter, S.L.; Stewart, C.; Mermel, C.H.; Roberts, S.A.; et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013, 499, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Leong, T.; Quam, L.; Billadeau, D.; Kay, N.E.; Greipp, P.; Kyle, R.A.; Oken, M.M.; Van Ness, B. Activating Mutations of N- And K-ras in Multiple Myeloma Show Different Clinical Associations: Analysis of the Eastern Cooperative Oncology Group Phase III Trial. Blood 1996, 88, 2699–2706. [Google Scholar] [CrossRef]
- Hoang, P.H.; Dobbins, S.E.; Cornish, A.J.; Chubb, D.; Law, P.J.; Kaiser, M.; Houlston, R.S. Whole-genome sequencing of multiple myeloma reveals oncogenic pathways are targeted somatically through multiple mechanisms. Leukemia 2018, 32, 2459–2470. [Google Scholar] [CrossRef]
- Annunziata, C.M.; Davis, R.E.; Demchenko, Y.; Bellamy, W.; Gabrea, A.; Zhan, F.; Lenz, G.; Hanamura, I.; Wright, G.; Xiao, W.; et al. Frequent Engagement of the Classical and Alternative NF-κB Pathways by Diverse Genetic Abnormalities in Multiple Myeloma. Cancer Cell 2007, 12, 115–130. [Google Scholar] [CrossRef]
- Keats, J.J.; Fonseca, R.; Chesi, M.; Schop, R.; Baker, A.; Chng, W.J.; Van Wier, S.; Tiedemann, R.; Shi, C.X.; Sebag, M.; et al. Promiscuous Mutations Activate the Noncanonical NF-κB Pathway in Multiple Myeloma. Cancer Cell 2007, 12, 131–144. [Google Scholar] [CrossRef]
- Mithraprabhu, S.; Hocking, J.; Ramachandran, M.; Choi, K.; Klarica, D.; Khong, T.; Reynolds, J.; Spencer, A. DNA-repair gene mutations are highly prevalent in circulating tumour dna from multiple myeloma patients. Cancers 2019, 11, 917. [Google Scholar] [CrossRef]
- Jovanović, K.K.; Escure, G.; Demonchy, J.; Willaume, A.; Van De Wyngaert, Z.; Farhat, M.; Chauvet, P.; Facon, T.; Quesnel, B.; Manier, S. Deregulation and targeting of TP53 pathway in multiple myeloma. Front. Oncol. 2019, 9, 665. [Google Scholar] [CrossRef]
- Kunz, V.; Bommert, K.S.; Kruk, J.; Schwinning, D.; Chatterjee, M.; Stühmer, T.; Bargou, R.; Bommert, K. Targeting of the E3 ubiquitin-protein ligase HUWE1 impairs DNA repair capacity and tumor growth in preclinical multiple myeloma models. Sci. Rep. 2020, 10, 18419. [Google Scholar] [CrossRef]
- Dumontet, C.; Landi, S.; Reiman, T.; Perry, T.; Plesa, A.; Bellini, I.; Barale, R.; Pilarski, L.M.; Troncy, J.; Tavtigian, S.; et al. Genetic polymorphisms associated with outcome in multiple myeloma patients receiving high-dose melphalan. Bone Marrow Transplant. 2010, 45, 1316–1324. [Google Scholar] [CrossRef]
- Kumar, S.; Talluri, S.; Pal, J.; Yuan, X.; Lu, R.; Nanjappa, P.; Samur, M.K.; Munshi, N.C.; Shammas, M.A. Role of apurinic/apyrimidinic nucleases in the regulation of homologous recombination in myeloma: Mechanisms and translational significance. Blood Cancer J. 2018, 8, 92. [Google Scholar] [CrossRef]
- Keats, J.J.; Speyer, G.; Christofferson, A.; Legendre, C.; Aldrich, J.; Russell, M.; Cuyugan, L.; Adkins, J.; Blanski, A.; Hodges, M.; et al. Molecular Predictors of Outcome and Drug Response in Multiple Myeloma: An Interim Analysis of the Mmrf CoMMpass Study. Blood 2016, 128, 194. [Google Scholar] [CrossRef]
- Boyd, K.D.; Ross, F.M.; Walker, B.A.; Wardell, C.P.; Tapper, W.J.; Chiecchio, L.; Dagrada, G.P.; Konn, Z.J.; Gregory, W.M.; Jackson, G.H.; et al. Mapping of chromosome 1p deletions in myeloma identifies FAM46C at 1p12 and CDKN2C at 1p32.3 as being genes in regions associated with adverse survival. Clin. Cancer Res. 2011, 17, 7776–7784. [Google Scholar] [CrossRef]
- Barbieri, M.; Manzoni, M.; Fabris, S.; Ciceri, G.; Todoerti, K.; Simeon, V.; Musto, P.; Cortelezzi, A.; Baldini, L.; Neri, A.; et al. Compendium of FAM46C gene mutations in plasma cell dyscrasias. Br. J. Haematol. 2016, 174, 642–645. [Google Scholar] [CrossRef]
- Mroczek, S.; Chlebowska, J.; Kuliński, T.M.; Gewartowska, O.; Gruchota, J.; Cysewski, D.; Liudkovska, V.; Borsuk, E.; Nowis, D.; Dziembowski, A. The non-canonical poly(A) polymerase FAM46C acts as an onco-suppressor in multiple myeloma. Nat. Commun. 2017, 8, 619. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, S.; Hu, J.; Wu, Y.; Ma, X.; Chen, Y.; Yu, B.; Liao, S.; Huang, H.; Gao, S. Structural and functional characterization of multiple myeloma associated cytoplasmic poly(A) polymerase FAM46C. Cancer Commun. 2021, 41, 615. [Google Scholar] [CrossRef] [PubMed]
- Herrero, A.B.; Quwaider, D.; Corchete, L.A.; Mateos, M.V.; García-Sanz, R.; Gutiérrez, N.C. FAM46C controls antibody production by the polyadenylation of immunoglobulin mRNAs and inhibits cell migration in multiple myeloma. J. Cell. Mol. Med. 2020, 24, 4171–4182. [Google Scholar] [CrossRef] [PubMed]
- Kuchta, K.; Muszewska, A.; Knizewski, L.; Steczkiewicz, K.; Wyrwicz, L.S.; Pawlowski, K.; Rychlewski, L.; Ginalski, K. FAM46 proteins are novel eukaryotic non-canonical poly(A) polymerases. Nucleic Acids Res. 2016, 44, 3534–3548. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Gong, Q.; Wang, Y.; Li, M.; Wang, L.; Ding, H.; Li, P. The biological function and clinical significance of SF3B1 mutations in cancer. Biomark. Res. 2020, 8, 38. [Google Scholar] [CrossRef]
- Hofman, I.J.F.; Patchett, S.; Van Duin, M.; Geerdens, E.; Verbeeck, J.; Michaux, L.; Delforge, M.; Sonneveld, P.; Johnson, A.W.; De Keersmaecker, K. Low frequency mutations in ribosomal proteins RPL10 and RPL5 in multiple myeloma. Haematologica 2017, 102, e317–e320. [Google Scholar] [CrossRef] [PubMed]
- Pawlyn, C.; Kaiser, M.F.; Heuck, C.; Melchor, L.; Wardell, C.P.; Murison, A.; Chavan, S.S.; Johnson, D.C.; Begum, D.B.; Dahir, N.M.; et al. The spectrum and clinical impact of epigenetic modifier mutations in Myeloma. Clin. Cancer Res. 2016, 22, 5783–5794. [Google Scholar] [CrossRef]
- Fyodorov, D.V.; Zhou, B.R.; Skoultchi, A.I.; Bai, Y. Emerging roles of linker histones in regulating chromatin structure and function. Nat. Rev. Mol. Cell Biol. 2018, 19, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Dupéré-Richer, D.; Licht, J.D. Epigenetic regulatory mutations and epigenetic therapy for multiple myeloma. Curr. Opin. Hematol. 2017, 24, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, E.; Popovic, R.; Min, D.J.; Sweet, S.M.M.; Thomas, P.M.; Zamdborg, L.; Heffner, A.; Will, C.; Lamy, L.; Staudt, L.M.; et al. The MMSET histone methyl transferase switches global histone methylation and alters gene expression in t(4;14) multiple myeloma cells. Blood 2011, 117, 211–220. [Google Scholar] [CrossRef]
- Popovic, R.; Martinez-Garcia, E.; Giannopoulou, E.G.; Zhang, Q.; Zhang, Q.; Ezponda, T.; Shah, M.Y.; Zheng, Y.; Will, C.M.; Small, E.C.; et al. Histone Methyltransferase MMSET/NSD2 Alters EZH2 Binding and Reprograms the Myeloma Epigenome through Global and Focal Changes in H3K36 and H3K27 Methylation. PLoS Genet. 2014, 10, e1004566. [Google Scholar] [CrossRef]
- Van Haaften, G.; Dalgliesh, G.L.; Davies, H.; Chen, L.; Bignell, G.; Greenman, C.; Edkins, S.; Hardy, C.; O’Meara, S.; Teague, J.; et al. Somatic mutations of the histone H3K27 demethylase gene UTX in human cancer. Nat. Genet. 2009, 41, 521–523. [Google Scholar] [CrossRef]
- Attar, N.; Kurdistani, S.K. Exploitation of EP300 and CREBBP lysine acetyltransferases by cancer. Cold Spring Harb. Perspect. Med. 2017, 7, a026534. [Google Scholar] [CrossRef]
- Heuser, M.; Yun, H.; Thol, F. Epigenetics in myelodysplastic syndromes. Semin. Cancer Biol. 2018, 51, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Conery, A.R.; Centore, R.C.; Neiss, A.; Keller, P.J.; Joshi, S.; Spillane, K.L.; Sandy, P.; Hatton, C.; Pardo, E.; Zawadzke, L.; et al. Bromodomain inhibition of the transcriptional coactivators CBP/EP300 as a therapeutic strategy to target the IRF4 network in multiple myeloma. Elife 2016, 5, e10483. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, J.; Suwa, T.; Murase, Y.; Tateno, S.; Mizutome, H.; Asatsuma-Okumura, T.; Shimizu, N.; Kishi, T.; Momose, S.; Kizaki, M.; et al. ARID2 is a pomalidomide-dependent CRL4CRBN substrate in multiple myeloma cells. Nat. Chem. Biol. 2020, 16, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.A.; Wardell, C.P.; Chiecchio, L.; Smith, E.M.; Boyd, K.D.; Neri, A.; Davies, F.E.; Ross, F.M.; Morgan, G.J. Aberrant global methylation patterns affect the molecular pathogenesis and prognosis of multiple myeloma. Blood 2011, 117, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Salhia, B.; Baker, A.; Ahmann, G.; Auclair, D.; Fonseca, R.; Carpten, J.D. DNA methylation analysis determines the high frequency of genic hypomethylation and low frequency of hypermethylation events in plasma cell tumors. Cancer Res. 2010, 70, 6934–6944. [Google Scholar] [CrossRef] [PubMed]
- Heuck, C.J.; Mehta, J.; Bhagat, T.; Gundabolu, K.; Yu, Y.; Khan, S.; Chrysofakis, G.; Schinke, C.; Tariman, J.; Vickrey, E.; et al. Myeloma Is Characterized by Stage-Specific Alterations in DNA Methylation That Occur Early during Myelomagenesis. J. Immunol. 2013, 190, 2966–2975. [Google Scholar] [CrossRef]
- Agirre, X.; Castellano, G.; Pascual, M.; Heath, S.; Kulis, M.; Segura, V.; Bergmann, A.; Esteve, A.; Merkel, A.; Raineri, E.; et al. Whole-epigenome analysis in multiple myeloma reveals DNA hypermethylation of B cell-specific enhancers. Genome Res. 2015, 25, 478–487. [Google Scholar] [CrossRef]
- Choudhury, S.R.; Ashby, C.; Tytarenko, R.; Bauer, M.; Wang, Y.; Deshpande, S.; Den, J.; Schinke, C.; Zangari, M.; Thanendrarajan, S.; et al. The functional epigenetic landscape of aberrant gene expression in molecular subgroups of newly diagnosed multiple myeloma. J. Hematol. Oncol. 2020, 13, 108. [Google Scholar] [CrossRef]
- Alberge, J.B.; Magrangeas, F.; Wagner, M.; Denié, S.; Guérin-Charbonnel, C.; Campion, L.; Attal, M.; Avet-Loiseau, H.; Carell, T.; Moreau, P.; et al. DNA hydroxymethylation is associated with disease severity and persists at enhancers of oncogenic regions in multiple myeloma. Clin. Epigenetics 2020, 12, 163. [Google Scholar] [CrossRef]
- Mullighan, C.G. TET2 mutations in myelodysplasia and myeloid malignancies. Nat. Genet. 2009, 41, 766–767. [Google Scholar] [CrossRef]
- Duployez, N.; Goursaud, L.; Fenwarth, L.; Bories, C.; Marceau-Renaut, A.; Boyer, T.; Fournier, E.; Nibourel, O.; Roche-Lestienne, C.; Huet, G.; et al. Familial myeloid malignancies with germline TET2 mutation. Leukemia 2020, 34, 1450–1453. [Google Scholar] [CrossRef]
- Solary, E.; Bernard, O.A.; Tefferi, A.; Fuks, F.; Vainchenker, W. The Ten-Eleven Translocation-2 (TET2) gene in hematopoiesis and hematopoietic diseases. Leukemia 2014, 28, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Lio, C.W.J.; Shukla, V.; Samaniego-Castruita, D.; González-Avalos, E.; Chakraborty, A.; Yue, X.; Schatz, D.G.; Ay, F.; Rao, A. TET enzymes augment activation-induced deaminase (AID) expression via 5-hydroxymethylcytosine modifications at the Aicda superenhancer. Sci. Immunol. 2019, 4, eaau7523. [Google Scholar] [CrossRef]
- Rogozin, I.B.; Pavlov, Y.I.; Bebenek, K.; Matsuda, T.; Kunkel, T.A. Somatic mutation hotspots correlate with DNA polymerase eta error spectrum. Nat. Immunol. 2001, 2, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Choi, M.; Heuck, C.; Mane, S.; Barlogie, B.; Lifton, R.P.; Dhodapkar, M. V Serial exome analysis of disease progression in premalignant gammopathies. Leukemia 2014, 28, 1548–1552. [Google Scholar] [CrossRef] [PubMed]
- Bustoros, M.; Park, J.; Salem, K.Z.; Liu, C.-J.; Capelletti, M.; Huynh, D.; Tai, Y.-T.; Mouhieddine, T.H.; Freeman, S.; Ha, G.; et al. Next Generation Sequencing Identifies Smoldering Multiple Myeloma Patients with a High Risk of Disease Progression. Blood 2017, 130, 392. [Google Scholar]
- Maura, F.; Petljak, M.; Lionetti, M.; Cifola, I.; Liang, W.; Pinatel, E.; Alexandrov, L.B.; Fullam, A.; Martincorena, I.; Dawson, K.J.; et al. Biological and prognostic impact of APOBEC-induced mutations in the spectrum of plasma cell dyscrasias and multiple myeloma cell lines. Leukemia 2018, 32, 1043–1047. [Google Scholar] [CrossRef]
- Manzoni, M.; Marchica, V.; Storti, P.; Ziccheddu, B.; Sammarelli, G.; Todaro, G.; Pelizzoni, F.; Salerio, S.; Notarfranchi, L.; Pompa, A.; et al. Application of next-generation sequencing for the genomic characterization of patients with smoldering myeloma. Cancers 2020, 12, 1332. [Google Scholar] [CrossRef]
- Egan, J.B.; Kortuem, K.M.; Kurdoglu, A.; Izatt, T.; Aldrich, J.; Reiman, R.; Phillips, L.; Baker, A.; Shi, C.X.; Schmidt, J.; et al. Extramedullary myeloma whole genome sequencing reveals novel mutations in Cereblon, proteasome subunit G2 and the glucocorticoid receptor in multi drug resistant disease. Br. J. Haematol. 2013, 161, 748–751. [Google Scholar] [CrossRef]
- Kortüm, K.M.; Mai, E.K.; Hanafiah, N.H.; Shi, C.X.; Zhu, Y.X.; Bruins, L.; Barrio, S.; Jedlowski, P.; Merz, M.; Xu, J.; et al. Targeted sequencing of refractory myeloma reveals a high incidence of mutations in CRBN and Ras pathway genes. Blood 2016, 128, 1226–1233. [Google Scholar] [CrossRef]
- Barrio, S.; Stühmer, T.; Da-Viá, M.; Barrio-Garcia, C.; Lehners, N.; Besse, A.; Cuenca, I.; Garitano-Trojaola, A.; Fink, S.; Leich, E.; et al. Spectrum and functional validation of PSMB5 mutations in multiple myeloma. Leukemia 2019, 33, 447–456. [Google Scholar] [CrossRef]
- Gooding, S.; Ansari-Pour, N.; Towfic, F.; Estévez, M.O.; Chamberlain, P.P.; Tsai, K.T.; Flynt, E.; Hirst, M.; Rozelle, D.; Dhiman, P.; et al. Multiple cereblon genetic changes are associated with acquired resistance to lenalidomide or pomalidomide in multiple myeloma. Blood 2021, 137, 232–237. [Google Scholar] [CrossRef]
- Cohen, Y.C.; Zada, M.; Wang, S.Y.; Bornstein, C.; David, E.; Moshe, A.; Li, B.; Shlomi-Loubaton, S.; Gatt, M.E.; Gur, C.; et al. Identification of resistance pathways and therapeutic targets in relapsed multiple myeloma patients through single-cell sequencing. Nat. Med. 2021, 27, 491–503. [Google Scholar] [CrossRef]
- Iskierka-Jażdżewska, E.; Canzian, F.; Stępień, A.; Martino, A.; Campa, D.; Stein, A.; Krawczyk-Kuliś, M.; Rybicka-Ramos, M.; Kyrcz-Krzemień, S.; Butrym, A.; et al. Cereblon (CRBN) gene polymorphisms predict clinical response and progression-free survival in relapsed/refractory multiple myeloma patients treated with lenalidomide: A pharmacogenetic study from the IMMEnSE consortium. Leuk. Lymphoma 2020, 61, 699–706. [Google Scholar] [CrossRef]
- Haertle, L.; Barrio, S.; Munawar, U.; Han, S.; Zhou, X.; Vogt, C.; Alonso, R.; Bittrich, M.; Ruiz-Heredia, Y.; Da-Via, M.; et al. Cereblon Enhancer Methylation and IMiD Resistance in Multiple Myeloma. Blood 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Rogozin, I.B.; Pavlov, Y.I.; Goncearenco, A.; De, S.; Lada, A.G.; Poliakov, E.; Panchenko, A.R.; Cooper, D.N. Mutational signatures and mutable motifs in cancer genomes. Brief. Bioinform. 2017, 19, 1085–1101. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, L.B.; Kim, J.; Haradhvala, N.J.; Huang, M.N.; Tian Ng, A.W.; Wu, Y.; Boot, A.; Covington, K.R.; Gordenin, D.A.; Bergstrom, E.N.; et al. The repertoire of mutational signatures in human cancer. Nature 2020, 578, 94–101. [Google Scholar] [CrossRef]
- Roberts, S.A.; Gordenin, D.A. Hypermutation in human cancer genomes: Footprints and mechanisms. Nat. Rev. Cancer 2014, 14, 786–800. [Google Scholar] [CrossRef]
- Conticello, S.G. The AID/APOBEC family of nucleic acid mutators. Genome Biol. 2008, 9, 229. [Google Scholar] [CrossRef] [PubMed]
- Pham, P.; Bransteitter, R.; Petruska, J.; Goodman, M.F. Processive AID-catalysed cytosine deamination on single-stranded DNA simulates somatic hypermutation. Nature 2003, 424, 103–107. [Google Scholar] [CrossRef]
- Nik-Zainal, S.; Alexandrov, L.B.; Wedge, D.C.; Van Loo, P.; Greenman, C.D.; Raine, K.; Jones, D.; Hinton, J.; Marshall, J.; Stebbings, L.A.; et al. Mutational processes molding the genomes of 21 breast cancers. Cell 2012, 149, 979–993. [Google Scholar] [CrossRef]
- Lada, A.G.; Dhar, A.; Boissy, R.J.; Hirano, M.; Rubel, A.A.; Rogozin, I.B.; Pavlov, Y.I. AID/APOBEC cytosine deaminase induces genome-wide kataegis. Biol. Direct 2012, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Saini, N.; Gordenin, D.A. Hypermutation in single-stranded DNA. DNA Repair (Amst) 2020, 91–92, 102868. [Google Scholar] [CrossRef]
- Mas-Ponte, D.; Supek, F. DNA mismatch repair promotes APOBEC3-mediated diffuse hypermutation in human cancers. Nat. Genet. 2020, 52, 958–968. [Google Scholar] [CrossRef]
- Walker, B.A.; Wardell, C.P.; Murison, A.; Boyle, E.M.; Begum, D.B.; Dahir, N.M.; Proszek, P.Z.; Melchor, L.; Pawlyn, C.; Kaiser, M.F.; et al. APOBEC family mutational signatures are associated with poor prognosis translocations in multiple myeloma. Nat. Commun. 2015, 6, 6997. [Google Scholar] [CrossRef]
- Maura, F.; Degasperi, A.; Nadeu, F.; Leongamornlert, D.; Davies, H.; Moore, L.; Royo, R.; Ziccheddu, B.; Puente, X.S.; Avet-Loiseau, H.; et al. A practical guide for mutational signature analysis in hematological malignancies. Nat. Commun. 2019, 10, 2969. [Google Scholar] [CrossRef]
- Maura, F.; Landgren, O.; Morgan, G.J. Designing Evolutionary-based Interception Strategies to Block the Transition from Precursor Phases to Multiple Myeloma. Clin. Cancer Res. 2021, 27, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.A.; Sterling, J.; Thompson, C.; Harris, S.; Mav, D.; Shah, R.; Klimczak, L.J.; Kryukov, G.V.; Malc, E.; Mieczkowski, P.A.; et al. Clustered Mutations in Yeast and in Human Cancers Can Arise from Damaged Long Single-Strand DNA Regions. Mol. Cell 2012, 46, 424–435. [Google Scholar] [CrossRef]
- Rogozin, I.B.; Kolchanov, N.A. Somatic hypermutagenesis in immunoglobulin genes. II. Influence of neighbouring base sequences on mutagenesis. BBA-Gene Struct. Expr. 1992, 1171, 11–18. [Google Scholar] [CrossRef]
- Pavlov, Y.I.; Rogozin, I.B.; Galkin, A.P.; Aksenova, A.Y.; Hanaoka, F.; Rada, C.; Kunkel, T.A. Correlation of somatic hypermutation specificity and A-T base pair substitution errors by DNA polymerase eta during copying of a mouse immunoglobulin kappa light chain transgene. Proc. Natl. Acad. Sci. USA 2002, 99, 9954–9959. [Google Scholar] [CrossRef] [PubMed]
- Rogozin, I.B.; Roche-Lima, A.; Tyryshkin, K.; Carrasquillo-Carrión, K.; Lada, A.G.; Poliakov, L.Y.; Schwartz, E.; Saura, A.; Yurchenko, V.; Cooper, D.N.; et al. DNA Methylation, Deamination, and Translesion Synthesis Combine to Generate Footprint Mutations in Cancer Driver Genes in B-Cell Derived Lymphomas and Other Cancers. Front. Genet. 2021, 12, 783. [Google Scholar] [CrossRef]
- Taylor, B.J.M.; Nik-Zainal, S.; Wu, Y.L.; Stebbings, L.A.; Raine, K.; Campbell, P.J.; Rada, C.; Stratton, M.R.; Neuberger, M.S. DNA deaminases induce break-associated mutation showers with implication of APOBEC3B and 3A in breast cancer kataegis. Elife 2013, 2, e00534. [Google Scholar] [CrossRef]
- Chan, K.; Roberts, S.A.; Klimczak, L.J.; Sterling, J.F.; Saini, N.; Malc, E.P.; Kim, J.; Kwiatkowski, D.J.; Fargo, D.C.; Mieczkowski, P.A.; et al. An APOBEC3A hypermutation signature is distinguishable from the signature of background mutagenesis by APOBEC3B in human cancers. Nat. Genet. 2015, 47, 1067–1072. [Google Scholar] [CrossRef]
- Rogozin, I.; Roche-Lima, A.; Lada, A.; Belinky, F.; Sidorenko, I.; Glazko, G.; Babenko, V.; Cooper, D.; Pavlov, Y. Nucleotide Weight Matrices Reveal Ubiquitous Mutational Footprints of AID/APOBEC Deaminases in Human Cancer Genomes. Cancers 2019, 11, 211. [Google Scholar] [CrossRef]
- Álvarez-Prado, Á.F.; Pérez-Durán, P.; Pérez-García, A.; Benguria, A.; Torroja, C.; de Yébenes, V.G.; Ramiro, A.R. A broad atlas of somatic hypermutation allows prediction of activation-induced deaminase targets. J. Exp. Med. 2018, 215, 761–771. [Google Scholar] [CrossRef]
- Rogozin, I.B.; Lada, A.G.; Goncearenco, A.; Green, M.R.; De, S.; Nudelman, G.; Panchenko, A.R.; Koonin, E.V.; Pavlov, Y.I. Activation induced deaminase mutational signature overlaps with CpG methylation sites in follicular lymphoma and other cancers. Sci. Rep. 2016, 6, 38133. [Google Scholar] [CrossRef] [PubMed]
- Maura, F.; Rustad, E.H.; Yellapantula, V.; Łuksza, M.; Hoyos, D.; Maclachlan, K.H.; Diamond, B.T.; Greenbaum, B.D.; Morgan, G.; Lesokhin, A.; et al. Role of AID in the temporal pattern of acquisition of driver mutations in multiple myeloma. Leukemia 2020, 34, 1476–1480. [Google Scholar] [CrossRef] [PubMed]
- Maul, R.W.; Gearhart, P.J. AID and somatic hypermutation. In Advances in Immunology; Academic Press Inc.: AEAmsterdam, The Netherlands, 2010; Volume 105, pp. 159–191. [Google Scholar]
- Ross, F.M.; Chiecchio, L.; Dagrada, G.; Protheroe, R.K.M.; Stockley, D.M.; Harrison, C.J.; Cross, N.C.; Szubert, A.J.; Drayson, M.T.; Morgan, G.J. The t(14;20) is a poor prognostic factor in myeloma but is associated with long-term stable disease in monoclonal gammopathies of undetermined significance. Haematologica 2010, 95, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Rouf Banday, A.; Onabajo, O.O.; Lin, S.H.Y.; Obajemu, A.; Vargas, J.M.; Delviks-Frankenberry, K.A.; Lamy, P.; Bayanjargal, A.; Zettelmeyer, C.; Florez-Vargas, O.; et al. Targeting natural splicing plasticity of APOBEC3B restricts its expression and mutagenic activity. Commun. Biol. 2021, 4, 386. [Google Scholar] [CrossRef]
- Flactif, M.; Zandecki, M.; Laï, J.L.; Bernardi, F.; Obein, V.; Bauters, F.; Facon, T. Interphase fluorescence in situ hybridization (FISH) as a powerful tool for the detection of aneuploidy in multiple myeloma. Leukemia 1995, 9, 2109–2114. [Google Scholar]
- Drach, J.; Schuster, J.; Nowotny, H.; Angerler, J.; Rosenthal, F.; Fiegl, M.; Rothermundt, C.; Gsur, A.; Jager, U.; Heinz, R.; et al. Multiple myeloma: High incidence of chromosomal aneuploidy as detected by interphase fluorescence in situ hybridization. Cancer Res. 1995, 55, 3854–3859. [Google Scholar]
- Lee, W.; Han, K.; Drut, R.M.; Harris, C.P.; Meisner, L.F. Use of fluorescence in situ hybridization for retrospective detection of aneuploidy in multiple myeloma. Genes Chromosom. Cancer 1993, 7, 137–143. [Google Scholar] [CrossRef]
- Pratt, G. Molecular aspects of multiple myeloma. J. Clin. Pathol.-Mol. Pathol. 2002, 55, 273–283. [Google Scholar] [CrossRef]
- Walker, B.A.; Leone, P.E.; Chiecchio, L.; Dickens, N.J.; Jenner, M.W.; Boyd, K.D.; Johnson, D.C.; Gonzalez, D.; Dagrada, G.P.; Protheroe, R.K.M.; et al. A compendium of myeloma-associated chromosomal copy number abnormalities and their prognostic value. Blood 2010, 116, e56–e65. [Google Scholar] [CrossRef] [PubMed]
- Chretien, M.L.; Corre, J.; Lauwers-Cances, V.; Magrangeas, F.; Cleynen, A.; Yon, E.; Hulin, C.; Leleu, X.; Orsini-Piocelle, F.; Blade, J.S.; et al. Understanding the role of hyperdiploidy in myeloma prognosis: Which trisomies really matter? Blood 2015, 126, 2713–2719. [Google Scholar] [CrossRef]
- Aktas Samur, A.; Minvielle, S.; Shammas, M.; Fulciniti, M.; Magrangeas, F.; Richardson, P.G.; Moreau, P.; Attal, M.; Anderson, K.C.; Parmigiani, G.; et al. Deciphering the chronology of copy number alterations in Multiple Myeloma. Blood Cancer J. 2019, 9, 39. [Google Scholar] [CrossRef]
- Barilà, G.; Bonaldi, L.; Grassi, A.; Martines, A.; Liço, A.; Macrì, N.; Nalio, S.; Pavan, L.; Berno, T.; Branca, A.; et al. Identification of the true hyperdiploid multiple myeloma subset by combining conventional karyotyping and FISH analysis. Blood Cancer J. 2020, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Rasillo, A.; Tabernero, M.D.; Sánchez, M.L.; Pérez, D.A.M.; Martín, A.M.; Hernández, J.; Moro, M.J.; Fernández-Calvo, J.; Sayagués, J.M.; Bortoluci, A.; et al. Fluorescence in situ hybridization analysis of aneuploidization patterns in monoclonal gammopathy of undetermined significance versus multiple myeloma and plasma cell leukemia. Cancer 2003, 97, 601–609. [Google Scholar] [PubMed]
- Brousseau, M.; Leleu, X.; Gerard, J.; Gastinne, T.; Godon, A.; Genevieve, F.; Dib, M.; Lai, J.L.; Facon, T.; Zandecki, M. Hyperdiploidy is a common finding in monoclonal gammopathy of undetermined significance and monosomy 13 is restricted to these hyperdiploid patients. Clin. Cancer Res. 2007, 13, 6026–6031. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, R.; Barlogie, B.; Bataille, R.; Bastard, C.; Bergsagel, P.L.; Chesi, M.; Davies, F.E.; Drach, J.; Greipp, P.R.; Kirsch, I.R.; et al. Genetics and Cytogenetics of Multiple Myeloma: A Workshop Report. Cancer Res. 2004, 64, 1546–1558. [Google Scholar] [CrossRef]
- Bergsagel, P.L.; Chesi, M.; Nardini, E.; Brents, L.A.; Kirby, S.L.; Kuehl, W.M. Promiscuous translocations into immunoglobulin heavy chain switch regions in multiple myeloma. Proc. Natl. Acad. Sci. USA 1996, 93, 13931–13936. [Google Scholar] [CrossRef] [PubMed]
- Leif Bergsagel, P.; Michael Kuehl, W. Chromosome translocations in multiple myeloma. Oncogene 2001, 20, 5611–5622. [Google Scholar] [CrossRef] [PubMed]
- Chesi, M.; Nardini, E.; Lim, R.S.C.; Smith, K.D.; Michael Kuehl, W.; Bergsagel, P.L. The t(4;14) translocation in myeloma dysregulates both FGFR3 and a novel gene, MMSET, resulting in IgH/MMSET hybrid transcripts. Blood 1998, 92, 3025–3034. [Google Scholar] [CrossRef] [PubMed]
- Kalff, A.; Spencer, A. The t(4;14) translocation and FGFR3 overexpression in multiple myeloma: Prognostic implications and current clinical strategies. Blood Cancer J. 2012, 2, e89. [Google Scholar] [CrossRef]
- Plowright, E.E.; Li, Z.; Bergsagel, P.L.; Chesi, M.; Barber, D.L.; Branch, D.R.; Hawley, R.G.; Stewart, A.K. Ectopic expression of fibroblast growth factor receptor 3 promotes myeloma cell proliferation and prevents apoptosis. Blood 2000, 95, 992–998. [Google Scholar] [CrossRef]
- Alvarez, J.V.; Frank, D.A. Genome-wide analysis of STAT target genes: Elucidating the mechanism of STAT-mediated oncogenesis. Cancer Biol. Ther. 2004, 3, 1045–1050. [Google Scholar] [CrossRef]
- Ramlee, M.K.; Wang, J.; Toh, W.X.; Li, S. Transcription regulation of the human telomerase reverse transcriptase (hTERT) gene. Genes 2016, 7, 50. [Google Scholar] [CrossRef]
- Bharti, A.C.; Shishodia, S.; Reuben, J.M.; Weber, D.; Alexanian, R.; Raj-Vadhan, S.; Estrov, Z.; Talpaz, M.; Aggarwal, B.B. Nuclear factor-κB and STAT3 are constitutively active in CD138 + cells derived from multiple myeloma patients, and suppression of these transcription factors leads to apoptosis. Blood 2004, 103, 3175–3184. [Google Scholar] [CrossRef]
- Catlett-Falcone, R.; Landowski, T.H.; Oshiro, M.M.; Turkson, J.; Levitzki, A.; Savino, R.; Ciliberto, G.; Moscinski, L.; Fernández-Luna, J.L.; Nuñez, G.; et al. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity 1999, 10, 105–115. [Google Scholar] [CrossRef]
- Brocke-Heidrich, K.; Kretzschmar, A.K.; Pfeifer, G.; Henze, C.; Löffler, D.; Koczan, D.; Thiesen, H.J.; Burger, R.; Gramatzki, M.; Horn, F. Interleukin-6-dependent gene expression profiles in multiple myeloma INA-6 cells reveal a Bcl-2 family-independent survival pathway closely associated with Stat3 activation. Blood 2004, 103, 242–251. [Google Scholar] [CrossRef]
- Avery, D.T.; Deenick, E.K.; Ma, C.S.; Suryani, S.; Simpson, N.; Chew, G.Y.; Chan, T.D.; Palendira, U.; Bustamante, J.; Boisson-Dupuis, S.; et al. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. J. Exp. Med. 2010, 207, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Faris, M.; Kokot, N.; Stahl, N.; Nel, A.E. Involvement of Stat3 in interleukin-6-induced IgM production in a human B-cell line. Immunology 1997, 90, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Ronchetti, D.; Greco, A.; Compasso, S.; Colombo, G.; Dell’Era, P.; Otsuki, T.; Lombardi, L.; Neri, A. Deregulated FGFR3 mutants in multiple myeloma cell lines with t(4;14): Comparative analysis of Y373C, K650E and the novel G384D mutations. Oncogene 2001, 20, 3553–3562. [Google Scholar] [CrossRef] [PubMed]
- Kuo, A.J.; Cheung, P.; Chen, K.; Zee, B.M.; Kioi, M.; Lauring, J.; Xi, Y.; Park, B.H.; Shi, X.; Garcia, B.A.; et al. NSD2 Links Dimethylation of Histone H3 at Lysine 36 to Oncogenic Programming. Mol. Cell 2011, 44, 609–620. [Google Scholar] [CrossRef]
- Shah, M.Y.; Martinez-Garcia, E.; Phillip, J.M.; Chambliss, A.B.; Popovic, R.; Ezponda, T.; Small, E.C.; Will, C.; Phillip, M.P.; Neri, P.; et al. MMSET/WHSC1 enhances DNA damage repair leading to an increase in resistance to chemotherapeutic agents. Oncogene 2016, 35, 5905–5915. [Google Scholar] [CrossRef]
- Park, J.W.; Chae, Y.C.; Kim, J.Y.; Oh, H.; Seo, S.B. Methylation of Aurora kinase A by MMSET reduces p53 stability and regulates cell proliferation and apoptosis. Oncogene 2018, 37, 6212–6224. [Google Scholar] [CrossRef]
- Pei, H.; Zhang, L.; Luo, K.; Qin, Y.; Chesi, M.; Fei, F.; Bergsagel, P.L.; Wang, L.; You, Z.; Lou, Z. MMSET regulates histone H4K20 methylation and 53BP1 accumulation at DNA damage sites. Nature 2011, 470, 124–129. [Google Scholar] [CrossRef]
- Evans, D.L.; Zhang, H.; Ham, H.; Pei, H.; Lee, S.B.; Kim, J.J.; Billadeau, D.D.; Lou, Z. MMSET is dynamically regulated during cell-cycle progression and promotes normal DNA replication. Cell Cycle 2016, 15, 95–105. [Google Scholar] [CrossRef]
- de Krijger, I.; van der Torre, J.; Peuscher, M.H.; Eder, M.; Jacobs, J.J.L. H3K36 dimethylation by MMSET promotes classical non-homologous end-joining at unprotected telomeres. Oncogene 2020, 39, 4814–4827. [Google Scholar] [CrossRef]
- Nguyen, H.V.; Dong, J.; Panchakshari, R.A.; Kumar, V.; Alt, F.W.; Bories, J.C. Histone methyltransferase MMSET promotes AID-mediated DNA breaks at the donor switch region during class switch recombination. Proc. Natl. Acad. Sci. USA 2017, 114, E10560–E10567. [Google Scholar] [CrossRef]
- Gao, G.; Smith, D.I. WWOX, large common fragile site genes, and cancer. Exp. Biol. Med. 2015, 240, 285–295. [Google Scholar] [CrossRef]
- Mangelsdorf, M.; Ried, K.; Woollatt, E.; Dayan, S.; Eyre, H.; Finnis, M.; Hobson, L.; Nancarrow, J.; Venter, D.; Baker, E.; et al. Chromosomal fragile site FRA16D and DNA instability in cancer. Cancer Res. 2000, 60, 1683–1689. [Google Scholar]
- Boersma-Vreugdenhil, G.R.; Kuipers, J.; Van Stralen, E.; Peeters, T.; Michaux, L.; Hagemeijer, A.; Pearson, P.L.; Clevers, H.C.; Bast, B.J.E.G. The recurrent translocation t(14;20)(q32;q12) in multiple myeloma results in aberrant expression of MAFB: A molecular and genetic analysis of the chromosomal breakpoint. Br. J. Haematol. 2004, 126, 355–363. [Google Scholar] [CrossRef]
- Chesi, M.; Bergsagel, P.L. Molecular pathogenesis of multiple myeloma: Basic and clinical updates. Int. J. Hematol. 2013, 97, 313–323. [Google Scholar] [CrossRef]
- Bergsagel, P.L.; Kuehl, W.M. Molecular pathogenesis and a consequent classification of multiple myeloma. J. Clin. Oncol. 2005, 23, 6333–6338. [Google Scholar] [CrossRef] [PubMed]
- Dolloff, N.G. Discovery platform for inhibitors of IgH gene enhancer activity. Cancer Biol. Ther. 2019, 20, 571–581. [Google Scholar] [CrossRef]
- Königsberg, R.; Ackermann, J.; Kaufmann, H.; Zojer, N.; Urbauer, E.; Krömer, E.; Jäger, U.; Gisslinger, H.; Schreiber, S.; Heinz, R.; et al. Deletions of chromosome 13q in monoclonal gammopathy of undetermined significance. Leukemia 2000, 14, 1975–1979. [Google Scholar] [CrossRef] [PubMed]
- Gertz, M.A.; Lacy, M.Q.; Dispenzieri, A.; Greipp, P.R.; Litzow, M.R.; Henderson, K.J.; Van Wier, S.A.; Ahmann, G.J.; Fonseca, R. Clinical implications of t(11;14)(q13;q32), t(4;14)(p16.3;q32), and -17p13 in myeloma patients treated with high-dose therapy. Blood 2005, 106, 2837–2840. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, R.; Blood, E.; Rue, M.; Harrington, D.; Oken, M.M.; Kyle, R.A.; Dewald, G.W.; Van Ness, B.; Van Wier, S.A.; Henderson, K.J.; et al. Clinical and biologic implications of recurrent genomic aberrations in myeloma. Blood 2003, 101, 4569–4575. [Google Scholar] [CrossRef]
- Hanamura, I.; Stewart, J.P.; Huang, Y.; Zhan, F.; Santra, M.; Sawyer, J.R.; Hollmig, K.; Zangarri, M.; Pineda-Roman, M.; Van Rhee, F.; et al. Frequent gain of chromosome band 1q21 in plasma-cell dyscrasias detected by fluorescence in situ hybridization: Incidence increases from MGUS to relapsed myeloma and is related to prognosis and disease progression following tandem stem-cell transplantatio. Blood 2006, 108, 1724–1732. [Google Scholar] [CrossRef]
- Avet-Loiseau, H.; Attal, M.; Moreau, P.; Charbonnel, C.; Garban, F.; Hulin, C.; Leyvraz, S.; Michallet, M.; Yakoub-Agha, I.; Garderet, L.; et al. Genetic abnormalities and survival in multiple myeloma: The experience of the Intergroupe Francophone du Myélome. Blood 2007, 109, 3489–3495. [Google Scholar] [CrossRef] [PubMed]
- Avet-Loiseau, H.; Attal, M.; Campion, L.; Caillot, D.; Hulin, C.; Marit, G.; Stoppa, A.M.; Voillat, L.; Wetterwald, M.; Pegourie, B.; et al. Long-term analysis of the ifm 99 trials for myeloma: Cytogenetic abnormalities [t(4;14), del(17p), 1q gains] play a major role in defining long-term survival. J. Clin. Oncol. 2012, 30, 1949–1952. [Google Scholar] [CrossRef]
- Corre, J.; Perrot, A.; Caillot, D.; Belhadj, K.; Hulin, C.; Leleu, X.; Mohty, M.; Facon, T.; Buisson, L.; Do Souto, L.; et al. del(17p) without TP53 mutation confers a poor prognosis in intensively treated newly diagnosed patients with multiple myeloma. Blood 2021, 137, 1192–1195. [Google Scholar] [CrossRef] [PubMed]
- Chalopin, T.; Vallet, N.; Theisen, O.; Ochmann, M.; Tiab, M.; Godmer, P.; Barin, C.; Hérault, O.; Gyan, E.; Le Gouill, S.; et al. No survival improvement in patients with high-risk multiple myeloma harbouring del(17p) and/or t(4;14) over the two past decades. Br. J. Haematol. 2021, 194, 635–638. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Shi, C.X.; Bruins, L.A.; Jedlowski, P.; Wang, X.; Kortüm, K.M.; Luo, M.; Ahmann, J.M.; Braggio, E.; Stewart, A.K. Loss of FAM46C promotes cell survival in myeloma. Cancer Res. 2017, 77, 4317–4327. [Google Scholar] [CrossRef] [PubMed]
- Kanasugi, J.; Hanamura, I.; Ota, A.; Karnan, S.; Lam, V.Q.; Mizuno, S.; Wahiduzzaman, M.; Rahman, M.L.; Hyodo, T.; Konishi, H.; et al. Biallelic loss of FAM46C triggers tumor growth with concomitant activation of Akt signaling in multiple myeloma cells. Cancer Sci. 2020, 111, 1663–1675. [Google Scholar] [CrossRef]
- Varma, A.; Sui, D.; Milton, D.R.; Tang, G.; Saini, N.; Hasan, O.; Mukherjee, A.; Joseph, J.J.; Bashir, Q.; Rondon, G.; et al. Outcome of Multiple Myeloma with Chromosome 1q Gain and 1p Deletion after Autologous Hematopoietic Stem Cell Transplantation: Propensity Score Matched Analysis. Biol. Blood Marrow Transplant. 2020, 26, 665–671. [Google Scholar] [CrossRef]
- Shah, G.L.; Landau, H.; Londono, D.; Devlin, S.M.; Kosuri, S.; Lesokhin, A.M.; Lendvai, N.; Hassoun, H.; Chung, D.J.; Koehne, G.; et al. Gain of chromosome 1q portends worse prognosis in multiple myeloma despite novel agent-based induction regimens and autologous transplantation. Leuk. Lymphoma 2017, 58, 1823–1831. [Google Scholar] [CrossRef]
- Chakraborty, R.; Gertz, M.A. + 1q: Amplifying the bad genes in myeloma. Leuk. 8 Lymphoma 2017, 58, 1771–1773. [Google Scholar] [CrossRef]
- Schmidt, T.M.; Barwick, B.G.; Joseph, N.; Heffner, L.T.; Hofmeister, C.C.; Bernal, L.; Dhodapkar, M.V.; Gupta, V.A.; Jaye, D.L.; Wu, J.; et al. Gain of Chromosome 1q is associated with early progression in multiple myeloma patients treated with lenalidomide, bortezomib, and dexamethasone. Blood Cancer J. 2019, 9, 94. [Google Scholar] [CrossRef]
- Sawyer, J.R.; Tricot, G.; Mattox, S.; Jagannath, S.; Barlogie, B. Jumping translocations of chromosome 1q in multiple myeloma: Evidence for a mechanism involving decondensation of pericentromeric heterochromatin. Blood 1998, 91, 1732–1741. [Google Scholar] [CrossRef]
- Jamet, D.; Marzin, Y.; Douet-Guilbert, N.; Morel, F.; Le Bris, M.-J.J.; Herry, A.; Banzakour, S.; Bourquard, P.; Morice, P.; Abgrall, J.F.; et al. Jumping translocations in multiple myeloma. Cancer Genet. Cytogenet. 2005, 161, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.; Bernard, O.A. Jumping translocations. Genes Chromosom. Cancer 2007, 46, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, J.R.; Tian, E.; Walker, B.A.; Wardell, C.; Lukacs, J.L.; Sammartino, G.; Bailey, C.; Schinke, C.D.; Thanendrarajan, S.; Davies, F.E.; et al. An acquired high-risk chromosome instability phenotype in multiple myeloma: Jumping 1q Syndrome. Blood Cancer J. 2019, 9, 62. [Google Scholar] [CrossRef]
- Philip, P.; Drivsholm, A.; Hansen, N.E.; Jensen, M.K.; Killmann, S.A. Chromosomes and survival in multiple myeloma. A banding study of 25 cases. Cancer Genet. Cytogenet. 1980, 2, 243–257. [Google Scholar] [CrossRef]
- Sawyer, J.R.; Tricot, G.; Lukacs, J.L.; Binz, R.L.; Tian, E.; Barlogie, B.; Shaughnessy, J. Genomic instability in multiple myeloma: Evidence for jumping segmental duplications of chromosome arm 1q. Genes Chromosom. Cancer 2005, 42, 95–106. [Google Scholar] [CrossRef]
- Aksenova, A.Y.; Mirkin, S.M. At the beginning of the end and in the middle of the beginning: Structure and maintenance of telomeric DNA repeats and interstitial telomeric sequences. Genes 2019, 10, 118. [Google Scholar] [CrossRef]
- Walker, B.A.; Mavrommatis, K.; Wardell, C.P.; Ashby, T.C.; Bauer, M.; Davies, F.; Rosenthal, A.; Wang, H.; Qu, P.; Hoering, A.; et al. A high-risk, Double-Hit, group of newly diagnosed myeloma identified by genomic analysis. Leukemia 2019, 33, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Shaughnessy, J. Amplification and overexpression of CKS1B at chromosome band 1q21 is associated with reduced levels of p27 Kip1 and an aggressive clinical course in multiple myeloma. Hematology 2005, 10, 117–126. [Google Scholar] [CrossRef]
- Shi, W.; Huang, Q.; Xie, J.; Wang, H.; Yu, X.; Zhou, Y. CKS1B as Drug Resistance-Inducing Gene—A Potential Target to Improve Cancer Therapy. Front. Oncol. 2020, 10, 582451. [Google Scholar] [CrossRef]
- Hanamura, I. Gain/amplification of chromosome arm 1q21 in multiple myeloma. Cancers 2021, 13, 256. [Google Scholar] [CrossRef]
- An, G.; Xu, Y.; Shi, L.; Zhong, S.; Deng, S.; Xie, Z.; Sui, W.; Zhan, F.; Qiu, L. Chromosome 1q21 gains confer inferior outcomes in multiple myeloma treated with bortezomib but copy number variation and percentage of plasma cells involved have no additional prognostic value. Haematologica 2014, 99, 353–359. [Google Scholar] [CrossRef]
- Avet-Loiseau, H.; Li, J.Y.; Morineau, N.; Facon, T.; Brigaudeau, C.; Harousseau, J.L.; Grosbois, B.; Bataille, R. Monosomy 13 is associated with the transition of monoclonal gammopathy of undetermined significance to multiple myeloma. Intergroupe Francophone du Myélome. Blood 1999, 94, 2583–2589. [Google Scholar] [CrossRef]
- Shaughnessy, J.; Tian, E.; Sawyer, J.; Bumm, K.; Landes, R.; Badros, A.; Morris, C.; Tricot, G.; Epstein, J.; Barlogie, B. High incidence of chromosome 13 deletion in multiple myeloma detected by multiprobe interphase FISH. Blood 2000, 96, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Chavan, S.S.; He, J.; Tytarenko, R.; Deshpande, S.; Patel, P.; Bailey, M.; Stein, C.K.; Stephens, O.; Weinhold, N.; Petty, N.; et al. Bi-allelic inactivation is more prevalent at relapse in multiple myeloma, identifying RB1 as an independent prognostic marker. Blood Cancer J. 2017, 7, e535. [Google Scholar] [CrossRef] [PubMed]
- Lodé, L.; Eveillard, M.; Trichet, V.; Soussi, T.; Wuillème, S.; Richebourg, S.; Magrangeas, F.; Ifrah, N.; Campion, L.; Traullé, C.; et al. Mutations in TP53 are exclusively associated with del(17p) in multiple myeloma. Haematologica 2010, 95, 1973–1976. [Google Scholar] [CrossRef]
- Chin, M.; Sive, J.I.; Allen, C.; Roddie, C.; Chavda, S.J.; Smith, D.; Blombery, P.; Jones, K.; Ryland, G.L.; Popat, R.; et al. Prevalence and timing of TP53 mutations in del(17p) myeloma and effect on survival. Blood Cancer J. 2017, 7, e610. [Google Scholar] [CrossRef]
- Sewastianik, T.; Prochorec-Sobieszek, M.; Chapuy, B.; Juszczyński, P. MYC deregulation in lymphoid tumors: Molecular mechanisms, clinical consequences and therapeutic implications. Biochim. Biophys. Acta-Rev. Cancer 2014, 1846, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Grisanzio, C.; Freedman, M.L. Chromosome 8q24-associated cancers and MYC. Genes and Cancer 2010, 1, 555–559. [Google Scholar] [CrossRef]
- Huppi, K.; Pitt, J.J.; Wahlberg, B.M.; Caplen, N.J. The 8q24 gene desert: An oasis of non-coding transcriptional activity. Front. Genet. 2012, 3, 69. [Google Scholar] [CrossRef]
- Ghoussaini, M.; Song, H.; Koessler, T.; Al Olama, A.A.; Kote-Jarai, Z.; Driver, K.E.; Pooley, K.A.; Ramus, S.J.; Kjaer, S.K.; Hogdall, E.; et al. Multiple loci with different cancer specificities within the 8q24 gene desert. J. Natl. Cancer Inst. 2008, 100, 962–966. [Google Scholar] [CrossRef] [PubMed]
- Chesi, M.; Robbiani, D.F.; Sebag, M.; Chng, W.J.; Affer, M.; Tiedemann, R.; Valdez, R.; Palmer, S.E.; Haas, S.S.; Stewart, A.K.; et al. AID-Dependent Activation of a MYC Transgene Induces Multiple Myeloma in a Conditional Mouse Model of Post-Germinal Center Malignancies. Cancer Cell 2008, 13, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Avet-Loiseau, H.; Gerson, F.; Magrangeas, F.; Minvielle, S.; Harousseau, J.L.; Bataille, R. Rearrangements of the c-myc oncogene are present in 15% of primary human multiple myeloma tumors. Blood 2001, 98, 3082–3086. [Google Scholar] [CrossRef] [PubMed]
- Misund, K.; Keane, N.; Stein, C.K.; Asmann, Y.W.; Day, G.; Welsh, S.; Van Wier, S.A.; Riggs, D.L.; Ahmann, G.; Chesi, M.; et al. MYC dysregulation in the progression of multiple myeloma. Leukemia 2020, 34, 322–326. [Google Scholar] [CrossRef]
- Affer, M.; Chesi, M.; Chen, W.D.; Keats, J.J.; Demchenko, Y.N.; Tamizhmani, K.; Garbitt, V.M.; Riggs, D.L.; Brents, L.A.; Roschke, A.V.; et al. Promiscuous MYC locus rearrangements hijack enhancers but mostly super-enhancers to dysregulate MYC expression in multiple myeloma. Leukemia 2014, 28, 1725–1735. [Google Scholar] [CrossRef]
- Barwick, B.G.; Neri, P.; Bahlis, N.J.; Nooka, A.K.; Dhodapkar, M.V.; Jaye, D.L.; Hofmeister, C.C.; Kaufman, J.L.; Gupta, V.A.; Auclair, D.; et al. Multiple myeloma immunoglobulin lambda translocations portend poor prognosis. Nat. Commun. 2019, 10, 1911. [Google Scholar] [CrossRef] [PubMed]
- Seliger, S.; Geirhos, V.; Haferlach, T.; Kern, W.; Walter, W.; Meggendorfer, M.; Baer, C.; Stengel, A.; Haferlach, C. Comprehensive Analysis of MYC Translocations in Multiple Myeloma By Whole Genome Sequencing and Whole Transcriptome Sequencing. Blood 2019, 134, 1774. [Google Scholar] [CrossRef]
- Walker, B.A.; Wardell, C.P.; Brioli, A.; Boyle, E.; Kaiser, M.F.; Begum, D.B.; Dahir, N.B.; Johnson, D.C.; Ross, F.M.; Davies, F.E.; et al. Translocations at 8q24 juxtapose MYC with genes that harbor superenhancers resulting in overexpression and poor prognosis in myeloma patients. Blood Cancer J. 2014, 4, e191. [Google Scholar] [CrossRef] [PubMed]
- Mikulasova, A.; Ashby, C.; Tytarenko, R.G.; Qu, P.; Rosenthal, A.; Dent, J.A.; Ryan, K.R.; Bauer, M.A.; Wardell, C.P.; Hoering, A.; et al. Microhomology-mediated end joining drives complex rearrangements and overexpression of MYC and PVT1 in multiple myeloma. Haematologica 2020, 105, 1055–1060. [Google Scholar] [CrossRef]
- Shaffer, A.L.; Emre, N.C.T.; Lamy, L.; Ngo, V.N.; Wright, G.; Xiao, W.; Powell, J.; Dave, S.; Yu, X.; Zhao, H.; et al. IRF4 addiction in multiple myeloma. Nature 2008, 454, 226–231. [Google Scholar] [CrossRef]
- Walker, B.A.; Wardell, C.P.; Johnson, D.C.; Kaiser, M.F.; Begum, D.B.; Dahir, N.B.; Ross, F.M.; Davies, F.E.; Gonzalez, D.; Morgan, G.J. Characterization of IGH locus breakpoints in multiple myeloma indicates a subset of translocations appear to occur in pregerminal center B cells. Blood 2013, 121, 3413–3419. [Google Scholar] [CrossRef]
- Jimenez, C.; Jara-Acevedo, M.; Corchete, L.A.L.A.; Castillo, D.; Ordonez, G.R.; Sarasquete, M.E.M.E.; Puig, N.; Martinez-Lopez, J.; Prieto-Conde, M.I.M.I.; Garcia-Alvarez, M.; et al. A Next-Generation Sequencing Strategy for Evaluating the Most Common Genetic Abnormalities in Multiple Myeloma. J. Mol. Diagnostics 2017, 19, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Fear, D.J.; McCloskey, N.; O’Connor, B.; Felsenfeld, G.; Gould, H.J. Transcription of Ig Germline Genes in Single Human B Cells and the Role of Cytokines in Isotype Determination. J. Immunol. 2004, 173, 4529–4538. [Google Scholar] [CrossRef] [PubMed]
- Jumper, M.D.; Splawski, J.B.; Lipsky, P.E.; Meek, K. Ligation of CD40 induces sterile transcripts of multiple Ig H chain isotypes in human B cells. J. Immunol. 1994, 152, 438–445. [Google Scholar]
- Pène, J.; Gauchat, J.-F.; Lécart, S.; Drouet, E.; Guglielmi, P.; Boulay, V.; Delwail, A.; Foster, D.; Lecron, J.-C.; Yssel, H. Cutting Edge: IL-21 Is a Switch Factor for the Production of IgG 1 and IgG 3 by Human B Cells. J. Immunol. 2004, 172, 5154–5157. [Google Scholar] [CrossRef]
- Smith, C.I.E.; Baskin, B.; Pattersson, E.; Hammarstrom, L.; Islam, K.B. In Vivo Switching: Identification of Germline Transcripts For Human IgA. Adv. Mucosal Immunol. Part A 1995, 371A, 9–13. [Google Scholar]
- Fujieda, S.; Zhang, K.; Saxon, A. IL-4 plus CD40 monoclonal antibody induces human B cells gamma subclass-specific isotype switch: Switching to gamma 1, gamma 3, and gamma 4, but not gamma 2. J. Immunol. 1995, 155, 2318–2328. [Google Scholar] [PubMed]
- Splawski, J.B.; Fu, S.M.; Lipsky, P.E. Immunoregulatory role of CD40 in human B cell differentiation. J. Immunol. 1993, 150, 1276–1285. [Google Scholar] [PubMed]
- Boumendjel, A.; Tawk, L.; Malefijt, R. de W.; Boulay, V.; Yssel, H.; Pène, J. IL-27 induces the production of IgG1 by human B cells. Eur. Cytokine Netw. 2006, 17, 281–289. [Google Scholar]
- Lundgren, M.; Persson, U.; Larsson, P.; Magnusson, C.; Smith, C.I.E.; Hammarström, L.; Severinson, E. Interleukin 4 induces synthesis of IgE and IgG4 in human B cells. Eur. J. Immunol. 1989, 19, 1311–1315. [Google Scholar] [CrossRef]
- Cerutti, A.; Zan, H.; Schaffer, A.; Bergsagel, L.; Harindranath, N.; Max, E.E.; Casali, P. CD40 Ligand and Appropriate Cytokines Induce Switching to IgG, IgA, and IgE and Coordinated Germinal Center and Plasmacytoid Phenotypic Differentiation in a Human Monoclonal IgM+IgD+ B Cell Line. J. Immunol. 1998, 160, 2145–2157. [Google Scholar]
- Stavnezer-Nordgren, J.; Sirlin, S. Specificity of immunoglobulin heavy chain switch correlates with activity of germline heavy chain genes prior to switching. EMBO J. 1986, 5, 95–102. [Google Scholar] [CrossRef]
- Brière, F.; Servet-Delprat, C.; Bridon, J.M.; Saint-Remy, J.M.; Banchereau, J. Human interleukin 10 induces naive surface immunoglobulin D+ (sIgD+) B cells to secrete IgG1 and IgG3. J. Exp. Med. 1994, 179, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Fujieda, S.; Saxon, A.; Zhang, K.E. Direct evidence that γ1 and γ3 switching in human B cells is interleukin-10 dependent. Mol. Immunol. 1996, 33, 1335–1343. [Google Scholar] [CrossRef]
- De Jong, B.G.; Ijspeert, H.; Marques, L.; Van Der Burg, M.; Van Dongen, J.J.M.; Loos, B.G.; Van Zelm, M.C. Human IgG2- and IgG4-expressing memory B cells display enhanced molecular and phenotypic signs of maturity and accumulate with age. Immunol. Cell Biol. 2017, 95, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Horns, F.; Vollmers, C.; Croote, D.; Mackey, S.F.; Swan, G.E.; Dekker, C.L.; Davis, M.M.; Quake, S.R. Lineage tracing of human B cells reveals the in vivo landscape of human antibody class switching. Elife 2016, 5, e16578. [Google Scholar] [CrossRef]
- Jackson, K.J.L.; Wang, Y.; Collins, A.M. Human immunoglobulin classes and subclasses show variability in VDJ gene mutation levels. Immunol. Cell Biol. 2014, 92, 729–733. [Google Scholar] [CrossRef]
- Kitaura, K.; Yamashita, H.; Ayabe, H.; Shini, T.; Matsutani, T.; Suzuki, R. Different somatic hypermutation levels among antibody subclasses disclosed by a new next-generation sequencing-based antibody repertoire analysis. Front. Immunol. 2017, 8, 389. [Google Scholar] [CrossRef]
- Dayal, S.; Nedbal, J.; Hobson, P.; Cooper, A.M.; Gould, H.J.; Gellert, M.; Felsenfeld, G.; Fear, D.J. High resolution analysis of the chromatin landscape of the igE switch region in human B cells. PLoS ONE 2011, 6, e24571. [Google Scholar] [CrossRef]
- Nambu, Y.; Sugai, M.; Gonda, H.; Lee, C.G.; Katakai, T.; Agata, Y.; Yokota, Y.; Shimizu, A. Transcription-Coupled Events Associating with Immunoglobulin Switch Region Chromatin. Science 2003, 302, 2137–2140. [Google Scholar] [CrossRef]
- Li, Z.; Luo, Z.; Scharff, M.D. Differential regulation of histone acetylation and generation of mutations in switch regions is associated with Ig class switching. Proc. Natl. Acad. Sci. USA 2004, 101, 15428–15433. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Whang, N.; Wuerffel, R.; Kenter, A.L. AID-dependent histone acetylation is detected in immunoglobulin S regions. J. Exp. Med. 2006, 203, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.; Forouhi, O.; Dayal, S.; McCloskey, N.; Gould, H.J.; Felsenfeld, G.; Fear, D.J. Analysis of intergenic transcription and histone modification across the human immunoglobulin heavy-chain locus. Proc. Natl. Acad. Sci. USA 2008, 105, 15872–15877. [Google Scholar] [CrossRef]
- Wang, L.; Wuerffel, R.; Feldman, S.; Khamlichi, A.A.; Kenter, A.L. S region sequence, RNA polymerase II, and histone modifications create chromatin accessibility during class switch recombination. J. Exp. Med. 2009, 206, 1817–1830. [Google Scholar] [CrossRef] [PubMed]
- Kuang, F.L.; Luo, Z.; Scharff, M.D. Modifications Are Associated With Class Switch Recombination. Proc. Natl. Acad. Sci. 2009, 106, 5288–5293. [Google Scholar] [CrossRef]
- Perlot, T.; Alt, F.W.; Bassing, C.H.; Sun, H.; Pinaud, E. Elucidation of IgH intronic enhancer functions via germ-line deletion. Proc. Natl. Acad. Sci. USA 2005, 102, 14362–14367. [Google Scholar] [CrossRef] [PubMed]
- Vincent-Fabert, C.; Fiancette, R.; Pinaud, E.; Truffinet, V.; Cogné, N.; Cogné, M.; Denizot, Y. Genomic deletion of the whole IgH 3′ regulatory region (hs3a, hs1,2, hs3b, and hs4) dramatically affects class switch recombination and Ig secretion to all isotypes. Blood 2010, 116, 1895–1898. [Google Scholar] [CrossRef]
- Pinaud, E.; Marquet, M.; Fiancette, R.; Péron, S.; Vincent-Fabert, C.; Denizot, Y.; Cogné, M. The IgH locus 3’ regulatory region: Pulling the strings from behind. Adv. Immunol. 2011, 110, 27–70. [Google Scholar]
- Birshtein, B.K. Epigenetic regulation of individual modules of the immunoglobulin heavy chain locus 3’ regulatory region. Front. Immunol. 2014, 5, 163. [Google Scholar] [CrossRef]
- Sepulveda, M.A.; Emelyanov, A.V.; Birshtein, B.K. NF-κB and Oct-2 Synergize to Activate the Human 3′ Igh hs4 Enhancer in B Cells. J. Immunol. 2004, 172, 1054–1064. [Google Scholar] [CrossRef]
- Sepulveda, M.A.; Garrett, F.E.; Price-Whelan, A.; Birshtein, B.K. Comparative analysis of human and mouse 3′ Igh regulatory regions identifies distinctive structural features. Mol. Immunol. 2005, 42, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Dalloul, I.; Boyer, F.; Dalloul, Z.; Pignarre, A.; Caron, G.; Fest, T.; Chatonnet, F.; Delaloy, C.; Durandy, A.; Jeannet, R.; et al. Locus suicide recombination actively occurs on the functionally rearranged IgH allele in B-cells from inflamed human lymphoid tissues. PLOS Genet. 2019, 15, e1007721. [Google Scholar] [CrossRef] [PubMed]
- Le Noir, S.; Bonaud, A.; Hervé, B.; Baylet, A.; Boyer, F.; Lecardeur, S.; Oruc, Z.; Sirac, C.; Cogné, M. IgH 3’ regulatory region increases ectopic class switch recombination. PLOS Genet. 2021, 17, e1009288. [Google Scholar] [CrossRef] [PubMed]
- Kuzin, I.I.; Bagaeva, L.; Young, F.M.; Bottaro, A. Requirement for Enhancer Specificity in Immunoglobulin Heavy Chain Locus Regulation. J. Immunol. 2008, 180, 7443–7450. [Google Scholar] [CrossRef]
- Kim, A.; Han, L.; Santiago, G.E.; Verdun, R.E.; Yu, K. Class-Switch Recombination in the Absence of the IgH 3′ Regulatory Region. J. Immunol. 2016, 197, 2930–2935. [Google Scholar] [CrossRef]
- Dauba, A.; Khamlichi, A.A. Long-Range Control of Class Switch Recombination by Transcriptional Regulatory Elements. Front. Immunol. 2021, 12, 3591. [Google Scholar] [CrossRef]
- Yu, K.; Lieber, M.R. Current insights into the mechanism of mammalian immunoglobulin class switch recombination. Crit. Rev. Biochem. Mol. Biol. 2019, 54, 333–351. [Google Scholar] [CrossRef]
- Stavnezer, J.; Schrader, C.E. IgH Chain Class Switch Recombination: Mechanism and Regulation. J. Immunol. 2014, 193, 5370–5378. [Google Scholar] [CrossRef]
- Montefiori, L.; Wuerffel, R.; Roqueiro, D.; Lajoie, B.; Guo, C.; Gerasimova, T.; De, S.; Wood, W.; Becker, K.G.; Dekker, J.; et al. Extremely Long-Range Chromatin Loops Link Topological Domains to Facilitate a Diverse Antibody Repertoire. Cell Rep. 2016, 14, 896–906. [Google Scholar] [CrossRef]
- Hill, L.; Ebert, A.; Jaritz, M.; Wutz, G.; Nagasaka, K.; Tagoh, H.; Kostanova-Poliakova, D.; Schindler, K.; Sun, Q.; Bönelt, P.; et al. Wapl repression by Pax5 promotes V gene recombination by Igh loop extrusion. Nature 2020, 584, 142–147. [Google Scholar] [CrossRef]
- Haarhuis, J.H.I.; van der Weide, R.H.; Blomen, V.A.; Yáñez-Cuna, J.O.; Amendola, M.; van Ruiten, M.S.; Krijger, P.H.L.; Teunissen, H.; Medema, R.H.; van Steensel, B.; et al. The Cohesin Release Factor WAPL Restricts Chromatin Loop Extension. Cell 2017, 169, 693–707.e14. [Google Scholar] [CrossRef]
- Wuerffel, R.; Wang, L.; Grigera, F.; Manis, J.; Selsing, E.; Perlot, T.; Alt, F.W.; Cogne, M.; Pinaud, E.; Kenter, A.L. S-S Synapsis during Class Switch Recombination Is Promoted by Distantly Located Transcriptional Elements and Activation-Induced Deaminase. Immunity 2007, 27, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Oudinet, C.; Braikia, F.Z.; Dauba, A.; Khamlichi, A.A. Mechanism and regulation of class switch recombination by IgH transcriptional control elements. In Advances in Immunology; Academic Press Inc.: AEAmsterdam, The Netherlands, 2020; Volume 147. [Google Scholar]
- Chatterjee, S.; Ju, Z.; Hassan, R.; Volpi, S.A.; Emelyanov, A.V.; Birshtein, B.K. Dynamic changes in binding of immunoglobulin heavy chain 3′ regulatory region to protein factors during class switching. J. Biol. Chem. 2011, 286, 29303–29312. [Google Scholar] [CrossRef] [PubMed]
- Kenter, A.L.; Feldman, S.; Wuerffel, R.; Achour, I.; Wang, L.; Kumar, S. Three-dimensional architecture of the IgH locus facilitates class switch recombination. Ann. New York Acad. Sci. 2012, 1267, 86–94. [Google Scholar] [CrossRef]
- Degner, S.C.; Verma-Gaur, J.; Wong, T.P.; Bossen, C.; Iverson, G.M.; Torkamani, A.; Vettermann, C.; Lin, Y.C.; Ju, Z.; Schulz, D.; et al. CCCTC-binding factor (CTCF) and cohesin influence the genomic architecture of the Igh locus and antisense transcription in pro-B cells. Proc. Natl. Acad. Sci. USA 2011, 108, 9566–9571. [Google Scholar] [CrossRef]
- Zhang, X.; Yoon, H.S.; Chapdelaine-Williams, A.M.; Kyritsis, N.; Alt, F.W. Physiological role of the 3′IgH CBEs super-anchor in antibody class switching. Proc. Natl. Acad. Sci. USA 2021, 118, 2024392118. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Ba, Z.; Kyritsis, N.; Casellas, R.; Alt, F.W. Fundamental roles of chromatin loop extrusion in antibody class switching. Nature 2019, 575, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, D.N.; Namiki, Y.; Chen, C.; Morshead, K.B.; Wood, A.L.; Johnston, C.M.; Morris, J.W.; Wang, Y.; Sadreyev, R.; Corcoran, A.E.; et al. The murine IgH locus contains a distinct DNA sequence motif for the chromatin regulatory factor CTCF. J. Biol. Chem. 2019, 294, 13580–13592. [Google Scholar] [CrossRef] [PubMed]
- Schwab, K.R.; Patel, S.R.; Dressler, G.R. Role of PTIP in Class Switch Recombination and Long-Range Chromatin Interactions at the Immunoglobulin Heavy Chain Locus. Mol. Cell. Biol. 2011, 31, 1503–1511. [Google Scholar] [CrossRef]
- Daniel, J.A.; Santos, M.A.; Wang, Z.; Zang, C.; Schwab, K.R.; Jankovic, M.; Filsuf, D.; Chen, H.T.; Gazumyan, A.; Yamane, A.; et al. PTIP promotes chromatin changes critical for immunoglobulin class switch recombination. Science 2010, 329, 917–923. [Google Scholar] [CrossRef]
- Giambra, V.; Volpi, S.; Emelyanov, A.V.; Pflugh, D.; Bothwell, A.L.M.; Norio, P.; Fan, Y.; Ju, Z.; Skoultchi, A.I.; Hardy, R.R.; et al. Pax5 and Linker Histone H1 Coordinate DNA Methylation and Histone Modifications in the 3′ Regulatory Region of the Immunoglobulin Heavy Chain Locus. Mol. Cell. Biol. 2008, 28, 6123–6133. [Google Scholar] [CrossRef]
- Minnich, M.; Tagoh, H.; Bönelt, P.; Axelsson, E.; Fischer, M.; Cebolla, B.; Tarakhovsky, A.; Nutt, S.L.; Jaritz, M.; Busslinger, M. Multifunctional role of the transcription factor Blimp-1 in coordinating plasma cell differentiation. Nat. Immunol. 2016, 17, 331–343. [Google Scholar] [CrossRef]
- Hauser, J.; Grundström, C.; Kumar, R.; Grundström, T. Regulated localization of an AID complex with E2A, PAX5 and IRF4 at the Igh locus. Mol. Immunol. 2016, 80, 78–90. [Google Scholar] [CrossRef]
- Grundström, C.; Kumar, A.; Priya, A.; Negi, N.; Grundström, T. ETS1 and PAX5 transcription factors recruit AID to Igh DNA. Eur. J. Immunol. 2018, 48, 1687–1697. [Google Scholar] [CrossRef]
- Perlot, T.; Li, G.; Alt, F.W. Antisense transcripts from immunoglobulin heavy-chain locus V(D)J and switch regions. Proc. Natl. Acad. Sci. USA 2008, 105, 3843–3848. [Google Scholar] [CrossRef] [PubMed]
- Osipovich, O.A.; Subrahmanyam, R.; Pierce, S.; Sen, R.; Oltz, E.M. Cutting Edge: SWI/SNF Mediates Antisense Igh Transcription and Locus-Wide Accessibility in B Cell Precursors. J. Immunol. 2009, 183, 1509–1513. [Google Scholar] [CrossRef] [PubMed]
- Yen, W.F.; Sharma, R.; Cols, M.; Lau, C.M.; Chaudhry, A.; Chowdhury, P.; Yewdell, W.T.; Vaidyanathan, B.; Sun, A.; Coffre, M.; et al. Distinct Requirements of CHD4 during B Cell Development and Antibody Response. Cell Rep. 2019, 27, 1472–1486.e5. [Google Scholar] [CrossRef]
- Lavery, W.J.; Barski, A.; Wiley, S.; Schorry, E.K.; Lindsley, A.W. KMT2C/D COMPASS complex-associated diseases [KCDCOM-ADs]: An emerging class of congenital regulopathies. Clin. Epigenetics 2020, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Eckner, R.; Yao, T.P.; Oldread, E.; Livingston, D.M. Interaction and functional collaboration of p300/CBP and bHLH proteins in muscle and B-cell differentiation. Genes Dev. 1996, 10, 2478–2490. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; Wu, X.; Liu, T.; Yu, K.; Jelinek, D.F.; Lou, Z. The Histone Methyltransferase MMSET Regulates Class Switch Recombination. J. Immunol. 2013, 190, 756–763. [Google Scholar] [CrossRef]
- Pefanis, E.; Wang, J.; Rothschild, G.; Lim, J.; Chao, J.; Rabadan, R.; Economides, A.N.; Basu, U. Noncoding RNA transcription targets AID to divergently transcribed loci in B cells. Nature 2014, 514, 389–393. [Google Scholar] [CrossRef]
- Medvedovic, J.; Ebert, A.; Tagoh, H.; Busslinger, M. Pax5: A Master Regulator of B Cell Development and Leukemogenesis. In Advances in Immunology; Academic Press Inc.: AEAmsterdam, The Netherlands, 2011; Volume 111, pp. 179–206. [Google Scholar]
- O’Brien, P.; Morin, P.; Ouellette, R.J.; Robichaud, G.A. The Pax-5 gene: A pluripotent regulator of B-cell differentiation and cancer disease. Cancer Res. 2011, 71, 7345–7350. [Google Scholar] [CrossRef]
- Okuyama, K.; Strid, T.; Kuruvilla, J.; Somasundaram, R.; Cristobal, S.; Smith, E.; Prasad, M.; Fioretos, T.; Lilljebjörn, H.; Soneji, S.; et al. PAX5 is part of a functional transcription factor network targeted in lymphoid leukemia. PLoS Genet. 2019, 15, e1008280. [Google Scholar] [CrossRef] [PubMed]
- Foltz, S.M.; Gao, Q.; Yoon, C.J.; Sun, H.; Yao, L.; Li, Y.; Jayasinghe, R.G.; Cao, S.; King, J.; Kohnen, D.R.; et al. Evolution and structure of clinically relevant gene fusions in multiple myeloma. Nat. Commun. 2020, 11, 2666. [Google Scholar] [CrossRef]
- Rustad, E.H.; Yellapantula, V.D.; Glodzik, D.; Maclachlan, K.H.; Diamond, B.; Boyle, E.M.; Ashby, C.; Blaney, P.; Gundem, G.; Hultcrantz, M.; et al. Revealing the Impact of Structural Variants in Multiple Myeloma. Blood Cancer Discov. 2020, 1, 258–273. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Wang, Q.; Dose, M.; Pruett, N.; Kieffer-Kwon, K.R.; Resch, W.; Liang, G.; Tang, Z.; Mathé, E.; Benner, C.; et al. B cell super-enhancers and regulatory clusters recruit AID tumorigenic activity. Cell 2014, 159, 1524–1537. [Google Scholar] [CrossRef] [PubMed]
- Borson, N.D.; Lacy, M.Q.; Wettstein, P.J. Altered mRNA expression of Pax5 and Blimp-1 in B cells in multiple myeloma. Blood 2002, 100, 4629–4639. [Google Scholar] [CrossRef]
- Fuxa, M.; Skok, J.; Souabni, A.; Salvagiotto, G.; Roldan, E.; Busslinger, M. Pax5 induces V-to-DJ rearrangements and locus contraction of the immunoglobulin heavy-chain gene. Genes Dev. 2004, 18, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Nutt, S.L.; Urbánek, P.; Relink, A.; Busslinger, M. Essential functions of Pax5 (BSAP) in pro-B cell development: Difference between fetal and adult B lymphopoiesis and reduced V-to-DJ recombination at the IgH locus. Genes Dev. 1997, 11, 476–491. [Google Scholar] [CrossRef]
- Waters, S.H.; Saikh, K.U.; Stavnezer, J. A B-cell-specific nuclear protein that binds to DNA sites 5’ to immunoglobulin S alpha tandem repeats is regulated during differentiation. Mol. Cell. Biol. 1989, 9, 5594–5601. [Google Scholar]
- Liao, F.; Giannini, S.L.; Birshtein, B.K. A nuclear DNA-binding protein expressed during early stages of B cell differentation interacts with diverse segments within and 3’ of the Ig H chain gene cluster. J. Immunol. 1992, 148, 2909–2917. [Google Scholar] [PubMed]
- Xu, L.; Kim, M.G.; Marcu, K.B. Properties of B cell stage specific and ubiquitous nuclear factors binding to immunoglobulin heavy chain gene switch regions. Int. Immunol. 1992, 4, 875–887. [Google Scholar] [CrossRef]
- Kosak, S.T.; Skok, J.A.; Medina, K.L.; Riblet, R.; Le Beau, M.M.; Fisher, A.G.; Singh, H. Subnuclear compartmentalization of immunoglobulin loci during lymphocyte development. Science 2002, 296, 158–162. [Google Scholar] [CrossRef]
- Zullo, J.M.; Demarco, I.A.; Piqué-Regi, R.; Gaffney, D.J.; Epstein, C.B.; Spooner, C.J.; Luperchio, T.R.; Bernstein, B.E.; Pritchard, J.K.; Reddy, K.L.; et al. DNA sequence-dependent compartmentalization and silencing of chromatin at the nuclear lamina. Cell 2012, 149, 1474–1487. [Google Scholar] [CrossRef] [PubMed]
- Bowen, A.J.; Corcoran, A.E. How chromatin remodelling allows shuffling of immunoglobulin heavy chain genes. Mol. Biosyst. 2008, 4, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, A.E. The epigenetic role of non-coding RNA transcription and nuclear organization in immunoglobulin repertoire generation. Semin. Immunol. 2010, 22, 353–361. [Google Scholar] [CrossRef]
- Pichugin, A.; Iarovaia, O.V.; Gavrilov, A.; Sklyar, I.; Barinova, N.; Barinov, A.; Ivashkin, E.; Caron, G.; Aoufouchi, S.; Razin, S.V.; et al. The IGH locus relocalizes to a “recombination compartment” in the perinucleolar region of differentiating B-lymphocytes. Oncotarget 2017, 8, 40079–40089. [Google Scholar] [CrossRef]
- Seifert, M.; Scholtysik, R.; Küppers, R. Origin and pathogenesis of B cell lymphomas. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2019; Volume 1956, pp. 1–33. [Google Scholar]
- Ramiro, A.R.; Jankovic, M.; Eisenreich, T.; Difilippantonio, S.; Chen-Kiang, S.; Muramatsu, M.; Honjo, T.; Nussenzweig, A.; Nussenzweig, M.C. AID is required for c-myc/IgH chromosome translocations in vivo. Cell 2004, 118, 431–438. [Google Scholar] [CrossRef]
- Ghetti, M.; Vannini, I.; Storlazzi, C.T.; Martinelli, G.; Simonetti, G. Linear and circular PVT1 in hematological malignancies and immune response: Two faces of the same coin. Mol. Cancer 2020, 19, 69. [Google Scholar] [CrossRef] [PubMed]
- Derderian, C.; Orunmuyi, A.T.; Oluwabunmi Olapade-Olaopa, E.; Ogunwobi, O.O. PVT1 signaling is a mediator of cancer progression. Front. Oncol. 2019, 9, 502. [Google Scholar] [CrossRef]
- Cho, S.W.; Xu, J.; Sun, R.; Mumbach, M.R.; Carter, A.C.; Chen, Y.G.; Yost, K.E.; Kim, J.; He, J.; Nevins, S.A.; et al. Promoter of lncRNA Gene PVT1 Is a Tumor-Suppressor DNA Boundary Element. Cell 2018, 173, 1398–1412.e22. [Google Scholar] [CrossRef]
- Osborne, C.S.; Chakalova, L.; Mitchell, J.A.; Horton, A.; Wood, A.L.; Bolland, D.J.; Corcoran, A.E.; Fraser, P. Myc dynamically and preferentially relocates to a transcription factory occupied by Igh. PLoS Biol. 2007, 5, 1763–1772. [Google Scholar] [CrossRef]
- Strongin, D.E.; Groudine, M.; Politz, J.C.R. Nucleolar tethering mediates pairing between the IgH and Myc loci. Nucleus 2014, 5, 474–481. [Google Scholar] [CrossRef]
- Iarovaia, O.V.; Ioudinkova, E.S.; Razin, S.V.; Vassetzky, Y.S. Role of the Nucleolus in Rearrangements of the IGH Locus. Mol. Biol. (Mosk) 2018, 52, 210–219. [Google Scholar] [CrossRef]
- Sklyar, I.V.; Pichugin, A.M.; Razin, S.V.; Vassetzky, E.S.; Iarovaia, O.V. Nuclear localization of translocation partners in differentiating B-cells. Dokl. Biochem. Biophys. 2015, 464, 312–314. [Google Scholar] [CrossRef]
- Sklyar, I.; Iarovaia, O.V.; Gavrilov, A.A.; Pichugin, A.; Germini, D.; Tsfasman, T.; Caron, G.; Fest, T.; Lipinski, M.; Razin, S.V.; et al. Distinct Patterns of Colocalization of the CCND1 and CMYC Genes with Their Potential Translocation Partner IGH at Successive Stages of B-Cell Differentiation. J. Cell. Biochem. 2016, 117, 1506–1510. [Google Scholar] [CrossRef]
- Kaur, G.; Gupta, R.; Mathur, N.; Rani, L.; Kumar, L.; Sharma, A.; Singh, V.; Gupta, A.; Sharma, O.D. Clinical impact of chromothriptic complex chromosomal rearrangements in newly diagnosed multiple myeloma. Leuk. Res. 2019, 76, 58–64. [Google Scholar] [CrossRef]
- Smetana, J.; Oppelt, J.; Štork, M.; Pour, L.; Kuglík, P. Chromothripsis 18 in multiple myeloma patient with rapid extramedullary relapse. Mol. Cytogenet. 2018, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Magrangeas, F.; Avet-Loiseau, H.; Munshi, N.C.; Minvielle, S. Chromothripsis identifies a rare and aggressive entity among newly diagnosed multiple myeloma patients. Blood 2011, 118, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Roberts, N.D.; Wala, J.A.; Shapira, O.; Schumacher, S.E.; Kumar, K.; Khurana, E.; Waszak, S.; Korbel, J.O.; Haber, J.E.; et al. Patterns of somatic structural variation in human cancer genomes. Nature 2020, 578, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Baca, S.C.; Prandi, D.; Lawrence, M.S.; Mosquera, J.M.; Romanel, A.; Drier, Y.; Park, K.; Kitabayashi, N.; MacDonald, T.Y.; Ghandi, M.; et al. Punctuated evolution of prostate cancer genomes. Cell 2013, 153, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Marcozzi, A.; Pellestor, F.; Kloosterman, W.P. The genomic characteristics and origin of chromothripsis. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2018; Volume 1769, pp. 3–19. [Google Scholar]
- Campbell, P.J.; Getz, G.; Korbel, J.O.; Stuart, J.M.; Jennings, J.L.; Stein, L.D.; Perry, M.D.; Nahal-Bose, H.K.; Ouellette, B.F.F.; Li, C.H.; et al. Pan-cancer analysis of whole genomes. Nature 2020, 578, 82–93. [Google Scholar]
- Kramara, J.; Osia, B.; Malkova, A. Break-Induced Replication: The Where, The Why, and The How. Trends Genet. 2018, 34, 518–531. [Google Scholar] [CrossRef]
- Kockler, Z.W.; Osia, B.; Lee, R.; Musmaker, K.; Malkova, A. Repair of DNA Breaks by Break-Induced Replication. Annu. Rev. Biochem. 2021, 90, 165–191. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.P.; Tsaponina, O.; Greenwell, P.W.; Lee, C.S.; Du, W.; Petes, T.D.; Haber, J.E. Chromosome rearrangements via template switching between diverged repeated sequences. Genes Dev. 2014, 28, 2394–2406. [Google Scholar] [CrossRef]
- Rodgers, K.; McVey, M. Error-Prone Repair of DNA Double-Strand Breaks. J. Cell. Physiol. 2016, 231, 15–24. [Google Scholar] [CrossRef]
- Zhang, F.; Khajavi, M.; Connolly, A.M.; Towne, C.F.; Batish, S.D.; Lupski, J.R. The DNA replication FoSTeS/MMBIR mechanism can generate genomic, genic and exonic complex rearrangements in humans. Nat. Genet. 2009, 41, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.J.; Cleveland, D.W. Chromoanagenesis and cancer: Mechanisms and consequences of localized, complex chromosomal rearrangements. Nat. Med. 2012, 18, 1630–1638. [Google Scholar] [CrossRef]
- Hastings, P.J.; Ira, G.; Lupski, J.R. A Microhomology-Mediated Break-Induced Replication Model for the Origin of Human Copy Number Variation. PLoS Genet. 2009, 5, e1000327. [Google Scholar] [CrossRef]
- Stephens, P.J.; Greenman, C.D.; Fu, B.; Yang, F.; Bignell, G.R.; Mudie, L.J.; Pleasance, E.D.; Lau, K.W.; Beare, D.; Stebbings, L.A.; et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell 2011, 144, 27–40. [Google Scholar] [CrossRef]
- Lee, K.J.; Lee, K.H.; Yoon, K.-A.; Sohn, J.Y.; Lee, E.; Lee, H.; Eom, H.-S.; Kong, S.-Y. Chromothripsis in Treatment Resistance in Multiple Myeloma. Genom. Inform. 2017, 15, 87–97. [Google Scholar] [CrossRef]
- Maciejowski, J.; Li, Y.; Bosco, N.; Campbell, P.J.; De Lange, T. Chromothripsis and Kataegis Induced by Telomere Crisis. Cell 2015, 163, 1641–1654. [Google Scholar] [CrossRef]
- Mardin, B.R.; Drainas, A.P.; Waszak, S.M.; Weischenfeldt, J.; Isokane, M.; Stütz, A.M.; Raeder, B.; Efthymiopoulos, T.; Buccitelli, C.; Segura-Wang, M.; et al. A cell-based model system links chromothripsis with hyperploidy. Mol. Syst. Biol. 2015, 11, 828. [Google Scholar] [CrossRef]
- Li, Y.; Schwab, C.; Ryan, S.L.; Papaemmanuil, E.; Robinson, H.M.; Jacobs, P.; Moorman, A.V.; Dyer, S.; Borrow, J.; Griffiths, M.; et al. Constitutional and somatic rearrangement of chromosome 21 in acute lymphoblastic leukaemia. Nature 2014, 508, 98–102. [Google Scholar] [CrossRef]
- Garsed, D.W.; Marshall, O.J.; Corbin, V.D.A.; Hsu, A.; DiStefano, L.; Schröder, J.; Li, J.; Feng, Z.P.; Kim, B.W.; Kowarsky, M.; et al. The Architecture and Evolution of Cancer Neochromosomes. Cancer Cell 2014, 26, 653–667. [Google Scholar] [CrossRef]
- Liu, S.; Kwon, M.; Mannino, M.; Yang, N.; Renda, F.; Khodjakov, A.; Pellman, D. Nuclear envelope assembly defects link mitotic errors to chromothripsis. Nature 2018, 561, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Z.; Spektor, A.; Cornils, H.; Francis, J.M.; Jackson, E.K.; Liu, S.; Meyerson, M.; Pellman, D. Chromothripsis from DNA damage in micronuclei. Nature 2015, 522, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Crasta, K.; Ganem, N.J.; Dagher, R.; Lantermann, A.B.; Ivanova, E.V.; Pan, Y.; Nezi, L.; Protopopov, A.; Chowdhury, D.; Pellman, D. DNA breaks and chromosome pulverization from errors in mitosis. Nature 2012, 482, 53–58. [Google Scholar] [CrossRef]
- Ly, P.; Brunner, S.F.; Shoshani, O.; Kim, D.H.; Lan, W.; Pyntikova, T.; Flanagan, A.M.; Behjati, S.; Page, D.C.; Campbell, P.J.; et al. Chromosome segregation errors generate a diverse spectrum of simple and complex genomic rearrangements. Nat. Genet. 2019, 51, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.J.; Sharpe, Z.; Alemara, S.; Mackenzie, S.; Liu, G.; Abdallah, B.; Horne, S.; Regan, S.; Heng, H.H. Micronuclei and genome chaos: Changing the system inheritance. Genes 2019, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Maciejowski, J.; De Lange, T. Telomeres in cancer: Tumour suppression and genome instability. Nat. Rev. Mol. Cell Biol. 2017, 18, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Cleal, K.; Jones, R.E.; Grimstead, J.W.; Hendrickson, E.A.; Baird, D.M. Chromothripsis during telomere crisis is independent of NHEJ, and consistent with a replicative origin. Genome Res. 2019, 29, 737–749. [Google Scholar] [CrossRef]
- Cheng, C.; Zhou, Y.; Li, H.; Xiong, T.; Li, S.; Bi, Y.; Kong, P.; Wang, F.; Cui, H.; Li, Y.; et al. Whole-Genome Sequencing Reveals Diverse Models of Structural Variations in Esophageal Squamous Cell Carcinoma. Am. J. Hum. Genet. 2016, 98, 256–274. [Google Scholar] [CrossRef]
- Nik-Zainal, S.; Van Loo, P.; Wedge, D.C.; Alexandrov, L.B.; Greenman, C.D.; Lau, K.W.; Raine, K.; Jones, D.; Marshall, J.; Ramakrishna, M.; et al. The life history of 21 breast cancers. Cell 2012, 149, 994–1007. [Google Scholar] [CrossRef] [PubMed]
- Piazza, A.; Wright, W.D.; Heyer, W.D. Multi-invasions Are Recombination Byproducts that Induce Chromosomal Rearrangements. Cell 2017, 170, 760–773.e15. [Google Scholar] [CrossRef]
- Cottliar, A.; Pedrazzini, E.; Corrado, C.; Engelberger, M.I.; Narbaitz, M.; Slavutsky, I. Telomere shortening in patients with plasma cell disorders. Eur. J. Haematol. 2003, 71, 334–340. [Google Scholar] [CrossRef]
- de la Guardia, R.D.; Catalina, P.; Panero, J.; Elosua, C.; Pulgarin, A.; López, M.B.; Ayllón, V.; Ligero, G.; Slavutsky, I.; Leone, P.E. Expression profile of telomere-associated genes in multiple myeloma. J. Cell. Mol. Med. 2012, 16, 3009–3021. [Google Scholar] [CrossRef]
- Hyatt, S.; Jones, R.E.; Heppel, N.H.; Grimstead, J.W.; Fegan, C.; Jackson, G.H.; Hills, R.; Allan, J.M.; Pratt, G.; Pepper, C.; et al. Telomere length is a critical determinant for survival in multiple myeloma. Br. J. Haematol. 2017, 178, 94–98. [Google Scholar] [CrossRef]
- Wu, K.D.; Orme, L.M.; Shaughnessy, J.; Jacobson, J.; Barlogie, B.; Moore, M.A.S. Telomerase and telomere length in multiple myeloma: Correlations with disease heterogeneity, cytogenetic status, and overall survival. Blood 2003, 101, 4982–4989. [Google Scholar] [CrossRef]
- Panero, J.; Arbelbide, J.; Fantl, D.B.; Rivello, H.G.; Kohan, D.; Slavutsky, I. Altered mRNA expression of telomere-associated genes in monoclonal gammopathy of undetermined significance and multiple myeloma. Mol. Med. 2010, 16, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Shiratsuchi, M.; Muta, K.; Abe, Y.; Motomura, S.; Taguchi, F.; Takatsuki, H.; Uike, N.; Umemura, T.; Nawata, H.; Nishimura, J. Clinical significance of telomerase activity in multiple myeloma. Cancer 2002, 94, 2232–2238. [Google Scholar] [CrossRef] [PubMed]
- Nergadze, S.G.; Santagostino, M.A.; Salzano, A.; Mondello, C.; Giulotto, E. Contribution of telomerase RNA retrotranscription to DNA double-strand break repair during mammalian genome evolution. Genome Biol. 2007, 8, R260. [Google Scholar] [CrossRef]
- Marzec, P.; Armenise, C.; Pérot, G.; Roumelioti, F.-M.M.; Basyuk, E.; Gagos, S.; Chibon, F.; Déjardin, J. Nuclear-Receptor-Mediated Telomere Insertion Leads to Genome Instability in ALT Cancers. Cell 2015, 160, 913–927. [Google Scholar] [CrossRef]
- Onozawa, M.; Zhang, Z.; Kim, Y.J.; Goldberg, L.; Varga, T.; Bergsagel, P.L.; Kuehl, W.M.; Aplan, P.D. Repair of DNA double-strand breaks by templated nucleotide sequence insertions derived from distant regions of the genome. Proc. Natl. Acad. Sci. USA 2014, 111, 7729–7734. [Google Scholar] [CrossRef] [PubMed]
- Azzalin, C.M.; Mucciolo, E.; Bertoni, L.; Giulotto, E. Fluorescence in situ hybridization with a synthetic (T2AG3)n polynucleotide detects several intrachromosomal telomere-like repeats on human chromosomes. Cytogenet. Cell Genet. 1997, 78, 112–115. [Google Scholar] [CrossRef]
- Aksenova, A.Y.; Greenwell, P.W.; Dominska, M.; Shishkin, A.A.; Kim, J.C.; Petes, T.D.; Mirkin, S.M. Genome rearrangements caused by interstitial telomeric sequences in yeast. Proc. Natl. Acad. Sci. USA 2013, 110, 19866–19871. [Google Scholar] [CrossRef] [PubMed]
- Kilburn, A.E.; Shea, M.J.; Sargent, R.G.; Wilson, J.H. Insertion of a telomere repeat sequence into a mammalian gene causes chromosome instability. Mol. Cell. Biol. 2001, 21, 126–135. [Google Scholar] [CrossRef]
- Bosco, N.; de Lange, T. A TRF1-controlled common fragile site containing interstitial telomeric sequences. Chromosoma 2012, 121, 465–474. [Google Scholar] [CrossRef]
- Wood, A.M.; Danielsen, J.M.R.; Lucas, C.A.; Rice, E.L.; Scalzo, D.; Shimi, T.; Goldman, R.D.; Smith, E.D.; Le Beau, M.M.; Kosak, S.T. TRF2 and lamin A/C interact to facilitate the functional organization of chromosome ends. Nat. Commun. 2014, 5, 5467. [Google Scholar] [CrossRef]
- Wood, A.M.; Laster, K.; Rice, E.L.; Kosak, S.T. A beginning of the end: New insights into the functional organization of telomeres. Nucleus 2015, 6, 172–178. [Google Scholar] [CrossRef]
- Klewes, L.; Vallente, R.; Dupas, E.; Brand, C.; Grün, D.; Guffei, A.; Sathitruangsak, C.; Awe, J.A.; Kuzyk, A.; Lichtensztejn, D.; et al. Three-dimensional nuclear telomere organization in multiple myeloma. Transl. Oncol. 2013, 6, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.L.I.; Wang, R.R.; Johnston, G.; Wang, Y.; Tammur, P.; Tamm, A.; Punab, M.; Rangel-Pozzo, A.; Mai, S. Distinct Nuclear Organization of Telomeresand Centromeres in Monoclonal Gammopathyof Undetermined Significance and Multiple Myeloma. Cells 2019, 8, 723. [Google Scholar] [CrossRef] [PubMed]
- Campa, D.; Martino, A.; Varkonyi, J.; Lesueur, F.; Jamroziak, K.; Landi, S.; Jurczyszyn, A.; Marques, H.; Andersen, V.; Jurado, M.; et al. Risk of multiple myeloma is associated with polymorphisms within telomerase genes and telomere length. Int. J. Cancer 2015, 136, E351–E358. [Google Scholar] [CrossRef] [PubMed]
- Hosnijeh, F.S.; Matullo, G.; Russo, A.; Guarrera, S.; Modica, F.; Nieters, A.; Overvad, K.; Guldberg, P.; Tjønneland, A.; Canzian, F.; et al. Prediagnostic telomere length and risk of B-cell lymphoma-Results from the EPIC cohort study. Int. J. Cancer 2014, 135, 2910–2917. [Google Scholar] [CrossRef]
- Went, M.; Cornish, A.J.; Law, P.J.; Kinnersley, B.; van Duin, M.; Weinhold, N.; Försti, A.; Hansson, M.; Sonneveld, P.; Goldschmidt, H.; et al. Search for multiple myeloma risk factors using Mendelian randomization. Blood Adv. 2020, 4, 2172–2179. [Google Scholar] [CrossRef]
- Jones, A.M.; Beggs, A.D.; Carvajal-Carmona, L.; Farrington, S.; Tenesa, A.; Walker, M.; Howarth, K.; Ballereau, S.; Hodgson, S.V.; Zauber, A.; et al. TERC polymorphisms are associated both with susceptibility to colorectal cancer and with longer telomeres. Gut 2012, 61, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Houlston, R.S.; Cheadle, J.; Dobbins, S.E.; Tenesa, A.; Jones, A.M.; Howarth, K.; Spain, S.L.; Broderick, P.; Domingo, E.; Farrington, S.; et al. Meta-analysis of three genome-wide association studies identifies susceptibility loci for colorectal cancer at 1q41, 3q26.2, 12q13.13 and 20q13.33. Nat. Genet. 2010, 42, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Codd, V.; Nelson, C.P.; Albrecht, E.; Mangino, M.; Deelen, J.; Buxton, J.L.; Hottenga, J.J.; Fischer, K.; Esko, T.; Surakka, I.; et al. Identification of seven loci affecting mean telomere length and their association with disease. Nat. Genet. 2013, 45, 422–427. [Google Scholar] [CrossRef]
- Speedy, H.E.; Di Bernardo, M.C.; Sava, G.P.; Dyer, M.J.S.; Holroyd, A.; Wang, Y.; Sunter, N.J.; Mansouri, L.; Juliusson, G.; Smedby, K.E.; et al. A genome-wide association study identifies multiple susceptibility loci for chronic lymphocytic leukemia. Nat. Genet. 2014, 46, 56–60. [Google Scholar] [CrossRef]
- Michalek, J.E.; Kepa, A.; Vincent, J.; Frissa, S.; Goodwin, L.; Hotopf, M.; Hatch, S.L.; Breen, G.; Powell, T.R. Genetic predisposition to advanced biological ageing increases risk for childhood-onset recurrent major depressive disorder in a large UK sample. J. Affect. Disord. 2017, 213, 207–213. [Google Scholar] [CrossRef]
- Bojesen, S.E.; Pooley, K.A.; Johnatty, S.E.; Beesley, J.; Michailidou, K.; Tyrer, J.P.; Edwards, S.L.; Pickett, H.A.; Shen, H.C.; Smart, C.E.; et al. Multiple independent variants at the TERT locus are associated with telomere length and risks of breast and ovarian cancer. Nat. Genet. 2013, 45, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Coutts, F.; Palmos, A.B.; Duarte, R.R.R.; de Jong, S.; Lewis, C.M.; Dima, D.; Powell, T.R. The polygenic nature of telomere length and the anti-ageing properties of lithium. Neuropsychopharmacology 2019, 44, 757–765. [Google Scholar] [CrossRef]
- Palm, W.; de Lange, T. How shelterin protects mammalian telomeres. Annu. Rev. Genet. 2008, 42, 301–334. [Google Scholar] [CrossRef] [PubMed]
- Speedy, H.E.; Kinnersley, B.; Chubb, D.; Broderick, P.; Law, P.J.; Litchfield, K.; Jayne, S.; Dyer, M.J.S.; Dearden, C.; Follows, G.A.; et al. Germ line mutations in shelterin complex genes are associated with familial chronic lymphocytic leukemia. Blood 2016, 128, 2319–2326. [Google Scholar] [CrossRef] [PubMed]
- Panero, J.; Stanganelli, C.; Arbelbide, J.; Fantl, D.B.; Kohan, D.; García Rivello, H.; Rabinovich, G.A.; Slavutsky, I. Expression profile of shelterin components in plasma cell disorders. Clinical significance of POT1 overexpression. Blood Cells Mol. Dis. 2014, 52, 134–139. [Google Scholar] [CrossRef]
- Popuri, V.; Hsu, J.; Khadka, P.; Horvath, K.; Liu, Y.; Croteau, D.L.; Bohr, V.A. Human RECQL1 participates in telomere maintenance. Nucleic Acids Res. 2014, 42, 5671–5688. [Google Scholar] [CrossRef]
- Viziteu, E.; Klein, B.; Basbous, J.; Lin, Y.L.; Hirtz, C.; Gourzones, C.; Tiers, L.; Bruyer, A.; Vincent, L.; Grandmougin, C.; et al. RECQ1 helicase is involved in replication stress survival and drug resistance in multiple myeloma. Leukemia 2017, 31, 2104–2113. [Google Scholar] [CrossRef]
- Viziteu, E.; Kassambara, A.; Pasero, P.; Klein, B.; Moreaux, J. RECQ helicases are deregulated in hematological malignancies in association with a prognostic value. Biomark. Res. 2016, 4, 3. [Google Scholar] [CrossRef]
- Porro, A.; Feuerhahn, S.; Lingner, J. TERRA-reinforced association of LSD1 with MRE11 promotes processing of uncapped telomeres. Cell Rep. 2014, 6, 765–776. [Google Scholar] [CrossRef]
- Hirschi, A.; Martin, W.J.; Luka, Z.; Loukachevitch, L.V.; Reiter, N.J. G-quadruplex RNA binding and recognition by the lysine-specific histone demethylase-1 enzyme. RNA 2016, 22, 1250–1260. [Google Scholar] [CrossRef]
- Rahmanto, Y.S.; Jung, J.G.; Wu, R.C.; Kobayashi, Y.; Heaphy, C.M.; Meeker, A.K.; Wang, T.L.; Shih, I.M. Inactivating ARID1A tumor suppressor enhances TERT transcription and maintains telomere length in cancer cells. J. Biol. Chem. 2016, 291, 9690–9699. [Google Scholar] [CrossRef]
- Zhao, B.; Lin, J.; Rong, L.; Wu, S.; Deng, Z.; Fatkhutdinov, N.; Zundell, J.; Fukumoto, T.; Liu, Q.; Kossenkov, A.; et al. ARID1A promotes genomic stability through protecting telomere cohesion. Nat. Commun. 2019, 10, 4067. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.D.; Niedernhofer, L.; Kuster, B.; Mann, M.; Hoeijmakers, J.H.J.; De Lange, T. ERCC1/XPF Removes the 3′ Overhang from Uncapped Telomeres and Represses Formation of Telomeric DNA-Containing Double Minute Chromosomes. Mol. Cell 2003, 12, 1489–1498. [Google Scholar] [CrossRef]
- Tseng, C.K.; Wang, H.F.; Burns, A.M.M.; Schroeder, M.R.R.; Gaspari, M.; Baumann, P. Human Telomerase RNA Processing and Quality Control. Cell Rep. 2015, 13, 2232–2243. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.R.; Oliver, A.W.; Chevassut, T.J.; Newbury, S.F. The 3’ to 5’ exoribonuclease DIS3: From structure and mechanisms to biological functions and role in human disease. Biomolecules 2015, 5, 1515–1539. [Google Scholar] [CrossRef] [PubMed]
- Her, Y.R.; Chung, I.K. P300-mediated acetylation of TRF2 is required for maintaining functional telomeres. Nucleic Acids Res. 2013, 41, 2267–2283. [Google Scholar] [CrossRef]
- Cubiles, M.D.; Barroso, S.; Vaquero-Sedas, M.I.; Enguix, A.; Aguilera, A.; Vega-Palas, M.A. Epigenetic features of human telomeres. Nucleic Acids Res. 2018, 46, 2347–2355. [Google Scholar] [CrossRef]
- Shalem-Cohavi, N.; Beery, E.; Nordenberg, J.; Rozovski, U.; Raanani, P.; Lahav, M.; Uziel, O. The effects of proteasome inhibitors on telomerase activity and regulation in multiple myeloma cells. Int. J. Mol. Sci. 2019, 20, 2509. [Google Scholar] [CrossRef]
- Weiss, C.; Uziel, O.; Wolach, O.; Nordenberg, J.; Beery, E.; Bulvick, S.; Kanfer, G.; Cohen, O.; Ram, R.; Bakhanashvili, M.; et al. Differential downregulation of telomerase activity by bortezomib in multiple myeloma cells-multiple regulatory pathways in vitro and ex vivo. Br. J. Cancer 2012, 107, 1844–1852. [Google Scholar] [CrossRef]
- Frontzek, F.; Staiger, A.M.; Zapukhlyak, M.; Xu, W.; Bonzheim, I.; Borgmann, V.; Sander, P.; Baptista, M.J.; Heming, J.N.; Berning, P.; et al. Molecular and functional profiling identifies therapeutically targetable vulnerabilities in plasmablastic lymphoma. Nat. Commun. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Liu, Z.; Filip, I.; Gomez, K.; Engelbrecht, D.; Meer, S.; Lalloo, P.N.; Patel, P.; Perner, Y.; Zhao, J.; Wang, J.; et al. Genomic Characterization of HIV-Associated Plasmablastic Lymphoma Identifies Pervasive Mutations in the JAK–STAT Pathway. Blood Cancer Discov. 2020, 1, 112–125. [Google Scholar] [CrossRef]
- Leeman-Neill, R.J.; Soderquist, C.R.; Montanari, F.; Raciti, P.; Park, D.; Radeski, D.; Mansukhani, M.M.; Murty, V.V.; Hsiao, S.; Alobeid, B.; et al. Phenogenomic heterogeneity of post-transplant plasmablastic lymphomas. Haematologica 2020. online ahead of print. [Google Scholar]
- Paradzik, T.; Bandini, C.; Mereu, E.; Labrador, M.; Taiana, E.; Amodio, N.; Neri, A.; Piva, R. The landscape of signaling pathways and proteasome inhibitors combinations in multiple myeloma. Cancers 2021, 13, 1235. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Gu, H.; Dong, M.; Cai, Z. Emerging agents and regimens for multiple myeloma. J. Hematol. Oncol. 2020, 13, 150. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Ishibashi, M.; Sunakawa, M.; Inokuchi, K. Immunotherapy for Multiple Myeloma. Cancers 2019, 11, 2009. [Google Scholar] [CrossRef]
- Tian, Z.; D’Arcy, P.; Wang, X.; Ray, A.; Tai, Y.T.; Hu, Y.; Carrasco, R.D.; Richardson, P.; Linder, S.; Chauhan, D.; et al. A novel small molecule inhibitor of deubiquitylating enzyme USP14 and UCHL5 induces apoptosis in multiple myeloma and overcomes bortezomib resistance. Blood 2014, 123, 706–716. [Google Scholar] [CrossRef]
- John, L.; Krauth, M.T.; Podar, K.; Raab, M.S. Pathway-directed therapy in multiple myeloma. Cancers 2021, 13, 1668. [Google Scholar] [CrossRef]
- Alagpulinsa, D.A.; Szalat, R.E.; Poznansky, M.C.; Shmookler Reis, R.J. Genomic Instability in Multiple Myeloma. Trends in Cancer 2020, 6, 858–873. [Google Scholar] [CrossRef]
- Taiana, E.; Cantafio, M.E.G.; Favasuli, V.K.; Bandini, C.; Viglietto, G.; Piva, R.; Neri, A.; Amodio, N. Genomic instability in multiple myeloma: A “non-coding rna” perspective. Cancers 2021, 13, 2127. [Google Scholar] [CrossRef]
- Caprio, C.; Sacco, A.; Giustini, V.; Roccaro, A.M. Epigenetic aberrations in multiple myeloma. Cancers 2020, 12, 2996. [Google Scholar] [CrossRef] [PubMed]
- White, B.S.; Lanc, I.; O’Neal, J.; Gupta, H.; Fulton, R.S.; Schmidt, H.; Fronick, C.; Belter, E.A., Jr.; Fiala, M.; King, J.; et al. A multiple myeloma-specific capture sequencing platform discovers novel translocations and frequent, risk-associated point mutations in IGLL5. Blood Cancer J. 2018, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Yellapantula, V.; Hultcrantz, M.; Rustad, E.H.; Wasserman, E.; Londono, D.; Cimera, R.; Ciardiello, A.; Landau, H.; Akhlaghi, T.; Mailankody, S.; et al. Comprehensive detection of recurring genomic abnormalities: A targeted sequencing approach for multiple myeloma. Blood Cancer J. 2019, 9, 101. [Google Scholar] [CrossRef] [PubMed]
- Buedts, L.; Smits, S.; Ameye, G.; Lehnert, S.; Ding, J.; Delforge, M.; Vermeesch, J.; Boeckx, N.; Tousseyn, T.; Michaux, L.; et al. Ultra-low depth sequencing of plasma cell DNA for the detection of copy number aberrations in multiple myeloma. Genes Chromosom. Cancer 2020, 59, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.; Beno, I.; Melnekoff, D.; Leshchenko, V.; Madduri, D.; Ramdas, D.; Sanchez, L.; Niglio, S.; Perumal, D.; Kidd, B.A.; et al. Precision Medicine for Relapsed Multiple Myeloma on the Basis of an Integrative Multiomics Approach. JCO Precis. Oncol. 2018, 2018, PO.18.00019. [Google Scholar] [CrossRef]
- Jang, J.S.; Li, Y.; Mitra, A.K.; Bi, L.; Abyzov, A.; van Wijnen, A.J.; Baughn, L.B.; Van Ness, B.; Rajkumar, V.; Kumar, S.; et al. Molecular signatures of multiple myeloma progression through single cell RNA-Seq. Blood Cancer J. 2019, 9, 2. [Google Scholar] [CrossRef]
- Höllein, A.; Twardziok, S.; Walter, W.; Hutter, S.; Baer, C.; Hernandez-Sanchez, J.M.; Meggendorfer, M.; Haferlach, T.; Kern, W.; Haferlach, C.; et al. The combination of WGS and RNA-Seq is superior to conventional diagnostic tests in multiple myeloma: Ready for prime time? Cancer Genet. 2020, 242, 15–24. [Google Scholar] [CrossRef] [PubMed]
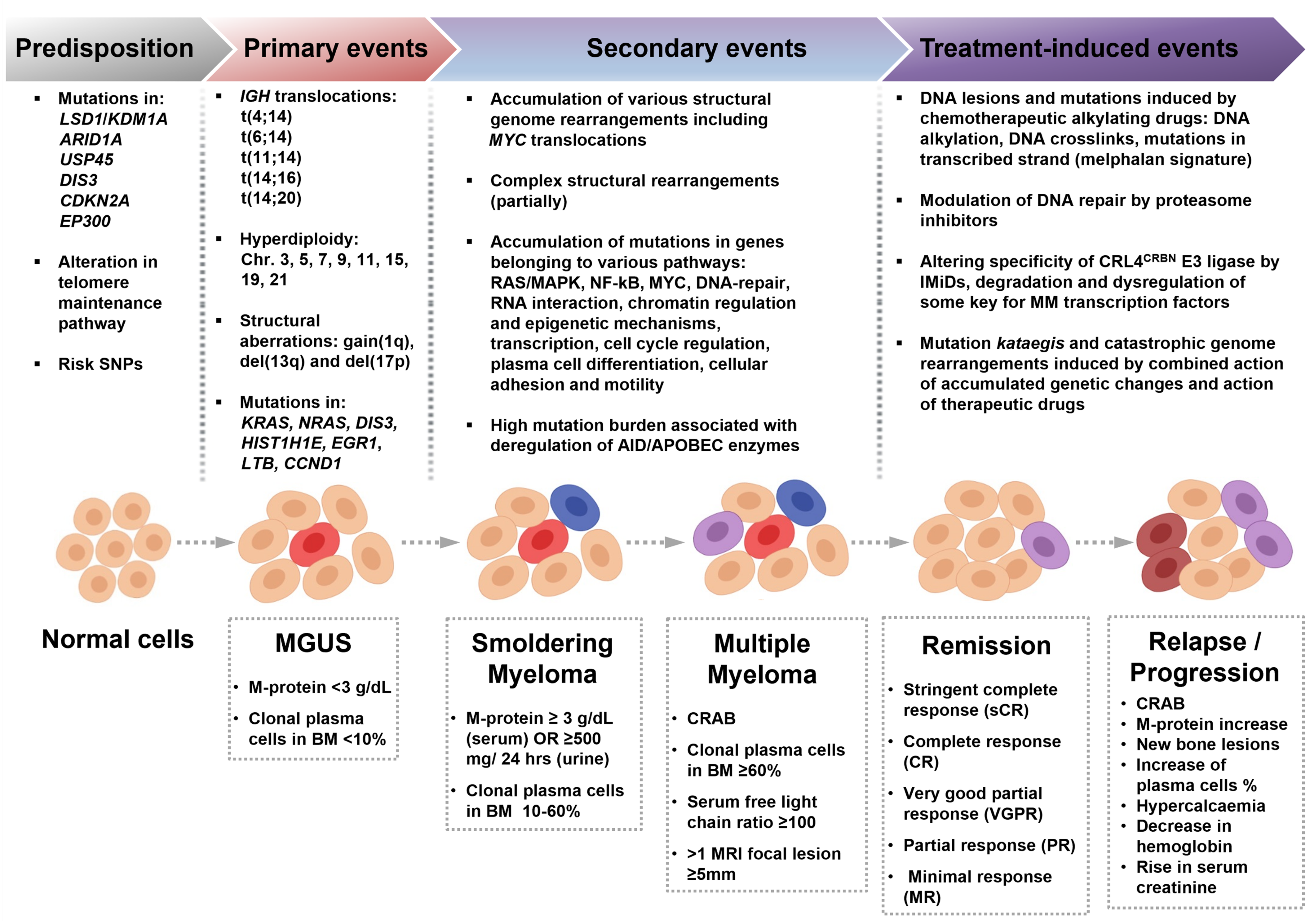





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aksenova, A.Y.; Zhuk, A.S.; Lada, A.G.; Zotova, I.V.; Stepchenkova, E.I.; Kostroma, I.I.; Gritsaev, S.V.; Pavlov, Y.I. Genome Instability in Multiple Myeloma: Facts and Factors. Cancers 2021, 13, 5949. https://doi.org/10.3390/cancers13235949
Aksenova AY, Zhuk AS, Lada AG, Zotova IV, Stepchenkova EI, Kostroma II, Gritsaev SV, Pavlov YI. Genome Instability in Multiple Myeloma: Facts and Factors. Cancers. 2021; 13(23):5949. https://doi.org/10.3390/cancers13235949
Chicago/Turabian StyleAksenova, Anna Y., Anna S. Zhuk, Artem G. Lada, Irina V. Zotova, Elena I. Stepchenkova, Ivan I. Kostroma, Sergey V. Gritsaev, and Youri I. Pavlov. 2021. "Genome Instability in Multiple Myeloma: Facts and Factors" Cancers 13, no. 23: 5949. https://doi.org/10.3390/cancers13235949





