An Improved Animal Model of Multiple Myeloma Bone Disease
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Intravenous Inoculation of 5TGM1-Luc Cells
2.3. In Vivo Bioluminescence Imaging
2.4. Blood Sampling and Serum IgG2b Level Measurement
2.5. BMD Measurement and Bone Histomorphometric Analyses on the Spine
2.6. Statistical Analysis
3. Results
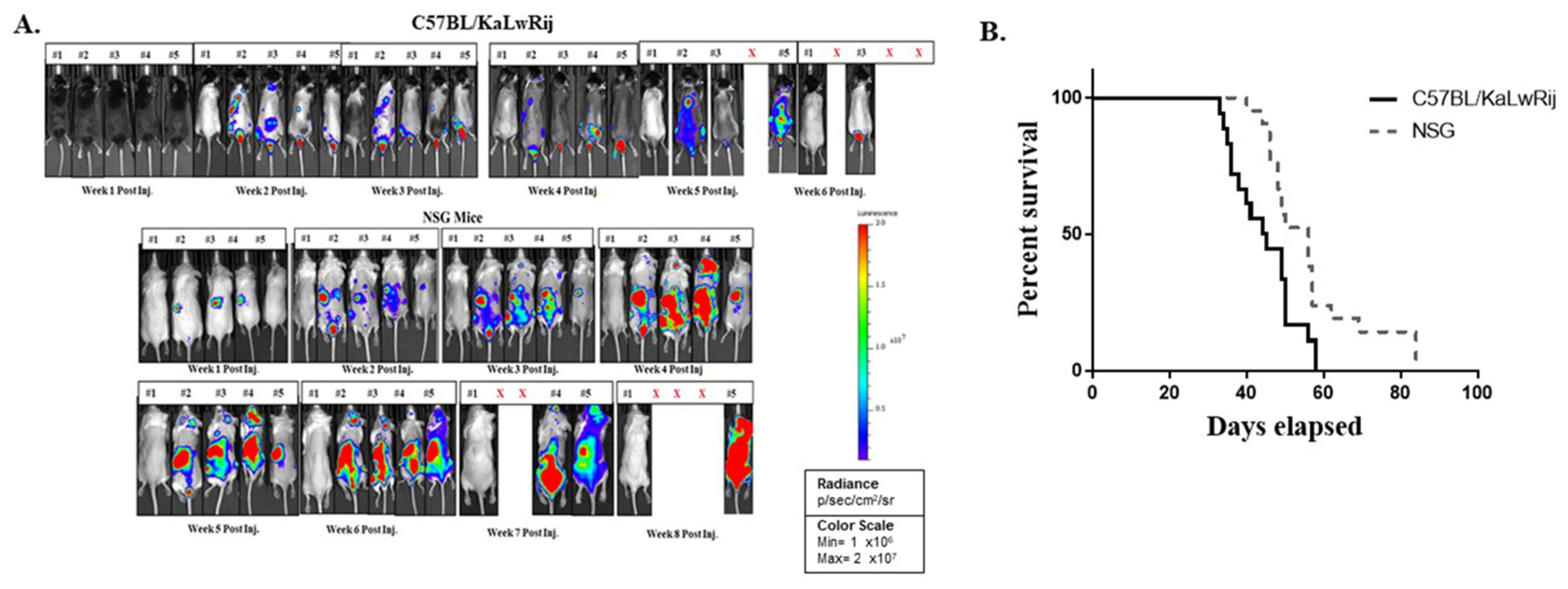
3.1. 5TGM1-Luc Transplanted NSG Mouse Shows Early BL Signs, but Longer Survival than C57BL/KaLwRij Mouse
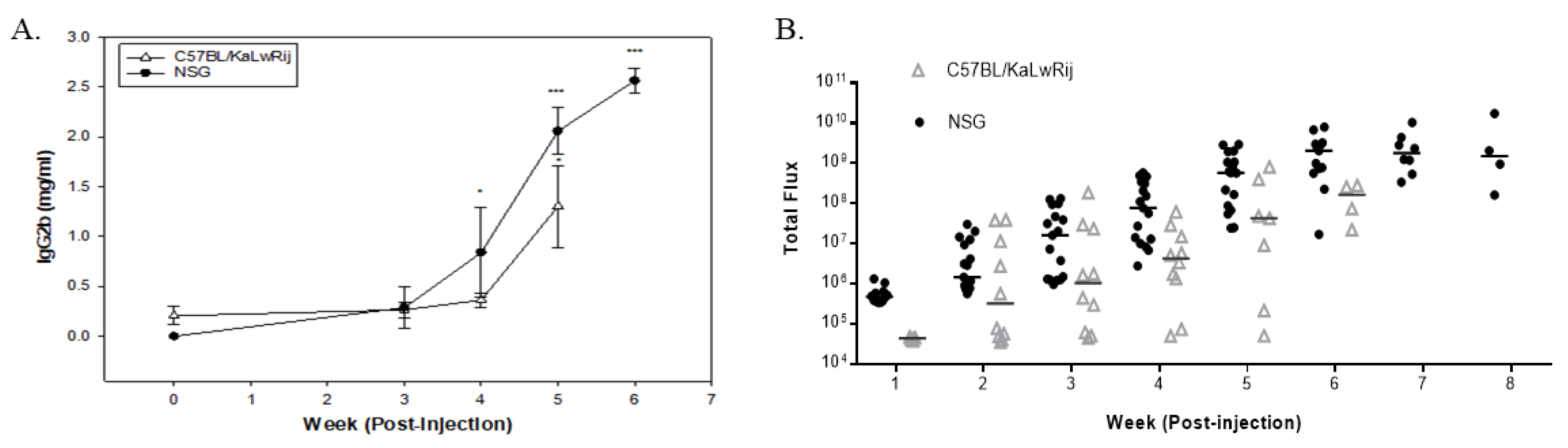
3.2. Tumor Burdens Are Detected as Early as Week 2 of Post-Injection by BL Analysis
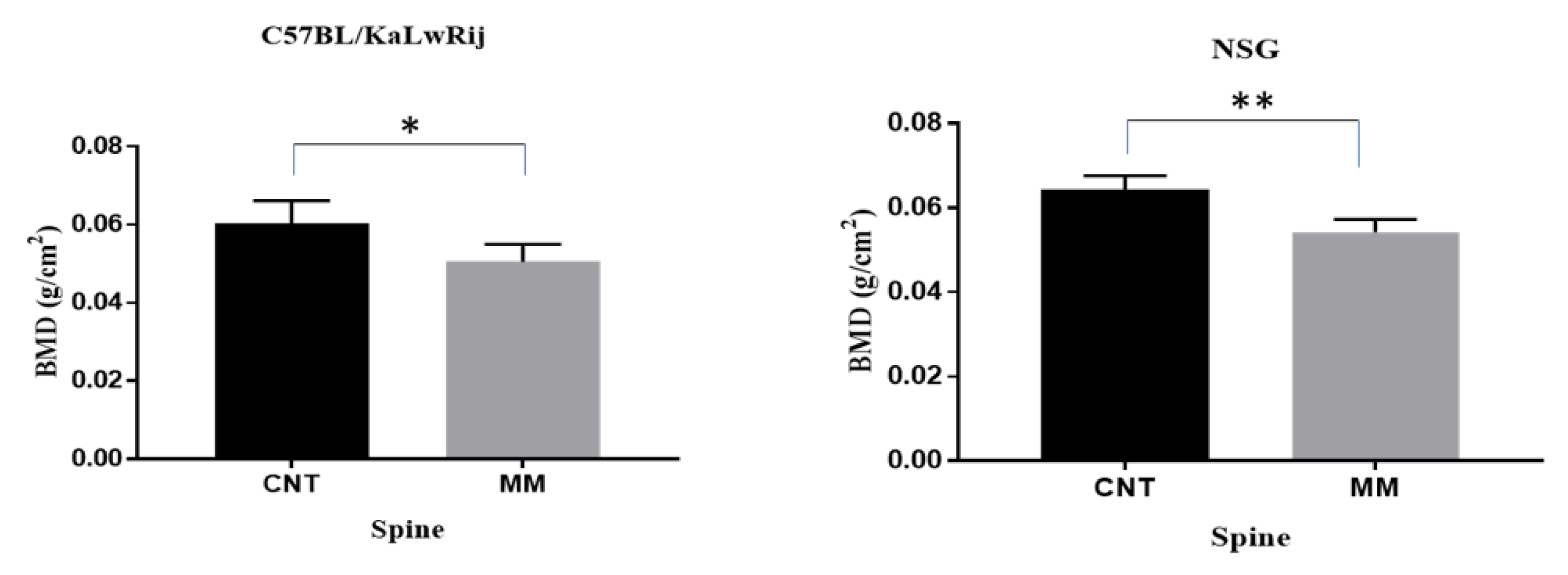
3.3. Both Models Demonstrate Loss of Bone Mineral Using the Ex Vivo DEXA Scan
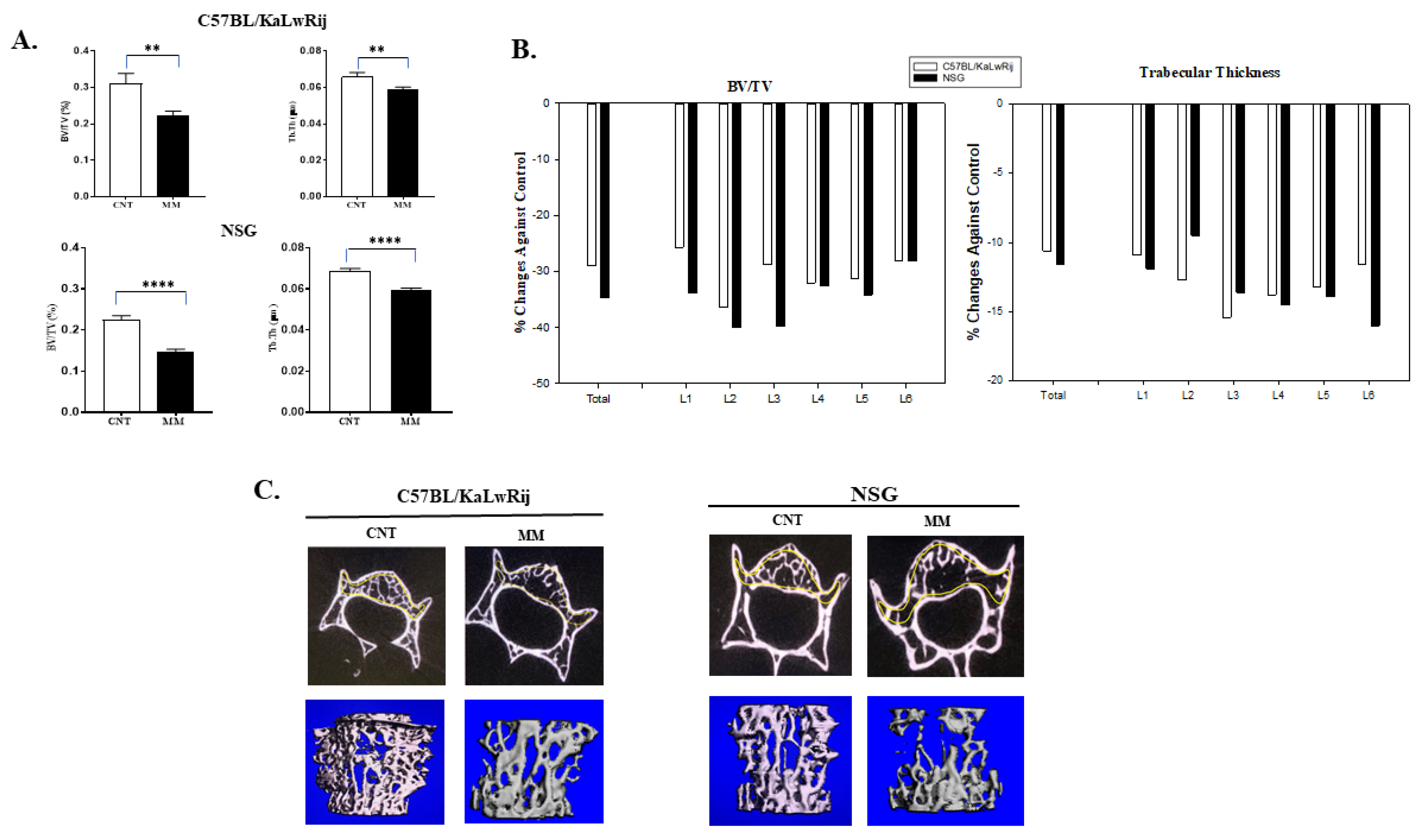
3.4. More Severe MMBD Developed in NSG Mice Than C57BL/KaLwRij
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Cancer Institute: Surveillance, Epidemiology, and End Results (SEER) Program. Cancer Stat Facts: Myeloma. 2020. Available online: https://seer.cancer.gov/statfacts/html/mulmy.html (accessed on 30 July 2021).
- Katz, B.Z. Adhesive interactions: The multi–task biochemical toolbox of cancer cells. Semin. Cancer. Biol. 2010, 20, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Zangari, M.; Suva, L.J. The effects of proteasome inhibitors on bone remodeling in multiple myeloma. Bone 2016, 86, 131–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyle, R.A.; Gertz, M.A.; Witzig, T.E.; Lust, J.A.; Lacy, M.Q.; Dispenzieri, A.; Fonseca, R.; Rajkumar, S.V.; Offord, J.R.; Larson, D.R.; et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo. Clin. Proc. 2003, 78, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Dispenzieri, A.; Lacy, M.Q.; Gertz, M.A.; Buadi, F.K.; Pandey, S.; Kapoor, P.; Dingli, D.; Hayman, S.R.; Leung, N.; et al. Continued improvement in survival in multiple myeloma: Changes in early mortality and outcomes in older patients. Leukemia 2014, 28, 1122–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ring, E.S.; Lawson, M.A.; Snowden, J.A.; Jolley, I.; Chantry, A.D. New agents in the treatment of myeloma bone disease. Calcif. Tissue Int. 2018, 102, 196–209. [Google Scholar] [CrossRef] [Green Version]
- Silbermann, R.; Roodman, G.D. Myeloma bone disease: Pathophysiology and management. J. Bone. Oncol. 2013, 2, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Dimopoulos, M.A. Myeloma bone disease: From biology findings to treatment approaches. Blood 2019, 133, 1534–1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arron, J.R.; Choi, Y. Bone versus immune system. Nature 2000, 408, 535–536. [Google Scholar] [CrossRef] [PubMed]
- Gnoni, A.; Brunetti, O.; Longo, V.; Calabrese, A.; Argentiero, A.L.; Calbi, R.; Solimando Antonio, G.; Licchetta, A. Immune system and bone microenvironment: Rationale for targeted cancer therapies. Oncotarget 2020, 11, 480–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehdi, S.H.; Nafees, S.; Mehdi, S.J.; Morris, C.A.; Mashouri, L.; Yoon, D. Animal Models of Multiple Myeloma Bone Disease. Front. Genet. 2021, 12, 640954. [Google Scholar] [CrossRef]
- Asosingh, K.; Radl, J.; Van Riet, I.; Van Camp, B.; Vanderkerken, K. The 5TMM series: A useful in vivo mouse model of human multiple myeloma. Hematol. J. 2000, 1, 351–356. [Google Scholar] [CrossRef]
- Garrett, I.R.; Dallas, S.; Radl, J.; Mundy, G.R. A murine model of human myeloma bone disease. Bone 1997, 20, 515–520. [Google Scholar] [CrossRef]
- Radl, J. Animal model of human disease. Benign monoclonal gammopathy (idiopathic paraproteinemia). Am. J. Pathol. 1981, 105, 91–93. [Google Scholar] [PubMed]
- Radl, J.; Croese, J.W.; Zurcher, C.; Van den Enden-Vieveen, M.H.; de Leeuw, A.M. Animal model of human disease. Multiple myeloma. Am. J. Pathol. 1988, 132, 593–597. [Google Scholar]
- Radl, J.; De Glopper, E.D.; Schuit, H.R.; Zurcher, C. Idiopathic paraproteinemia. II. Transplantation of the paraprotein-producing clone from old to young C57BL/KaLwRij mice. J. Immunol. 1979, 122, 609–613. [Google Scholar]
- Zangari, M.; Berno, T.; Yang, Y.; Zeng, M.; Xu, H.; Pappas, L.; Tricot, G.; Kamalakar, A.; Yoon, D.; Suva, L.J. Parathyroid hormone receptor mediates the anti–myeloma effect of proteasome inhibitors. Bone 2014, 61, 39–43. [Google Scholar] [CrossRef] [Green Version]
- Zangari, M.; Yoo, H.; Shin, I.; Kim, B.; Edmondson, R.; Morgan, G.J.; Suva, L.J.; Yoon, D. Thymic PTH Increases After Thyroparathyroidectomy in C57BL/KaLwRij Mice. Endocrinology 2018, 159, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Zangari, M.; Yoo, H.; Shin, I.J.; Yoon, D.; Suva, L.J. Surgical thyroparathyroidectomy prevents progression of 5TGM1 murine multiple myeloma in vivo. J. Bone Oncol. 2018, 12, 19–22. [Google Scholar] [CrossRef]
- Delgado-Calle, J.; Anderson, J.; Cregor, M.D.; Hiasa, M.; Chirgwin, J.M.; Carlesso, N.; Yoneda, T.; Mohammad, K.S.; Plotkin, L.I.; Roodman, G.D.; et al. Bidirectional Notch Signaling and Osteocyte-Derived Factors in the Bone Marrow Microenvironment Promote Tumor Cell Proliferation and Bone Destruction in Multiple Myeloma. Cancer. Res. 2016, 76, 1089–1100. [Google Scholar] [CrossRef] [Green Version]
- Mori, Y.; Shimizu, N.; Dallas, M.; Niewolna, M.; Story, B.; Williams, P.J.; Mundy, G.R.; Yoneda, T. Anti-alpha4 integrin antibody suppresses the development of multiple myeloma and associated osteoclastic osteolysis. Blood 2004, 104, 2149–2154. [Google Scholar] [CrossRef]
- Fryer, R.A.; Graham, T.J.; Smith, E.M.; Walker-Samuel, S.; Morgan, G.J.; Robinson, S.P.; Davies, F.E. Characterization of a novel mouse model of multiple myeloma and its use in preclinical therapeutic assessment. PLoS ONE 2013, 8, e57641. [Google Scholar] [CrossRef] [PubMed]
- Mitsiades, C.S.; Mitsiades, N.S.; Bronson, R.T.; Chauhan, D.; Munshi, N.; Treon, S.P.; Maxwell, C.A.; Pilarski, L.; Hideshima, T.; Hoffman, R.M.; et al. Fluorescence imaging of multiple myeloma cells in a clinically relevant SCID/NOD in vivo model: Biologic and clinical implications. Cancer Res. 2003, 63, 6689–6696. [Google Scholar] [PubMed]
- Yaccoby, S.; Barlogie, B.; Epstein, J. Primary myeloma cells growing in SCID-hu mice: A model for studying the biology and treatment of myeloma and its manifestations. Blood 1998, 92, 2908–2913. [Google Scholar] [CrossRef]
- Yaccoby, S.; Epstein, J. The proliferative potential of myeloma plasma cells manifest in the SCID-hu host. Blood 1999, 94, 3576–3582. [Google Scholar] [CrossRef] [PubMed]
- Yaccoby, S.; Johnson, C.L.; Mahaffey, S.C.; Wezeman, M.J.; Barlogie, B.; Epstein, J. Antimyeloma efficacy of thalidomide in the SCID-hu model. Blood 2002, 100, 4162–4168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yata, K.; Yaccoby, S. The SCID–rab model: A novel in vivo system for primary human myeloma demonstrating growth of CD138-expressing malignant cells. Leukemia 2004, 18, 1891–1897. [Google Scholar] [CrossRef] [Green Version]
- Bartee, E.; Chan, W.M.; Moreb, J.S.; Cogle, C.R.; McFadden, G. Selective purging of human multiple myeloma cells from autologous stem cell transplantation grafts using oncolytic myxoma virus. Biol. Blood Marrow Transplant. 2012, 18, 1540–1551. [Google Scholar] [CrossRef] [Green Version]
- Swift, B.E.; Williams, B.A.; Kosaka, Y.; Wang, X.H.; Medin, J.A.; Viswanathan, S.; Martinez-Lopez, J.; Keating, A. Natural killer cell lines preferentially kill clonogenic multiple myeloma cells and decrease myeloma engraftment in a bioluminescent xenograft mouse model. Haematologica 2012, 97, 1020–1028. [Google Scholar] [CrossRef] [Green Version]
- Fuhler, G.M.; Brooks, R.; Toms, B.; Iyer, S.; Gengo, E.A.; Park, M.Y.; Gumbleton, M.; Viernes, D.R.; Chisholm, J.D.; Kerr, W.G. Therapeutic potential of SH2 domain-containing inositol–5’–phosphatase 1 (SHIP1) and SHIP2 inhibition in cancer. Mol. Med. 2012, 18, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Udi, J.; Schuler, J.; Wider, D.; Ihorst, G.; Catusse, J.; Waldschmidt, J.; Schnerch, D.; Follo, M.; Wasch, R.; Engelhardt, M. Potent in vitro and in vivo activity of sorafenib in multiple myeloma: Induction of cell death, CD138-downregulation and inhibition of migration through actin depolymerization. Br. J. Haematol. 2013, 161, 104–116. [Google Scholar] [CrossRef]
- Schuler, J.; Ewerth, D.; Waldschmidt, J.; Wasch, R.; Engelhardt, M. Preclinical models of multiple myeloma: A critical appraisal. Expert Opin. Biol. Ther. 2013, 13 (Suppl. 1), S111–123. [Google Scholar] [CrossRef]
- Miyakawa, Y.; Ohnishi, Y.; Tomisawa, M.; Monnai, M.; Kohmura, K.; Ueyama, Y.; Ito, M.; Ikeda, Y.; Kizaki, M.; Nakamura, M. Establishment of a new model of human multiple myeloma using NOD/SCID/gammac(null) (NOG) mice. Biochem. Biophys. Res. Commun. 2004, 313, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Hurchla, M.A.; Garcia-Gomez, A.; Hornick, M.C.; Ocio, E.M.; Li, A.; Blanco, J.F.; Collins, L.; Kirk, C.J.; Piwnica-Worms, D.; Vij, R.; et al. The epoxyketone-based proteasome inhibitors carfilzomib and orally bioavailable oprozomib have anti-resorptive and bone-anabolic activity in addition to anti-myeloma effects. Leukemia 2013, 27, 430–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heath, D.J.; Chantry, A.D.; Buckle, C.H.; Coulton, L.; Shaughnessy, J.D., Jr.; Evans, H.R.; Snowden, J.A.; Stover, D.R.; Vanderkerken, K.; Croucher, P.I. Inhibiting Dickkopf–1 (Dkk1) removes suppression of bone formation and prevents the development of osteolytic bone disease in multiple myeloma. J. Bone Miner. Res. 2009, 24, 425–436. [Google Scholar] [CrossRef]
- Terpos, E.; Morgan, G.; Dimopoulos, M.A.; Drake, M.T.; Lentzsch, S.; Raje, N.; Sezer, O.; Garcia-Sanz, R.; Shimizu, K.; Turesson, I.; et al. International Myeloma Working Group recommendations for the treatment of multiple myeloma-related bone disease. J. Clin. Oncol. 2013, 31, 2347–2357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terpos, E.; Dimopoulos, M.A.; Sezer, O.; Roodman, D.; Abildgaard, N.; Vescio, R.; Tosi, P.; Garcia-Sanz, R.; Davies, F.; Chanan-Khan, A.; et al. The use of biochemical markers of bone remodeling in multiple myeloma: A report of the International Myeloma Working Group. Leukemia 2010, 24, 1700–1712. [Google Scholar] [CrossRef] [Green Version]
- Rossi, M.; Botta, C.; Arbitrio, M.; Grembiale, R.D.; Tagliaferri, P.; Tassone, P. Mouse models of multiple myeloma: Technologic platforms and perspectives. Oncotarget 2018, 9, 20119–20133. [Google Scholar] [CrossRef] [Green Version]
- Chesi, M.; Robbiani, D.F.; Sebag, M.; Chng, W.J.; Affer, M.; Tiedemann, R.; Valdez, R.; Palmer, S.E.; Haas, S.S.; Stewart, A.K.; et al. AID-dependent activation of a MYC transgene induces multiple myeloma in a conditional mouse model of post-germinal center malignancies. Cancer Cell 2008, 13, 167–180. [Google Scholar] [CrossRef] [Green Version]
- Boylan, K.L.; Gosse, M.A.; Staggs, S.E.; Janz, S.; Grindle, S.; Kansas, G.S.; Van Ness, B.G. A transgenic mouse model of plasma cell malignancy shows phenotypic, cytogenetic, and gene expression heterogeneity similar to human multiple myeloma. Cancer Res. 2007, 67, 4069–4078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrasco, D.R.; Sukhdeo, K.; Protopopova, M.; Sinha, R.; Enos, M.; Carrasco, D.E.; Zheng, M.; Mani, M.; Henderson, J.; Pinkus, G.S.; et al. The differentiation and stress response factor XBP-1 drives multiple myeloma pathogenesis. Cancer Cell 2007, 11, 349–360. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Pennisi, A.; Zhan, F.; Sawyer, J.R.; Shaughnessy, J.D.; Yaccoby, S. Establishment and exploitation of hyperdiploid and non-hyperdiploid human myeloma cell lines. Br. J. Haematol. 2007, 138, 802–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, F.; Huang, Y.; Colla, S.; Stewart, J.P.; Hanamura, I.; Gupta, S.; Epstein, J.; Yaccoby, S.; Sawyer, J.; Burington, B.; et al. The molecular classification of multiple myeloma. Blood 2006, 108, 2020–2028. [Google Scholar] [CrossRef] [Green Version]
- Groen, R.W.; Noort, W.A.; Raymakers, R.A.; Prins, H.J.; Aalders, L.; Hofhuis, F.M.; Moerer, P.; van Velzen, J.F.; Bloem, A.C.; van Kessel, B.; et al. Reconstructing the human hematopoietic niche in immunodeficient mice: Opportunities for studying primary multiple myeloma. Blood 2012, 120, e9–e16. [Google Scholar] [CrossRef] [Green Version]
- Schueler, J.; Wider, D.; Klingner, K.; Siegers, G.M.; May, A.M.; Wasch, R.; Fiebig, H.H.; Engelhardt, M. Intratibial injection of human multiple myeloma cells in NOD/SCID IL–2Rgamma(null) mice mimics human myeloma and serves as a valuable tool for the development of anticancer strategies. PLoS ONE 2013, 8, e79939. [Google Scholar] [CrossRef] [Green Version]
- Mirandola, L.; Yu, Y.; Jenkins, M.R.; Chiaramonte, R.; Cobos, E.; John, C.M.; Chiriva-Internati, M. Tracking human multiple myeloma xenografts in NOD–Rag–1/IL–2 receptor gamma chain-null mice with the novel biomarker AKAP–4. BMC. Cancer 2011, 11, 394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawson, M.A.; McDonald, M.M.; Kovacic, N.; Hua Khoo, W.; Terry, R.L.; Down, J.; Kaplan, W.; Paton–Hough, J.; Fellows, C.; Pettitt, J.A.; et al. Osteoclasts control reactivation of dormant myeloma cells by remodelling the endosteal niche. Nat. Commun. 2015, 6, 8983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postnov, A.A.; Rozemuller, H.; Verwey, V.; Lokhorst, H.; De Clerck, N.; Martens, A.C. Correlation of high-resolution X-ray micro-computed tomography with bioluminescence imaging of multiple myeloma growth in a xenograft mouse model. Calcif. Tissue Int. 2009, 85, 434–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyle, R.A. New strategies for MGUS and smoldering multiple myeloma. Clin. Adv. Hematol. Oncol. 2004, 2, 507–509. [Google Scholar] [PubMed]
- Lawson, M.A.; Paton-Hough, J.M.; Evans, H.R.; Walker, R.E.; Harris, W.; Ratnabalan, D.; Snowden, J.A.; Chantry, A.D. NOD/SCID-GAMMA mice are an ideal strain to assess the efficacy of therapeutic agents used in the treatment of myeloma bone disease. PLoS ONE 2015, 10, e0119546. [Google Scholar] [CrossRef]
- Radl, J.; Croese, J.W.; Zurcher, C.; van den Enden-Vieveen, M.H.; Brondijk, R.J.; Kazil, M.; Haaijman, J.J.; Reitsma, P.H.; Bijvoet, O.L. Influence of treatment with APD-bisphosphonate on the bone lesions in the mouse 5T2 multiple myeloma. Cancer 1985, 55, 1030–1040. [Google Scholar] [CrossRef]
- Croucher, P.I.; De Hendrik, R.; Perry, M.J.; Hijzen, A.; Shipman, C.M.; Lippitt, J.; Green, J.; Van Marck, E.; Van Camp, B.; Vanderkerken, K. Zoledronic acid treatment of 5T2MM-bearing mice inhibits the development of myeloma bone disease: Evidence for decreased osteolysis, tumor burden and angiogenesis, and increased survival. J. Bone Miner. Res. 2003, 18, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Croucher, P.I.; Shipman, C.M.; Lippitt, J.; Perry, M.; Asosingh, K.; Hijzen, A.; Brabbs, A.C.; van Beek, E.J.; Holen, I.; Skerry, T.M.; et al. Osteoprotegerin inhibits the development of osteolytic bone disease in multiple myeloma. Blood 2001, 98, 3534–3540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dallas, S.L.; Garrett, I.R.; Oyajobi, B.O.; Dallas, M.R.; Boyce, B.F.; Bauss, F.; Radl, J.; Mundy, G.R. Ibandronate reduces osteolytic lesions but not tumor burden in a murine model of myeloma bone disease. Blood 1999, 93, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Cocks, K.; Cohen, D.; Wisloff, F.; Sezer, O.; Lee, S.; Hippe, E.; Gimsing, P.; Turesson, I.; Hajek, R.; Smith, A.; et al. An international field study of the reliability and validity of a disease-specific questionnaire module (the QLQ-MY20) in assessing the quality of life of patients with multiple myeloma. Eur. J. Cancer 2007, 43, 1670–1678. [Google Scholar] [CrossRef]
- Shipman, C.M.; Rogers, M.J.; Vanderkerken, K.; Van Camp, B.; Graham, R.; Russell, G.; Croucher, P.I. Bisphosphonates--mechanisms of action in multiple myeloma. Acta. Oncol. 2000, 39, 829–835. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehdi, S.H.; Morris, C.A.; Lee, J.A.; Yoon, D. An Improved Animal Model of Multiple Myeloma Bone Disease. Cancers 2021, 13, 4277. https://doi.org/10.3390/cancers13174277
Mehdi SH, Morris CA, Lee JA, Yoon D. An Improved Animal Model of Multiple Myeloma Bone Disease. Cancers. 2021; 13(17):4277. https://doi.org/10.3390/cancers13174277
Chicago/Turabian StyleMehdi, Syed Hassan, Carol A Morris, Jung Ae Lee, and Donghoon Yoon. 2021. "An Improved Animal Model of Multiple Myeloma Bone Disease" Cancers 13, no. 17: 4277. https://doi.org/10.3390/cancers13174277




