Secreted Frizzled-Related Protein 1 as a Biomarker against Incomplete Age-Related Lobular Involution and Microcalcifications’ Development
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Study Population
2.2. Association between SFRP1 Expression and the Degree of Lobular Involution
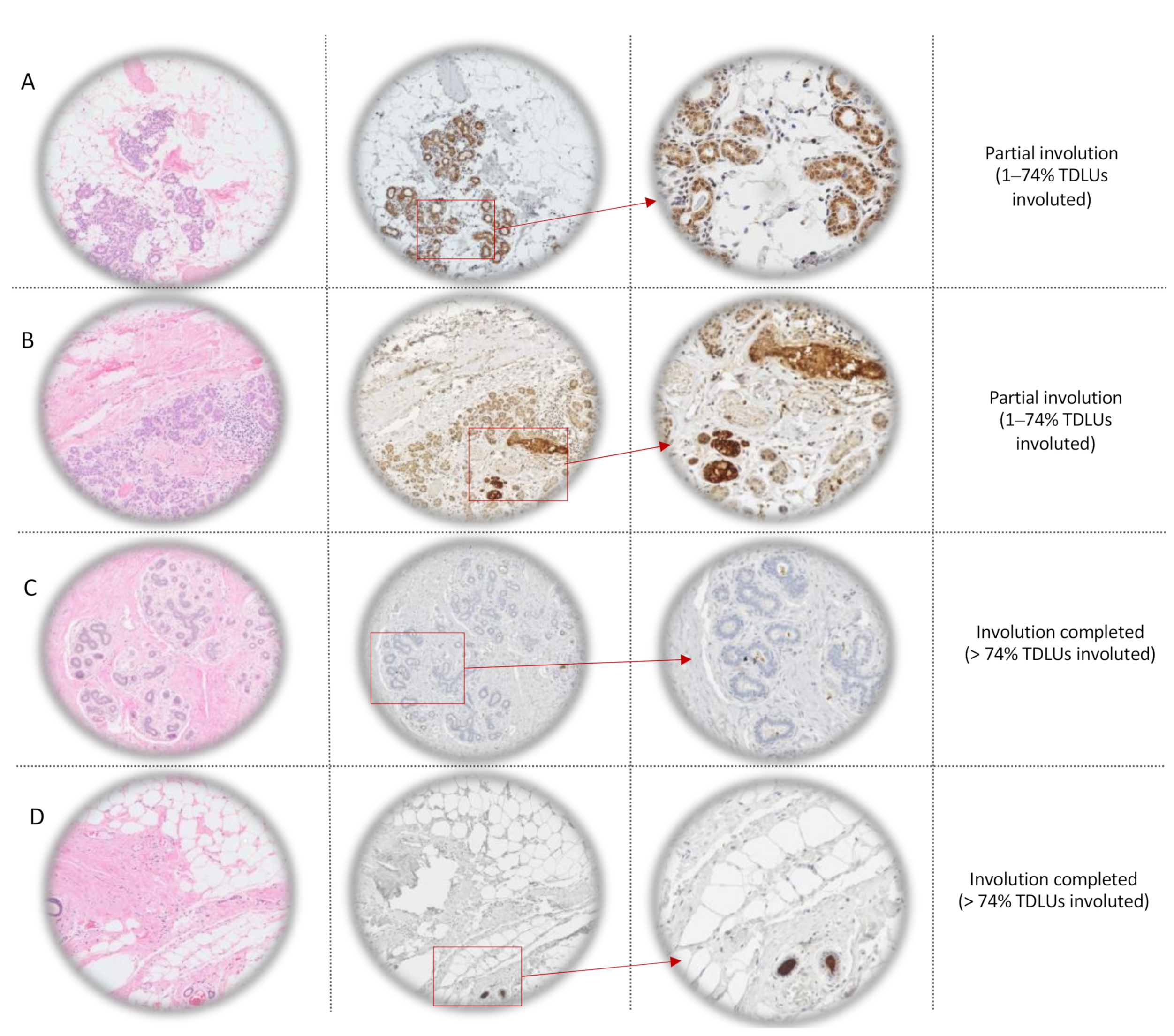
2.2.1. Immunohistochemistry (IHC)
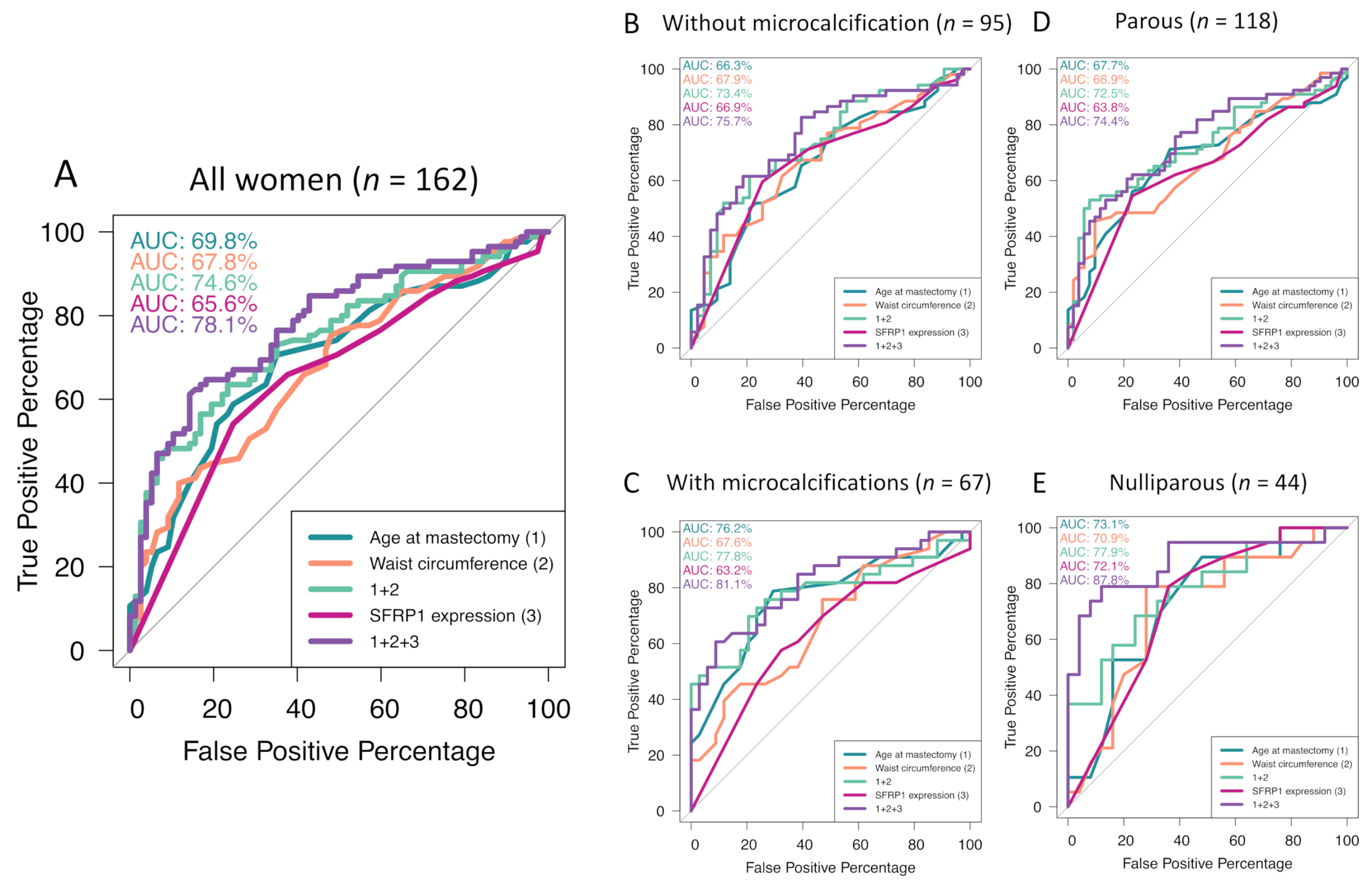
2.2.2. Association Study
2.2.3. Inflammatory Profile
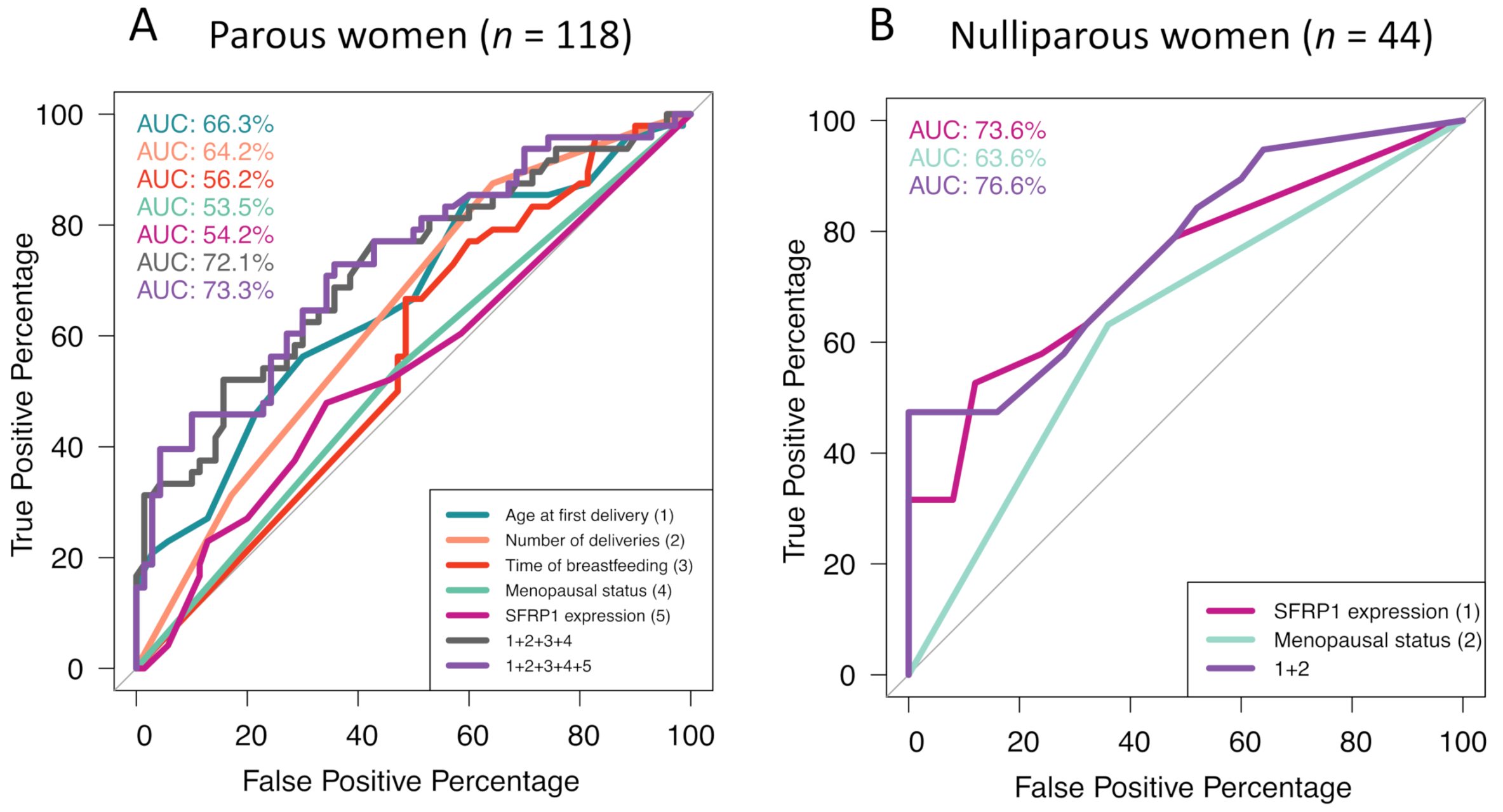
2.2.4. Reproductive History
2.3. Association between SFRP1 and the Presence of Microcalcifications
2.3.1. Association Study
2.3.2. Inflammatory Profile and Reproductive History
3. Discussion
4. Materials and Methods
4.1. Study Population and Data Collection
4.2. Age-Related Lobular Involution Assessment
4.3. Anti-SFRP1 Immunohistochemistry Staining
4.4. Interpretation of Anti-SFRP1 Immunostaining
4.5. Inflammatory Markers’ Immunostaining and Interpretation
4.6. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR)
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rehn, M.; Pihlajaniemi, T. Identification of three N-terminal ends of type XVIII collagen chains and tissue-specific differences in the expression of the corresponding transcripts. J. Biol. Chem. 1995, 270, 4705–4711. [Google Scholar] [CrossRef] [Green Version]
- Finch, P.W.; He, X.; Kelley, M.J.; Uren, A.; Schaudies, R.P.; Popescu, N.C.; Rudikoff, S.; Aaronson, S.A.; Varmus, H.E.; Rubin, J.S. Purification and molecular cloning of a secreted, frizzled-related antagonist of Wnt action. Proc. Natl. Acad. Sci. USA 1997, 94, 6770–6775. [Google Scholar] [CrossRef] [Green Version]
- Üren, A.; Reichsman, F.; Anest, V.; Taylor, W.G.; Muraiso, K.; Bottaro, D.P.; Cumberledge, S.; Rubin, J.S. Secreted frizzled-related protein-1 binds directly to wingless and is a biphasic modulator of Wnt signaling. J. Biol. Chem. 2000, 275, 4374–4382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joesting, M.S.; Cheever, T.R.; Volzing, K.G.; Yamaguchi, T.P.; Wolf, V.; Naf, D.; Rubin, J.S.; Marker, P.C. Secreted frizzled related protein 1 is a paracrine modulator of epithelial branching morphogenesis, proliferation, and secretory gene expression in the prostate. Dev. Biol. 2008, 317, 161–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawano, Y. Secreted antagonists of the Wnt signalling pathway. J. Cell Sci. 2003, 116, 2627–2634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kothari, C.; Ouellette, G.; Labrie, Y.; Jacob, S.; Diorio, C.; Durocher, F. Identification of a gene signature for different stages of breast cancer development that could be used for early diagnosis and specific therapy. Oncotarget 2018, 9, 37407–37420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregory, K.J.; Roberts, A.L.; Conlon, E.M.; Mayfield, J.A.; Hagen, M.J.; Crisi, G.M.; Bentley, B.A.; Kane, J.J.; Makari-Judson, G.; Mason, H.S.; et al. Gene expression signature of atypical breast hyperplasia and regulation by SFRP1. Breast Cancer Res. 2019, 21, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Clemenceau, A.; Diorio, C.; Durocher, F. Role of secreted frizzled-related protein 1 in early mammary gland tumorigenesis and its regulation in breast microenvironment. Cells 2020, 9, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.; Ning, C.; Dong, Y.; Zhao, P.; Li, J.; Fan, Z.; Li, J.; Yu, Y.; Mrode, R.; Liu, J. Quantitative proteome analysis of bovine mammary gland reveals protein dynamic changes involved in peak and late lactation stages. Biochem. Biophys. Res. Commun. 2017, 494, 292–297. [Google Scholar] [CrossRef]
- Gauger, K.J.; Shimono, A.; Crisi, G.M.; Schneider, S. Loss of sfrp1 promotes ductal branching in the murine mammary gland. BMC Dev. Biol. 2012, 12, 25. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, K.; Vachon, C.M.; Pankratz, V.S.; Vierkant, R.A.; Anderson, S.S.; Brandt, K.R.; Visscher, D.W.; Reynolds, C.; Frost, M.H.; Hartmann, L.C. Independent association of lobular involution and mammographic breast density with breast cancer risk. J. Natl. Cancer Inst. 2010, 102, 1716–1723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radisky, D.C.; Visscher, D.W.; Frank, R.D.; Vierkant, R.A.; Winham, S.; Stallings-Mann, M.; Hoskin, T.L.; Nassar, A.; Vachon, C.M.; Denison, L.A.; et al. Natural history of age-related lobular involution and impact on breast cancer risk. Breast Cancer Res. Treat. 2016, 155, 423–430. [Google Scholar] [CrossRef] [Green Version]
- Henson, D.E.; Tarone, R.E.; Nsouli, H. Lobular involution: The physiological prevention of breast cancer. J. Natl. Cancer Inst. 2006, 98, 1589–1590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furth, P.A.; Bar-Peled, U.; Li, M. Apoptosis and mammary gland involution: Reviewing the process. Apoptosis 1997, 2, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Hanna, M.; Dumas, I.; Orain, M.; Jacob, S.; Têtu, B.; Sanschagrin, F.; Bureau, A.; Poirier, B.; Diorio, C. Association between local inflammation and breast tissue age-related lobular involution among premenopausal and postmenopausal breast cancer patients. PLoS ONE 2017, 12, e0183579. [Google Scholar] [CrossRef]
- Gauger, K.J.; Chenausky, K.L.; Murray, M.E.; Schneider, S.S. SFRP1 reduction results in an increased sensitivity to TGF-β signaling. BMC Cancer 2011, 11, 59. [Google Scholar] [CrossRef] [Green Version]
- Li, C.H.; Amar, S. Inhibition of SFRP1 reduces severity of periodontitis. J. Dent. Res. 2007, 86, 873–877. [Google Scholar] [CrossRef]
- Gauger, K.J.; Schneider, S.S. Tumour supressor secreted frizzled related protein 1 regulates p53-mediated apoptosis: SFRP1 loss reduces p53-mediated apoptosis. Cell Biol. Int. 2014, 38, 124–130. [Google Scholar] [CrossRef]
- Seol, M.B.; Bong, J.J.; Baik, M. Expression profiles of apoptosis genes in mammary epithelial cells. Mol. Cells 2005, 20, 97–104. [Google Scholar]
- Lagathu, C.; Christodoulides, C.; Tan, C.Y.; Virtue, S.; Laudes, M.; Campbell, M.; Ishikawa, K.; Ortega, F.; Tinahones, F.J.; Fernández-Real, J.-M.; et al. Secreted frizzled-related protein 1 regulates adipose tissue expansion and is dysregulated in severe obesity. Int. J. Obes. 2010, 34, 1695–1705. [Google Scholar] [CrossRef] [Green Version]
- He, F.; Springer, N.L.; Whitman, M.A.; Pathi, S.P.; Lee, Y.; Mohanan, S.; Marcott, S.; Chiou, A.E.; Blank, B.S.; Iyengar, N.; et al. Hydroxyapatite mineral enhances malignant potential in a tissue-engineered model of ductal carcinoma in situ (DCIS). Biomaterials 2019, 224, 119489. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.W.; Merkel, A.R.; Page, J.M.; Ruppender, N.S.; Guelcher, S.A.; Sterling, J.A. Wnt signaling induces gene expression of factors associated with bone destruction in lung and breast cancer. Clin. Exp. Metastasis 2014, 31, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Boudin, E.; Fijalkowski, I.; Piters, E.; Van Hul, W. The role of extracellular modulators of canonical Wnt signaling in bone metabolism and diseases. Semin. Arthritis Rheum. 2013, 43, 220–240. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; He, C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018, 44, 38–50. [Google Scholar] [CrossRef]
- Xie, C.; Chen, Q. Adipokines: New therapeutic target for osteoarthritis? Curr. Rheumatol. Rep. 2019, 21, 71. [Google Scholar] [CrossRef]
- Hanna, M.; Dumas, I.; Orain, M.; Jacob, S.; Têtu, B.; Sanschagrin, F.; Bureau, A.; Poirier, B.; Diorio, C. Association between expression of inflammatory markers in normal breast tissue and mammographic density among premenopausal and postmenopausal women. Menopause 2017, 24, 524–535. [Google Scholar] [CrossRef]
- Hanna, M.; Dumas, I.; Jacob, S.; Têtu, B.; Diorio, C. Physical activity, mammographic density, and age-related lobular involution among premenopausal and postmenopausal women. Menopause 2015, 22, 964–975. [Google Scholar] [CrossRef]
- Ennour-Idrissi, K.; Têtu, B.; Maunsell, E.; Poirier, B.; Montoni, A.; Rochette, P.J.; Diorio, C. Association of telomere length with breast cancer prognostic factors. PLoS ONE 2016, 11, e0161903. [Google Scholar] [CrossRef] [Green Version]
- Lakhani, S.; Ellis, I.; Schnitt, S.; Tan, P.; Van de Vijver, M.J. WHO Classification of Tumours of the Breast, 4th ed.; World Health Organization: Lyon, France, 2012. [Google Scholar]
- Vierkant, R.A.; Hartmann, L.C.; Pankratz, V.S.; Anderson, S.S.; Radisky, D.; Frost, M.H.; Vachon, C.M.; Ghosh, K.; Distad, T.J.; Degnim, A.C.; et al. Lobular involution: Localized phenomenon or field effect? Breast Cancer Res. Treat. 2009, 117, 193–196. [Google Scholar] [CrossRef] [Green Version]
- Burguin, A.; Furrer, D.; Ouellette, G.; Jacob, S.; Diorio, C.; Durocher, F. Trastuzumab effects depend on HER2 phosphorylation in HER2-negative breast cancer cell lines. PLoS ONE 2020, 15, e0234991. [Google Scholar] [CrossRef]
- Slim, F.A.; Ouellette, G.; Ennour-Idrissi, K.; Jacob, S.; Diorio, C.; Durocher, F. An isoform of AIF1 involved in breast cancer. Cancer Cell Int. 2018, 18, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Beaulieu, J.-F.; Huggett, J.; Jaggi, R.; Kibenge, F.S.; Olsvik, P.A.; Penning, L.C.; Toegel, S. MIQE précis: Practical implementation of minimum standard guidelines for fluorescence-based quantitative real-time PCR experiments. BMC Mol. Biol. 2010, 11, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Characteristics | All (n = 162) | Premenopausal (n = 82) | Postmenopausal (n = 80) | p-Value |
|---|---|---|---|---|
| Age at mastectomy (years) | 52 ± 7.9 | 47 ± 5.8 | 58 ± 4.9 | <0.0001 |
| Degree of lobular involution (n) | ||||
| No or partial involution | 77 (48%) | 51 (62%) | 26 (32%) | 0.00012 |
| Complete involution | 85 (52%) | 31 (38%) | 54 (68%) | |
| Predominant lobule type (n) | ||||
| Predominant type 1 without any type 3 | 65 (40%) | 16 (20%) | 49 (61%) | <0.0001 |
| Predominant type 2–3 | 97 (60%) | 66 (80%) | 31 (39%) | |
| Microcalcifications (n) | 67 (41%) | 29 (35%) | 38 (48%) | 0.12 |
| Waist circumference (cm) | 87 ± 13 | 84 ± 12 | 90 ± 13 | 0.0018 |
| Parity | ||||
| Nulliparous | 44 (27%) | 23 (28%) | 21 (26%) | 0.80 |
| Parous | 118 (73%) | 59 (72%) | 59 (74%) | |
| Number of deliveries * (mean) | 2.1 ± 0.80 | 2.1 ± 0.81 | 2.0 ± 0.80 | 0.36 |
| Age at the first delivery * (years) | 26 ± 4.1 | 26 ± 4.3 | 26 ± 3.8 | 0.44 |
| Breastfeeding * (n) | 61 (52%) | 33 (56%) | 28 (47%) | 0.49 |
| Total breastfeeding duration * (months) | 4.6 ± 7 | 5.5 ± 8.3 | 3.6 ± 5.3 | 0.13 |
| Continuous Scores | All (n = 162) | Premenopausal (n = 82) | Postmenopausal (n = 80) | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| SFRP1 expression * | 0.85 (0.76–0.95) | 0.0053 | 0.80 (0.65–0.96) | 0.027 | 0.85 (0.72–0.99) | 0.036 |
| SFRP1 expression * adjusted for age at mastectomy | 0.83 (0.74–0.94) | 0.0031 | 0.80 (0.65–0.97) | 0.030 | 0.83 (0.70–0.98) | 0.030 |
| SFRP1 expression * adjusted for menopausal status | 0.83 (0.73–0.93) | 0.0024 | ||||
| SFRP1 expression * adjusted for age at mastectomy, waist circumference and presence microcalcifications | 0.83 (0.73–0.94) | 0.0047 | 0.83 (0.73–0.94) | 0.049 | 0.82 (0.66–0.99) | 0.011 |
| Continuous Variables | Cytokines | Epithelial Cells | Adipocytes | ||||
|---|---|---|---|---|---|---|---|
| N | Rho | p-Value | N | Rho | p-Value | ||
| SFRP1 | Pro-inflammatory markers: | ||||||
| Leptin | 155 | 0.32 | <0.0001 | 72 | 0.043 | 0.72 | |
| COX-2 | 156 | −0.060 | 0.45 | - | - | ||
| CRP | 158 | 0.14 | 0.090 | - | - | ||
| SAA1 | 152 | 0.15 | 0.070 | - | - | ||
| STAT3 | 156 | 0.038 | 0.64 | - | - | ||
| TNF-α | 157 | 0.21 | 0.007 | 62 | −0.078 | 0.54 | |
| IL-6 | 156 | 0.21 | 0.009 | 72 | 0.041 | 0.73 | |
| IL-8 | 157 | 0.040 | 0.64 | - | - | ||
| Anti-inflammatory markers: | |||||||
| Lactoferrin | 152 | 0.070 | 0.36 | - | - | ||
| IL-10 | 148 | 0.040 | 0.67 | - | - | ||
| TGF-β | 158 | −0.024 | 0.76 | - | - | ||
| Waist circumference | Pro-inflammatory markers: | ||||||
| Leptin | 155 | −0.16 | 0.046 | 72 | 0.45 | <0.0001 | |
| COX-2 | 156 | −0.080 | 0.31 | - | - | ||
| CRP | 158 | −0.22 | 0.0060 | - | - | ||
| SAA1 | 152 | 0.036 | 0.66 | - | - | ||
| STAT3 | 156 | 0.087 | 0.28 | - | - | ||
| TNF-α | 157 | −0.26 | 0.00091 | 62 | 0.16 | 0.21 | |
| IL-6 | 156 | −0.25 | 0.0016 | 72 | 0.37 | 0.001 | |
| IL-8 | 157 | −0.045 | 0.57 | - | - | ||
| Anti-inflammatory markers: | |||||||
| Lactoferrin | 152 | −0.070 | 0.37 | - | - | ||
| IL-10 | 148 | −0.11 | 0.20 | - | - | ||
| TGF-β | 158 | −0.0061 | 0.94 | - | - | ||
| Continuous Scores | Parous (n = 118) | Nulliparous (n = 44) | |||||
|---|---|---|---|---|---|---|---|
| Breastfeeding (n = 61) | Without Breastfeeding (n = 57) | ||||||
| Involuted (n = 28) | Non-Involuted (n = 33) | Involuted (n = 38) | Non-Involuted (n = 19) | Involuted (n = 19) | Non-Involuted (n = 25) | ||
| SFRP1 | Mean | 1.9 | 3.1 | 2.2 | 3.1 | 1.1 | 3.3 |
| p-value | 0.10 | 0.27 | 0.0030 | ||||
| Pro-inflammatory markers | |||||||
| IL-8 epithelium | Mean | 3.0 | 3.7 | 3.3 | 3.3 | 2.8 | 3.4 |
| p-value | 0.0083 | 0.98 | 0.019 | ||||
| IL-6 epithelium | Mean | 1.6 | 4.0 | 2.06 | 3.6 | 2.3 | 3.6 |
| p-value | 0.0013 | 0.037 | 0.11 | ||||
| Leptin epithelium | Mean | 3.7 | 6.2 | 3.5 | 5.7 | 3.9 | 6.4 |
| p-value | 0.00045 | 0.0067 | 0.0030 | ||||
| TNF-α epithelium | Mean | 1.6 | 3.8 | 1.7 | 3.4 | 1.7 | 4.1 |
| p-value | 0.0011 | 0.021 | 0.0022 | ||||
| COX-2 epithelium | Mean | 4.8 | 6.0 | 4.6 | 5.7 | 4.4 | 6.1 |
| p-value | 0.12 | 0.050 | 0.041 | ||||
| COX-2 stroma | Mean | 1.6 | 2.1 | 1.8 | 2.7 | 1.3 | 2.0 |
| p-value | 0.35 | 0.044 | 0.090 | ||||
| SAA1 epithelium | Mean | 2.8 | 3.3 | 3.2 | 4.2 | 2.8 | 3.7 |
| p-value | 0.17 | 0.11 | 0.033 | ||||
| STAT3 epithelium | Mean | 4.2 | 4.0 | 4.3 | 5.1 | 3.7 | 4.2 |
| p-value | 0.76 | 0.20 | 0.51 | ||||
| CRP epithelium | Mean | 1.8 | 4.3 | 1.6 | 2.6 | 1.2 | 2.8 |
| p-value | 0.00011 | 0.070 | 0.0070 | ||||
| Anti-inflammatory markers | |||||||
| TGF-β epithelium | Mean | 1.1 | 2.2 | 2.0 | 1.8 | 1.7 | 1.3 |
| p-value | 0.090 | 0.81 | 0.48 | ||||
| IL-10 epithelium | Mean | 3.5 | 5.0 | 4.3 | 5.3 | 3.3 | 5.4 |
| p-value | 0.0075 | 0.14 | 0.0021 | ||||
| Lactoferrin epithelium | Mean | 4.0 | 4.5 | 4.1 | 4.8 | 2.4 | 4.7 |
| p-value | 0.47 | 0.42 | 0.0062 | ||||
| Characteristics | T-Test: Mean Comparison | Generalized Linear Model | |||
|---|---|---|---|---|---|
| MC | No MC | p-Value | OR (95% CI) | p-Value | |
| Parous women (n = 118) | |||||
| Age at the first delivery (years) | 27 ± 4.4 | 25 ± 3.5 | 0.0019 | 1.2 (1.1–1.3) | 0.0029 |
| Number of deliveries (n) | 1.8 ± 0.69 | 2.3 ± 0.83 | 0.0033 | 0.63 (0.34–1.1) | 0.12 |
| Time of breastfeeding (month) | 3.2 ± 5.3 | 5.5 ± 7.9 | 0.05 | 0.95 (0.87–1.0) | 0.14 |
| Postmenopausal (%) | 44 | 56 | 0.47 | 1.3 (0.64–2.8) | 0.45 |
| SFRP1 expression (continuous) | 2.9 ± 3.1 | 2.3 ± 2.9 | 0.31 | 1.1 (0.94–1.2) | 0.30 |
| Nulliparous women (n = 44) | |||||
| Postmenopausal (%) | 57 | 43 | 0.080 | 3.1 (0.9–11) | 0.080 |
| SFRP1 expression (continuous) | 3.7 ± 3.1 | 1.3 ± 1.8 | 0.0057 | 1.5 (1.1–2.0) | 0.0075 |
| Continuous Scores | Parous (n = 118) | Nulliparous (n = 44) | |||||
|---|---|---|---|---|---|---|---|
| Breastfeeding (n = 61) | Without Breastfeeding (n = 57) | ||||||
| No MC (n = 37) | MC (n = 24) | No MC (n = 33) | MC (n = 24) | No MC (n = 25) | MC (n = 19) | ||
| SFRP1 | Mean | 2.7 | 2.3 | 1.9 | 3.4 | 1.3 | 3.7 |
| p-value | 0.63 | 0.061 | 0.0057 | ||||
| Pro-inflammatory markers | |||||||
| IL-8 epithelium | Mean | 3.4 | 3.4 | 3.3 | 3.3 | 2.9 | 3.4 |
| p-value | 0.75 | 0.90 | 0.15 | ||||
| IL-6 epithelium | Mean | 3.0 | 2.9 | 2.3 | 3.0 | 3.2 | 2.8 |
| p-value | 0.90 | 0.37 | 0.64 | ||||
| Leptin epithelium | Mean | 5.2 | 5.0 | 4.1 | 4.4 | 5.4 | 5.4 |
| p-value | 0.80 | 0.71 | 0.97 | ||||
| TNF-α epithelium | Mean | 3.2 | 2.4 | 2.1 | 2.5 | 3.2 | 3.0 |
| p-value | 0.24 | 0.49 | 0.79 | ||||
| COX-2 epithelium | Mean | 5.6 | 5.2 | 5.2 | 4.6 | 6.0 | 4.6 |
| p-value | 0.55 | 0.30 | 0.09 | ||||
| COX-2 stroma | Mean | 2.3 | 1.2 | 2.0 | 2.2 | 1.8 | 1.6 |
| p-value | 0.0085 | 0.63 | 0.48 | ||||
| SAA1 epithelium | Mean | 3.2 | 2.9 | 3.6 | 3.4 | 3.0 | 3.8 |
| p-value | 0.27 | 0.74 | 0.12 | ||||
| STAT3 epithelium | Mean | 4.0 | 4.2 | 4.4 | 4.7 | 4.1 | 3.8 |
| p-value | 0.73 | 0.64 | 0.68 | ||||
| CRP epithelium | Mean | 3.3 | 3.1 | 1.7 | 2.3 | 2.7 | 1.3 |
| p-value | 0.87 | 0.27 | 0.019 | ||||
| Anti-inflammatory markers | |||||||
| TGF-β epithelium | Mean | 1.9 | 1.4 | 1.3 | 2.8 | 1.8 | 1.1 |
| p-value | 0.50 | 0.025 | 0.20 | ||||
| IL-10 epithelium | Mean | 4.2 | 4.4 | 4.9 | 4.4 | 4.6 | 4.6 |
| p-value | 0.69 | 0.49 | 0.97 | ||||
| Lactoferrin epithelium | Mean | 4.4 | 4.1 | 4.5 | 4.1 | 4.2 | 3.1 |
| p-value | 0.71 | 0.65 | 0.21 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clemenceau, A.; Hanna, M.; Ennour-Idrissi, K.; Burguin, A.; Diorio, C.; Durocher, F. Secreted Frizzled-Related Protein 1 as a Biomarker against Incomplete Age-Related Lobular Involution and Microcalcifications’ Development. Cancers 2020, 12, 2693. https://doi.org/10.3390/cancers12092693
Clemenceau A, Hanna M, Ennour-Idrissi K, Burguin A, Diorio C, Durocher F. Secreted Frizzled-Related Protein 1 as a Biomarker against Incomplete Age-Related Lobular Involution and Microcalcifications’ Development. Cancers. 2020; 12(9):2693. https://doi.org/10.3390/cancers12092693
Chicago/Turabian StyleClemenceau, Alisson, Mirette Hanna, Kaoutar Ennour-Idrissi, Anna Burguin, Caroline Diorio, and Francine Durocher. 2020. "Secreted Frizzled-Related Protein 1 as a Biomarker against Incomplete Age-Related Lobular Involution and Microcalcifications’ Development" Cancers 12, no. 9: 2693. https://doi.org/10.3390/cancers12092693





