1. Introduction
Metastatic carcinomas express high levels of voltage-gated sodium channels (VGSCs), a feature that imparts an increased ability to invade normal tissue and to metastasize [
1,
2,
3]. This feature of epithelium-derived cancer cells provides the basis for proposed treatments using cytotoxic agents that target VGSCs with the goal to destroy or to block the function of these channels in an attempt to eliminate the cancer [
3,
4,
5,
6,
7,
8]. This approach has been able to slow tumor growth and metastasis by negatively modulating VGSC expression or function, but unfortunately, it does not kill the cancer cells [
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15].
By contrast, we have shown that the simultaneous administration of electrical or physiological stimulation of VGSCs and a pharmacological blockade of sodium, potassium-ATPase (Na
+, K
+-ATPase; sodium pumps) causes lysis of primary afferent neurons that over-express VGSCs without damaging neurons that express VGSCs at normal physiological levels [
16]. We have thus proposed that, as in primary peripheral afferent neurons, the simultaneous activation of VGSCs and pharmacological blockade of Na
+, K
+-ATPase in cancer cells will result in an excess of intracellular sodium and increased osmotic pressure. In advanced-stage cancers that greatly over-express VGSCs, the rise in osmotic pressure is sufficient to cause osmotic lysis of the cancer cells, leaving unaffected cells that express VGSCs normally. When this “targeted osmotic lysis” (TOL) is administered to several lines of malignant cells in vitro using Na
+, K
+-ATPase-blocking cardiac glycosides in combination with electrical stimulation, we have been able to affect 100% lysis of the malignant cells. However, when TOL is similarly applied to ectopic xenografts of malignant breast cancer MDA-MB-231 cells in vivo, the response to treatment has been less complete [
17].
Two features of the TOL treatment as previously applied may contribute to limiting TOL efficacy and precluding optimum treatment in vivo. The first is the form of stimulation that we have used to activate VGSCs. Direct contact electrical stimulation introduces electrons into body tissues that then flow along the shortest path between the positively charged anodal source and the negatively charged cathode. The path of electron flow is determined by the degree of resistance imposed by the tissues that support the cancer cells. In biology, the tissues that comprise the extracellular tumor environment and the interstitial/extracellular tumor matrix provide a heterogeneous source of resistance that, as in electronics, can alter or inhibit the degree of stimulation that any given cell will receive [
18,
19,
20]. Thus, as is evident in
Figure 1, electrical current is not the ideal method for stimulating VGSCs especially in solid and deeply seeded tumors.
The second feature that may limit the efficacy of TOL in vivo is the ability to deliver sufficient cardiac glycoside to effectively block all of the sodium pumps in the cancer cells within a solid tumor. Variable vascularization, refractory drug transporters, and the composition of the extracellular tumor matrix consisting of collagen, elastin, fibronectin and laminin and the resulting intratumoral hydrostatic pressure, can limit the distribution of many chemotherapeutic agents by impeding blood supply, thus diminishing the efficacy of promising treatment options [
20]. To this point, a number of agents designed to degrade the extracellular tumor matrix, such as losartan, an anti-hypertensive and anti-fibrotic drug that inhibits collagen I synthesis, and sildenafil, an agent that is used to treat erectile dysfunction by modulating the peripheral vascular tone and the distribution of blood supply through the inhibition of phosphodiesterase type 5 (PDE5), have been used to enhance drug penetration and the efficacy of chemotherapeutic agents in the treatment of cancer [
19,
21,
22]. We therefore hypothesized that it might be possible to improve the efficacy of TOL for destroying advanced stage cancer cells by utilizing a more effective method for stimulating VGSC activation and by improving the delivery of the cardiac glycoside that is used to block sodium pumps.
It is well known that pulsed magnetic fields (PMFs) can be used to activate action potentials in excitable tissues [
23,
24,
25,
26]. Transcranial magnetic stimulation of motor cortex has been used clinically for the treatment of depression, movement disorders, post-traumatic stress disorder, migraine and chronic pain and has been shown to produce evoked potentials and movement in contralateral limbs [
27]. As the delivery of magnetic field stimulation is much less influenced by tissue resistance than electric current stimulation [
23,
24,
25,
26], we hypothesized that PMFs would be a more efficient way to stimulate all cells in a body and improve lysis of invasive cancers. We therefore assessed the effect of TOL for treating highly malignant human (MDA-MB-231) and murine (4T1) breast cancer cells in vitro using PMF as the stimulus modality. PMF stimulation was also used in vivo to test the efficacy of TOL for treating ectopic xenografts and homografts of highly malignant murine breast cancer cells with and without losartan or sildenafil pretreatment to enhance the blood supply and delivery of cardiac glycoside.
3. Discussion
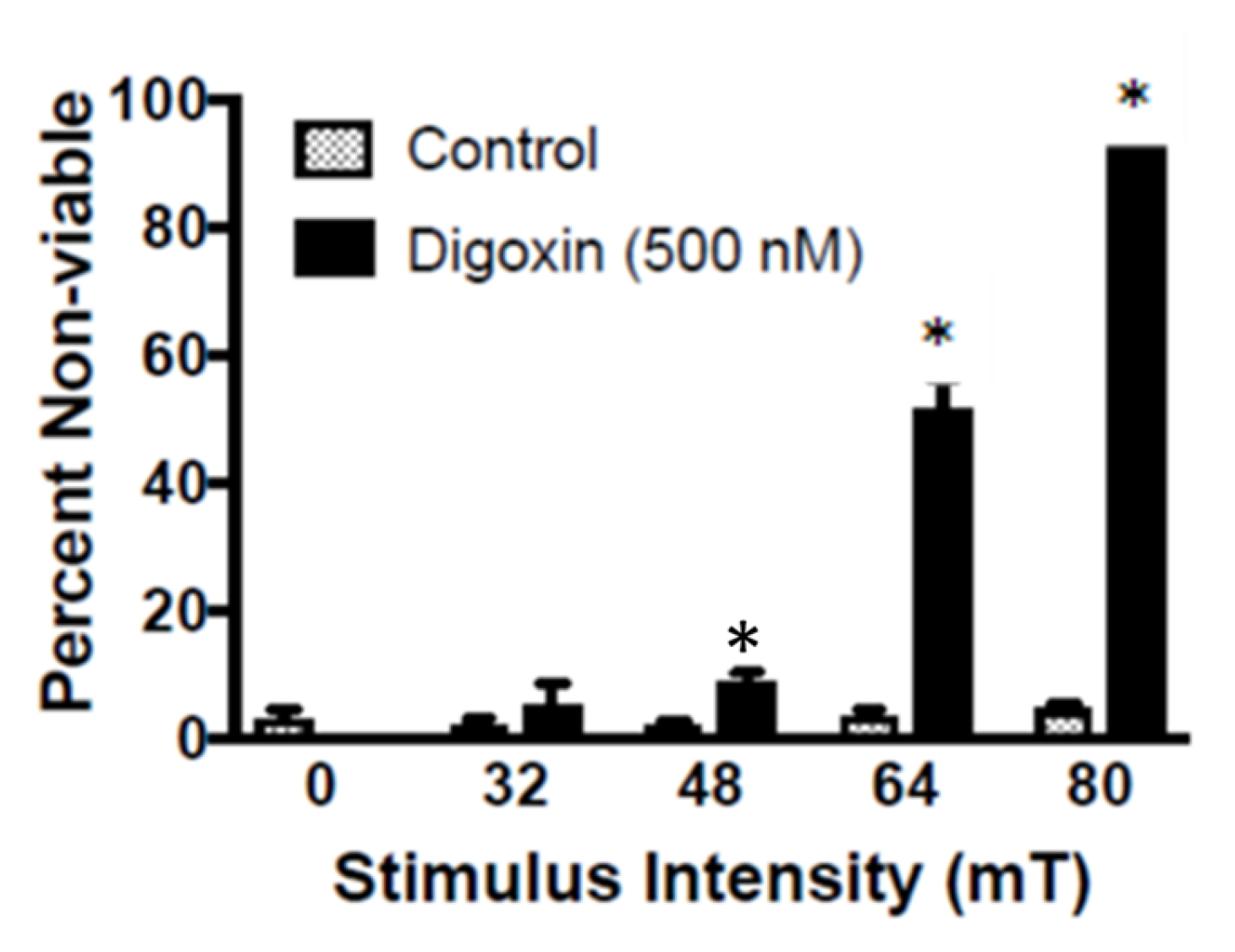
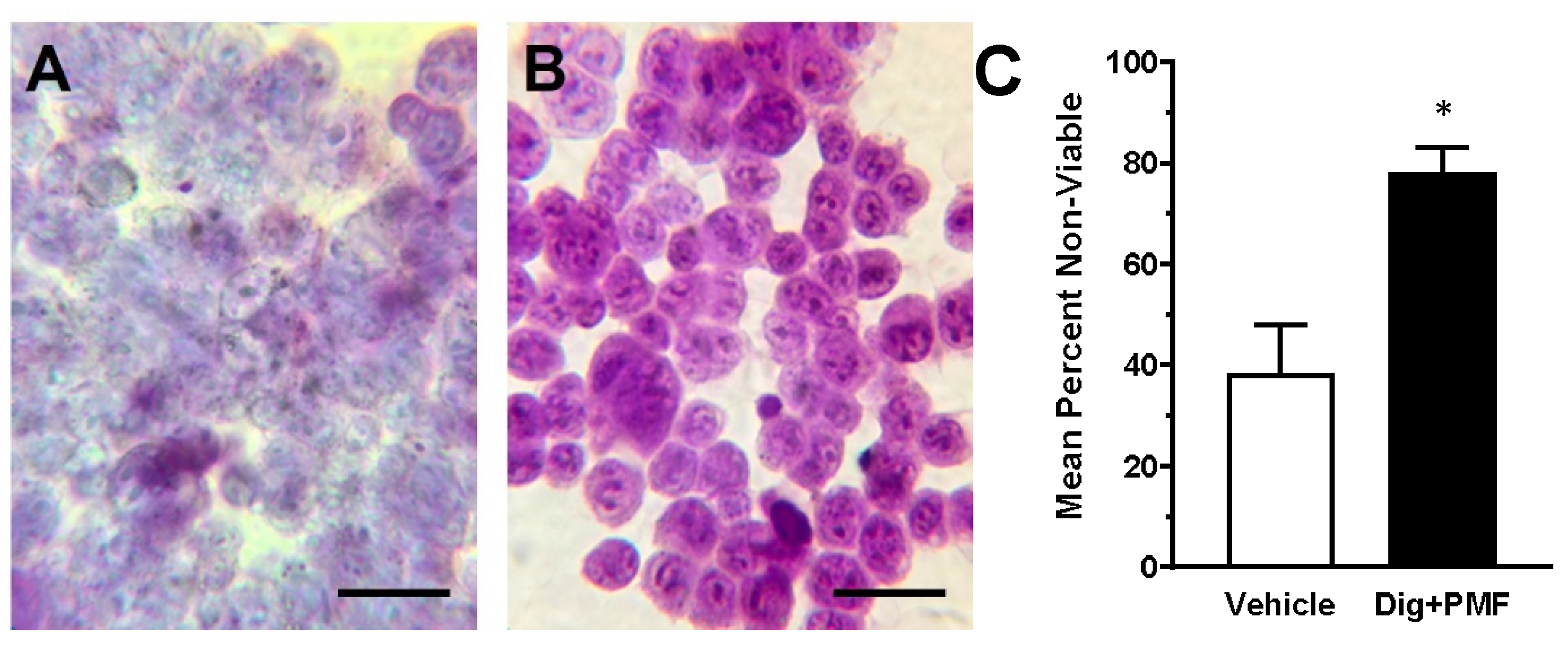
The results of the present study are in vitro and in vivo evidence that support our earlier observation that targeted osmotic lysis, the simultaneous stimulation of VGSCs and Na
+, K
+-ATPase blockade, is able to kill highly malignant MDA-MB-231 human and 4T1 mouse breast cancer cells while preserving similarly treated normal breast cells [
17]. In the in vitro experiments, the lysis of the cancer cells was dependent upon the intensity of the PMF, with greater than 95% of the digoxin treated cancer cells lysing within 15 min, with as little as 80 mT PMF. As with our previous report, when Na
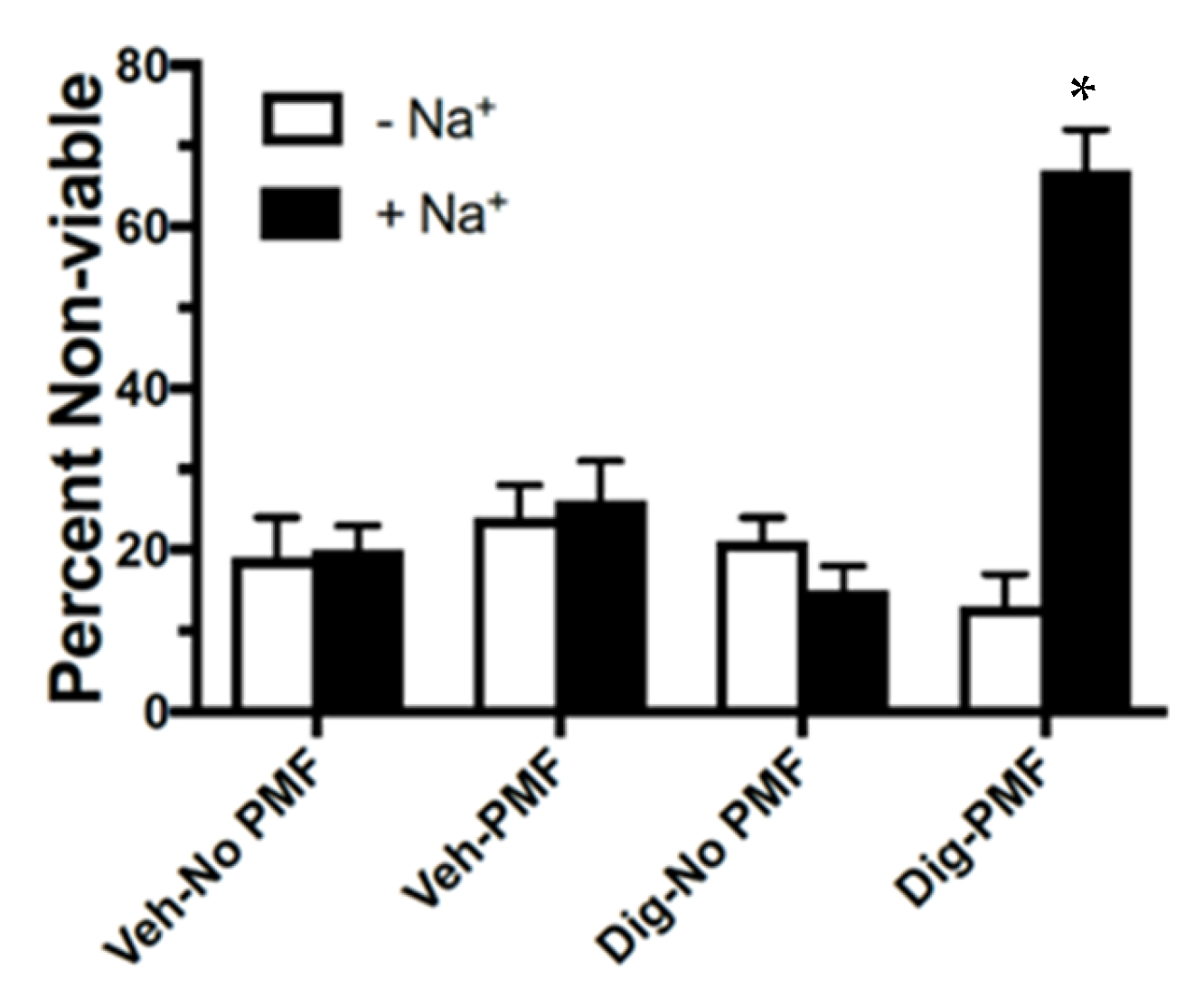
+ was eliminated from the incubation buffer, lysis of the cancer cells was no different than controls. Thus, TOL is Na
+-dependent.
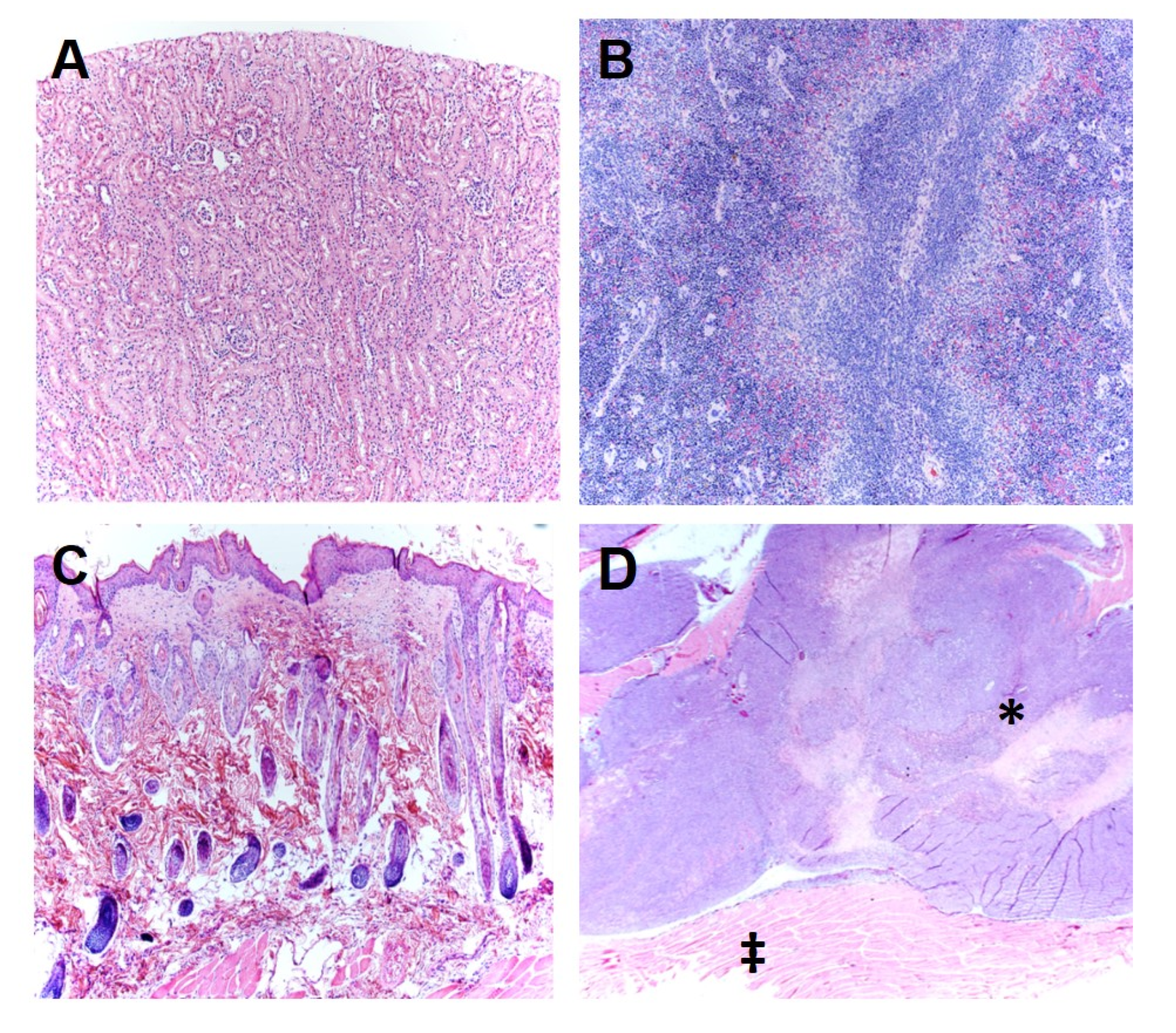
We also demonstrated that the delivery of TOL using PMFs slows the growth of ectopic xenografts of both highly malignant human and homografts of murine breast cancer cells and increases survival of the hosts. Importantly, noncancerous organs in these animals were unaffected by the TOL treatment. Therefore, the cytotoxicity in tumors was not due to Rho/Rho kinase mediated apoptosis as has been seen with long-term treatment with cardiac glycosides [
28]. The cells of normal tissues have only a fraction of the number of VGSCs compared to highly malignant cancer cells. Consequently, less Na
+ and thus less water enters the cells, and they do not lyse [
17].
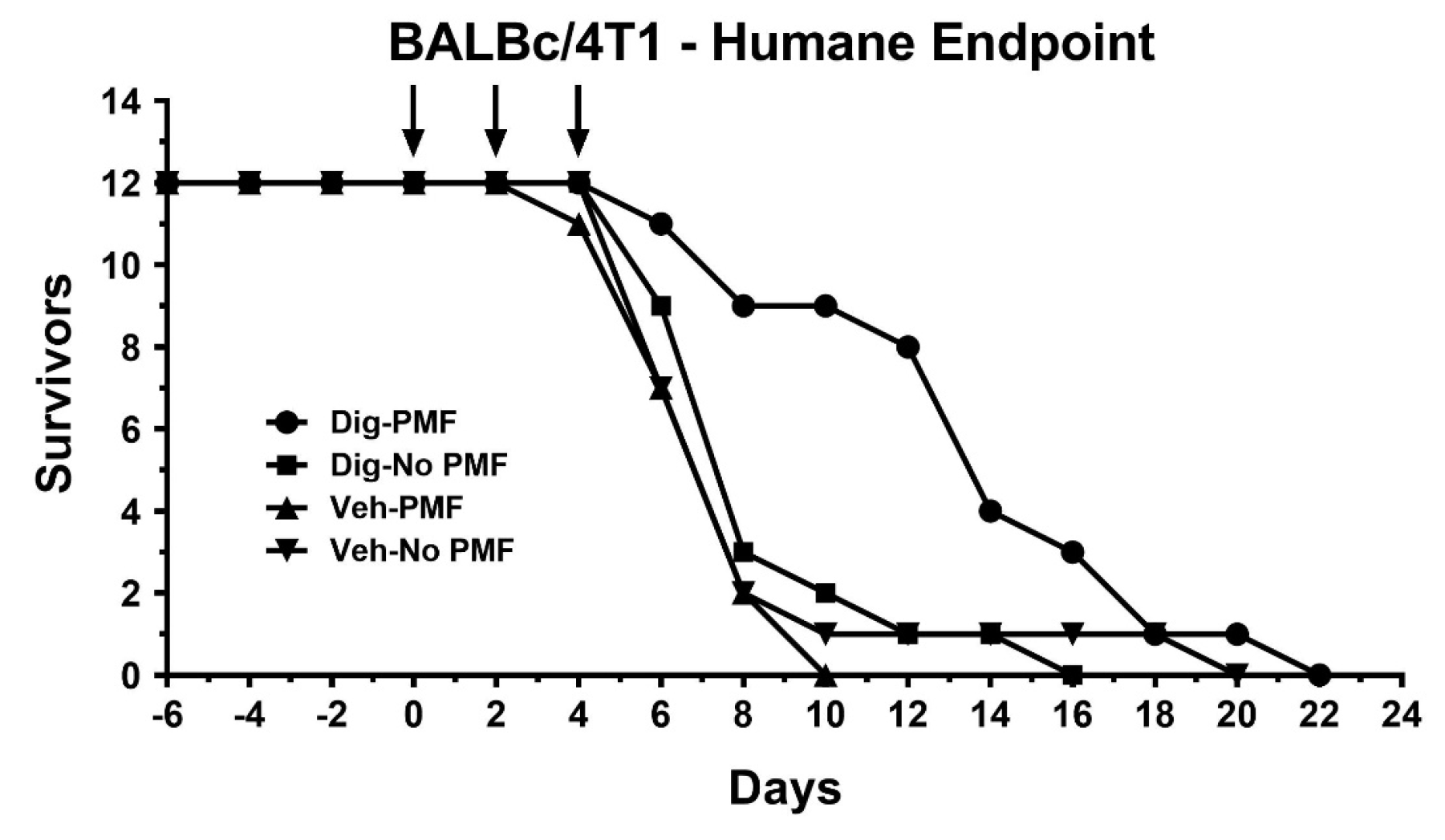
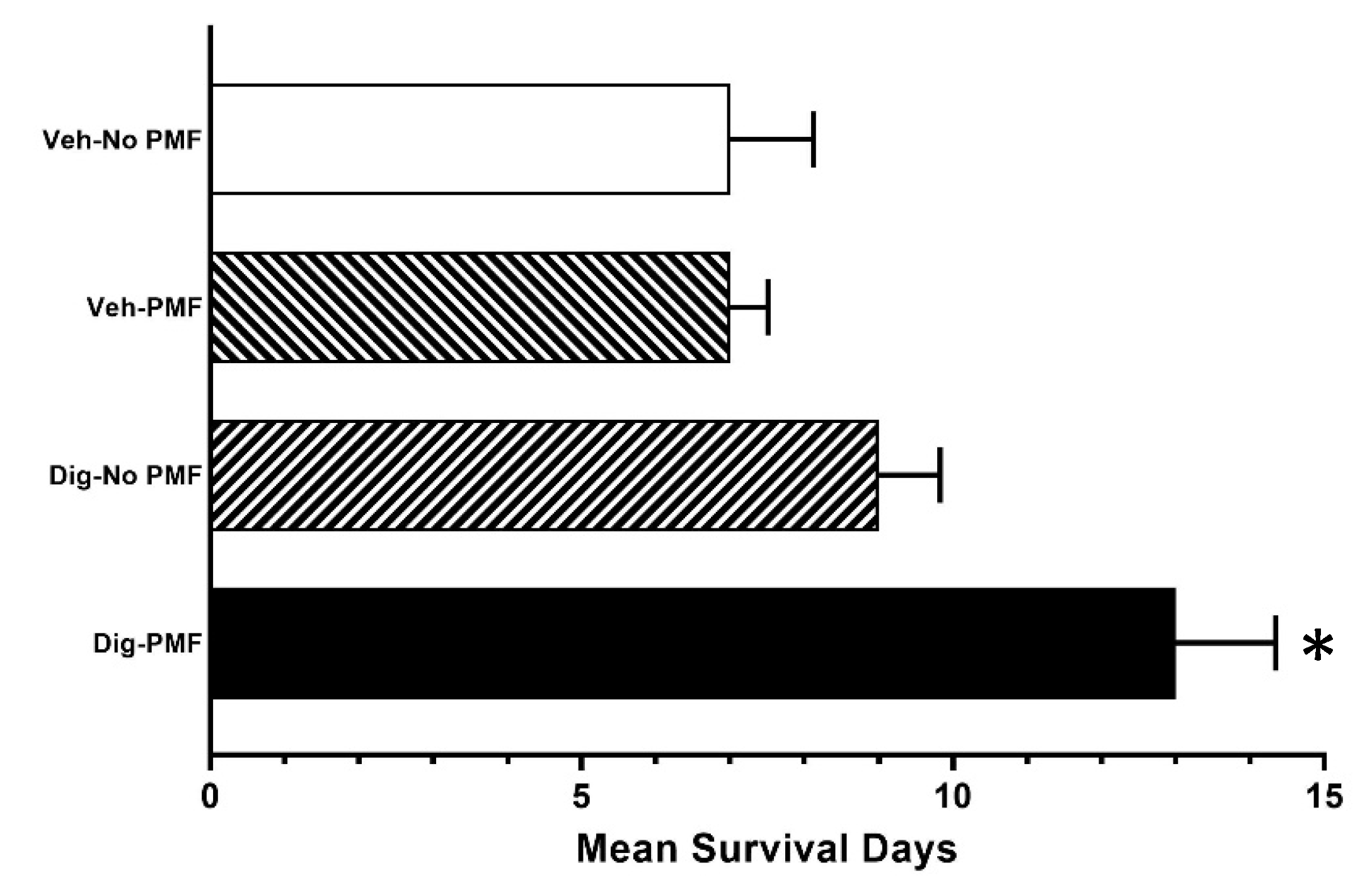
The efficacy of treating xenografts with TOL using PMFs was comparable to treating with TOL using electrical current for slowing the growth of both the xenograft and homograft tumors, and increasing the survival of the hosts than when the tumors were treated with PMF alone, digoxin alone or vehicle, but the effect of TOL seemed to be less focused when PMFs were used. Moreover in neither case did TOL negatively affect noncancerous organs. Thus, this treatment is likely to be selective for cancerous tissue, sparing healthy tissue and reducing morbidity compared to current cancer treatments.
To assess whether our digoxin administration protocol was insufficient to perfuse tumors sufficiently, we used two treatments shown to increase chemotherapeutic pharmacokinetics [
19,
21,
22]. Neither losartan, which degrades the extracellular tumor matrix [
21], nor sildenafil, which increases vascular permeability [
23], increased survival beyond our standard treatment protocol. Therefore, digoxin levels had achieved steady-state in all tissues of the body based on the drug’s pharmacokinetics, i.e., five doses separated by the digoxin t
1/2.
PMF stimulation has the added benefits of providing a noncontact mode of stimulation and enabling the simultaneous targeting of all tumors in metastatic disease, even when collections of metastatic cells are too small to be imaged. This is because the neoplastic cells that over-express VGSC carry both the targeting feature and the mechanism necessary for producing a therapeutic effect.
In summary, we have shown in mouse models of breast cancer that PMF is a sufficient stimulus method to lyse carcinomas in a sodium-mediated TOL paradigm. Together, these results are strong evidence that PMF stimulation of VGSCs combined with blockade of Na+, K+-ATPase is a potentially efficacious treatment for late stage, highly malignant breast carcinomas.
4. Materials and Methods
4.1. Drugs
Digoxin was purchased from Sigma-Aldrich (St. Louis, MO, USA). For in vitro experiments, the drugs were diluted in the appropriate medium to a concentration twice that of the final concentration for that experiment. For in vivo experiments, digoxin was diluted in 10% dimethylsulfoxide (DMSO)/saline. Losartan and sildenafil were dissolved in saline.
4.2. Cell Culture
MDA-MB-231 human breast cancer cells were purchased from ATCC (Manassas, VA, USA) and cultured in Dulbecco’s modified eagle’s medium (DMEM; Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA) and penicillin/streptomycin (pen/strep; Gibco, Grand Island, NY, USA). Insulin (10 µM; Sigma-Aldrich, St. Louis, MO, USA) was added 48 h before testing to assure sodium pump expression and activity. Mouse 4T1 breast cancer cells were also purchased from ATCC and cultured in RPMI-1640 media supplemented with FBS and pen/strep. MCF-10a normal breast epithelial cells were cultured in complete mammillary epithelial growth medium (MEGM; Lonza, Basel, Switzerland), which contains 10 µM insulin, supplemented with pen/strep.
4.3. Animals
All of our procedures were approved by the Louisiana State University Health Sciences Center Institutional Animal Care and Use Committee (IACUC), which is OLAW approved (Assurance #D16-00058), AAALAC accredited (#000037), and US Dept. of Agriculture certified (#72-R-0003). For MDA-MB-231 cell xenografts, 6-week-old, 15–20 g, female J/Nu mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA) and housed in a specific pathogen-free, climate-controlled colony room with a 12/12 h light/dark cycle with free access to water and lab chow. To establish xenografts, approximately 4 million cells were injected in a 50% matrigel/saline suspension. Tumors formed to 0.7–1.2 cm diameter in 3–4 weeks.
For 4T1 homografts, 6-week-old, 15–20 g, female BALB/c mice were purchased from Jackson Laboratories and housed as with the J/Nu mice, but in a room with modified barrier controls. To establish homografts, approximately 500,000 cells were injected subcutaneously between the scapulae in a saline suspension. Tumors formed to 0.7 to 1.2 cm in 7 days. Treatment day 1 was initiated when average tumor size attained 5–10 mm in length as measured with a digital caliper by at least 2 investigators, one of which was not blinded to the treatment groups.
4.4. Flow Cytometry
MDA-MB-231 and 4T1 cells were dissociated using Cellstripper (Corning Life Science, Corning, NY, USA) centrifuged, decanted and resuspended in RPMI. 4% paraformaldehyde was added for 10 min. The cells were then centrifuged, decanted and washed with saline. The cells were resuspended in 5% goat serum + 3% bovine serum albumin for 1 hr. After centrifugation and aspiration of the supernatant, the cells were resuspended in PBS and incubated overnight with a pan-specific VGSC antibody (Alomone Labs; Jerusalem, Israel) that was pre-conjugated to an Allophycocyanin (APC) fluorophore, excitation 594 and 633 nm/emission 660 nm. The cells were centrifuged and resuspended in PBS and analyzed using a FACSCanto II flow cytometer (BD Biosciences, San Jose, CA, USA).
4.5. Image Analysis
MDA-MB-231 and 4T1 cells were incubated in RPMI for 2 days on a 22 × 22 mm slide cover. Media was aspirated and the cells were fixed with 0.5% paraformaldehyde for 10 min, rinsed 3 times for 5 min with 1 × PBS and then blocked with 5% goat serum + 3% bovine serum albumin for 1 h. Cells were then incubated overnight with the pan-specific VGSC antibody (Alomone Labs), 1:200 dilution. After overnight incubation with the primary antibody, cells were washed 3 times for 5 min with 1 × PBS. Cells were then incubated at room temperature for 45 min with a goat-anti-rabbit 488 Alexa fluorophore secondary, 1:800 dilution. Cells were washed 3 times for 5 min with 1 × PBS. Cells were incubated 10 min with DRAQ5 as a nuclear counterstain, dilution 1:600. Cells were rinsed and set with ProLong Gold antifade mountant. Images were taken on a Leica DMi8 confocal microscope (Leica Microsystems, Wetzlar, Germany) at 40 × oil magnification. As a control for nonspecific fluorescence, the anti-sodium channel antibody was pre-blocked with a 500-fold excess of the peptide antigen to which it was raised.
4.6. In Vitro TOL Treatment
Before testing, cells were dissociated with Cellstripper and approximately 150,000 cells resuspended in each 1.5 mL microfuge tubes using DMEM with or without digoxin (Sigma-Aldrich). Tubes were placed in an 8” length, 2.5” inner diameter solenoid with 697 turns (500 ft.) of 12 ga copper wire that gives an expected peak field strength of 4.4 mT/A with an expected resistance of 0.825 Ω. Based on measurements performed by driving 0.76 A at 0.60 V, the measured peak field strength using a F.W. Bell (Bedford, MA, USA) Model 4048 Handheld Gauss/Tesla Meter was 3.99 mT/A. The calculated resistance was 0.79 Ω. For stimulus/response experiments, 1–5 VDC current from an AE Techron (Elkhart, IN, USA) 7224 DC-extended AC amplifier was pulsed at 25 Hz, with a 10 msec ramp and fall, controlled by a Tektronix (Beaverton, OR, USA) AFG 3021B waveform generator, which produced 35–80 mT magnetic pulses in the solenoid. Cells were stimulated for 10 min. For dose/response experiments, cells were resuspended in 500 nM digoxin and stimulated for 10 min at 80 mT. Following each experiment, cells were fixed with 0.5% paraformaldehyde in phosphate buffer, pH 7.6, then stained with Cresyl violet. An evaluator blind to the treatment counted live vs. dead cells in at least 10 fields and a total of more than 100 cells for each treatment. Cells with a centrally located nucleus were scored as viable. Cells that were pyknotic or had no nucleus were considered nonviable.
4.7. Sodium Dependency
For Na+ dependency studies, cells were resuspended in normal Ringer’s solution [125 mM NaCl, 5.0 mM KCl, 2.0 mM CaCl2, 1.0 mM MgSO4, 10.0 mM glucose, 10.0 mM HEPES ] or Na+-free Ringer’s [NaCl was replaced with 250 mM sucrose to maintain osmotic pressure] with or without 500 nM digoxin. Approximately 150,000 cells were distributed to each 1.5 mL microfuge tube. Tubes were treated with 0 or 80 mT PMF, then evaluated as with the stimulus-response curve.
4.8. In Vivo TOL Treatment
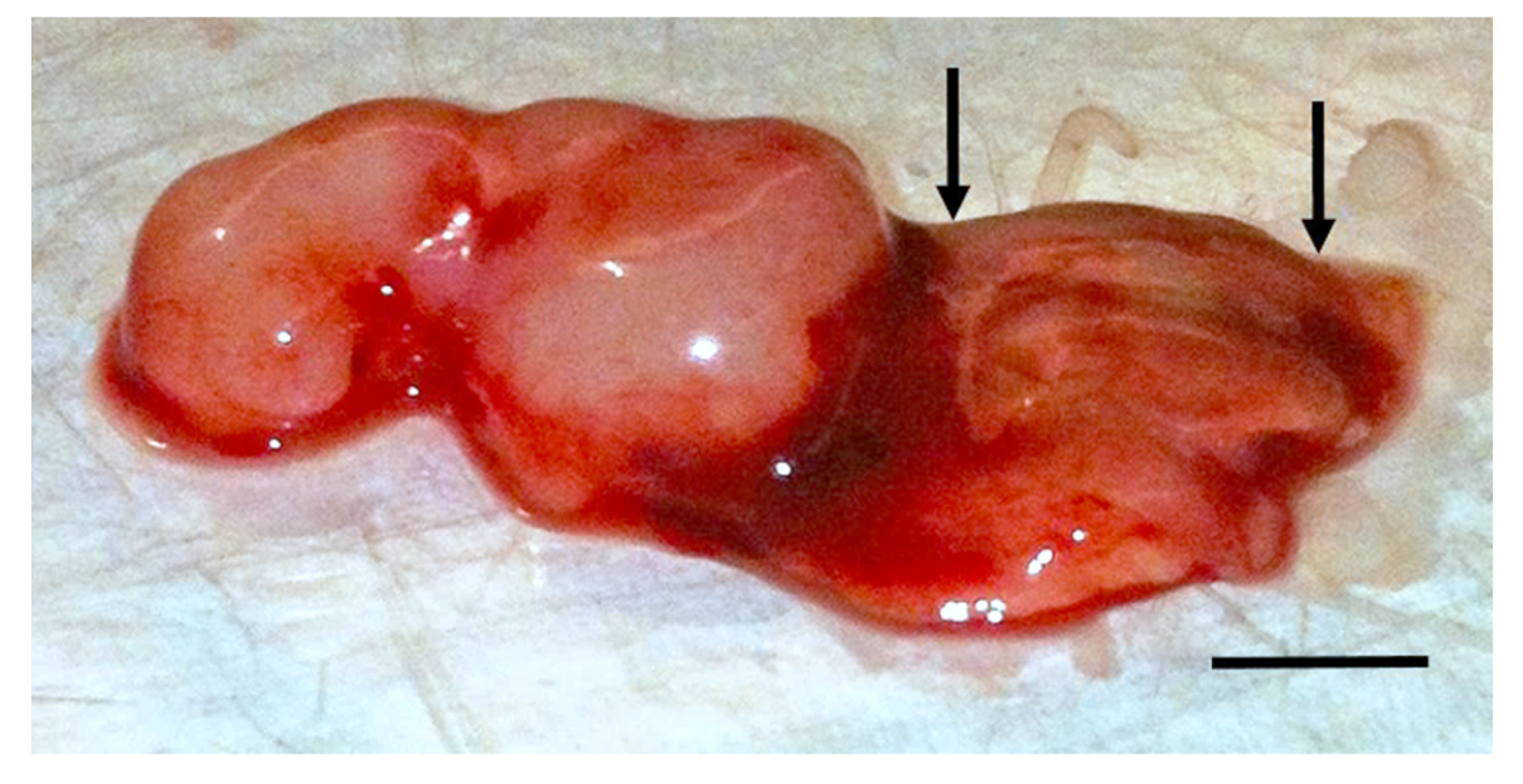
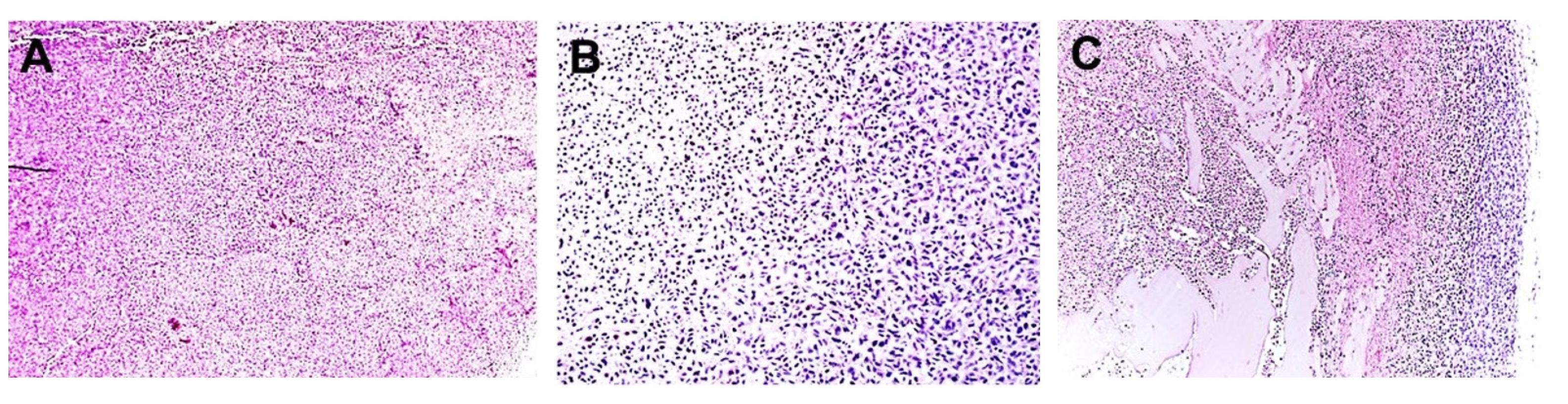
For in vivo validation, groups of J/Nu mice (n = 8) hosting MDA-MB-231 xenografts were injected subcutaneously between the scapulae, five times at 1 h intervals with 7 mg/kg digoxin or an equal volume of the 10% DMSO/saline vehicle to bring digoxin or vehicle levels to steady-state for testing. The mice were treated on day 1 or on days 1, 3 and 5. On each of these days, were exposed to the PMF for 15 min starting 30 min after the last injection. For pathological analysis, mice were sacrificed by cervical dislocation and fixed with 0.5% paraformaldehyde in phosphate buffer 24 h after the last treatment. Samples of tissue from the grafted tumors as well as representative samples of kidney, spleen, skin, and muscle tissues were harvested, embedded in paraffin, sectioned and stained with hematoxylin and eosin. The tissue sections were then evaluated with light microscopy by a veterinary pathologist who is board certified by the American College of Veterinary Pathologists and was blinded to the treatments provided. Because the ranking was not continuous, the effect of TOL on tumors was compared using a Χ2 test.
Groups of BALB/c mice with 4T1 homografts were injected with 7 mg/kg digoxin or the vehicle and tested as with the J/Nu mice.
4.9. Survival Studies
Post-treatment survival for MDA-MB-231-J/Nu and 4T1-BALB/c mice were evaluated using the same treatment parameters as above. The cross-sectional area of the xenografts were measured every third day for up to 33 days. Cross-sectional area was calculated by measuring the length and width of the tumors (d1 and d2, respectively) and calculating the assumed oval area using the formula: A = (d1/2) × (d2/2) × π) Mice were sacrificed when they met the NIH criteria for humane endpoint euthanasia of laboratory animals in cancer studies. In two experiments, one using MDA-MB-231 xenografts and another using 4T1 homografts, the tumor cross-sections were measured every other day and the mice were allowed to survive until humane endpoint criteria were met.
4.10. Enhancement of Drug Penetration
Ectopic homografts of 4T1 murine breast cancer cells were established in female BALB/c mice. For TOL, mice were injected with digoxin (7 mg/kg), then stimulated with PMF (80 mT at 25 Hz) for 30 min on days 1, 3 and 5 and compared with controls. Groups of TOL-treated or control mice received either losartan (20 mg/kg/d; Sigma-Aldrich) beginning 2 days before TOL or sildenafil (1 mg/kg; Sigma-Aldrich) 15 min prior to each digoxin dosing and were compared to groups of mice that did not receive supplementary drug. Tumor growth was measured every other day. Animals were euthanized when they met NIH criteria for humane endpoint euthanasia.