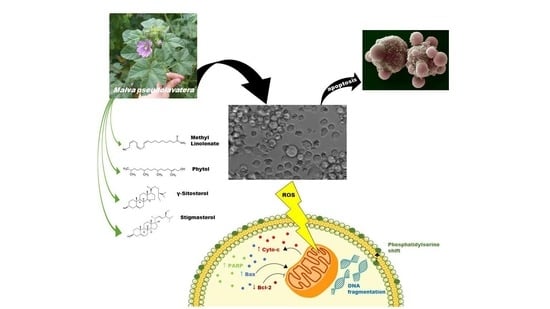
Malva pseudolavatera Leaf Extract Promotes ROS Induction Leading to Apoptosis in Acute Myeloid Leukemia Cells In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Isolation and Culture of Mesenchymal Stem Cells (MSCs) from Rat Bone Marrow
2.3. Isolation and Culture of Normal Mononuclear Cells (MNCs) from Human Bone Marrow (BM)
2.4. Plant Material
2.5. Preparation of Crude Leaf Extract (MMLE)
2.6. Cytotoxicity Assay
2.7. Cell Cycle Analysis
2.8. Apoptosis Detection Using Fluorescent Annexin V Staining
2.9. Apoptosis Quantification by Dual Annexin V/PI Staining
2.10. Cell Death ELISA
2.11. Western Blot
2.12. Reactive Oxygen Species Detection
2.13. Gas ChromatographyMass Spectrometry Analysis of the Methanolic Extract of M. Pseudolavatera Webb & Berthel. Leaves
2.14. Liquid ChromatographyMass Spectrometry Analysis of the Methanolic Extract of M. Pseudolavatera Webb & Berthel. Leaves
2.15. Statistical Analysis
3. Results
3.1. M. pseudolavatera Leaf Extract Exhibits Selective Anti-Proliferative Effects on AML Cell Lines
3.2. M. pseudolavatera Leaf Extract Induces Cellular Fragmentation in AML Cell Lines
3.3. M. pseudolavatera Leaf Extract Significantly Induces Apoptosis in AML Cell Lines
3.4. M. pseudolavatera Leaf Extract Causes Upregulation of Pro-Apoptotic Proteins
3.5. M. pseudolavatera Leaf Extract Induces Oxidative Stress in AML Cell Lines
3.6. Chemical Elucidation of M. pseudolavatera Leaf Extract Using GC-MS
3.7. Chemical Elucidation of M. Pseudolavatera Leaf Extract Using LC-MS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- The Jebson Herbarium University of California, Berkley. Available online: http://ucjeps.berkeley.edu/eflora/eflora_display.php?tid=89042 (accessed on 25 June 2019).
- Edgecombe, W.S. Weeds of Lebanon; American University of Beirut: Beirut, Lebanon, 1964; pp. 244–247. [Google Scholar]
- Webb, P.B.; Berthelot, S. Histoire Naturelle des Iles Canaries, Phytographia Canariensis; Bethune, editeur: Paris, France, 1836; pp. 1836–1850. [Google Scholar]
- The Plant List. Malva pseudolavatera Webb & Berthel. Available online: http://theplantlist.org/tpl1.1/record/tro-50337435 (accessed on 7 April 2019).
- Abdullatif, A. Malva: Food, medicine and chemistry. Eur. Chem. Bull. 2017, 6, 295–320. [Google Scholar]
- Singorini, M.A.; Piredda, M.; Bruschi, P. Plants and traditional knowledge: An ethnobotanical investigation on Monte Ortobene. J. Ethnobiol. Ethnomed. 2009, 5, 6. [Google Scholar]
- Rivera, N.D.; Obon, D.C.C. Medicaments et Aliments: l’approche ethnopharmacologique. Acte du 2eme Colloque Europeen d’Ethnopharamcologie et de la 1ere Coference Internationale d’Ethnomedicine. Heidelberg 1993, 2, 223. [Google Scholar]
- Gaspar, N.; Godinho, J.; Vasconcelos, T.; Caldas, D.; Mendes, P.; Barros, O. Ethnobotany in the center of Portugal. In Natural Products in the New Millennium: Prospects and Industrial Applications; Springer: Dordercht, The Netherlands, 2002; pp. 271–284. [Google Scholar]
- Ben-Nasr, S.; Aazza, S.; Mnif, W.; da Graca Costa Miguel, M. Antioxidant and anti-lipoxygenase activities of extracts from different parts of Lavatera cretica L. grown in Algarve (Portugal). Pharm. Mag. 2015, 11, 48–54. [Google Scholar]
- Commitee of Herbal Medicinal Products. Assessment Report on Malva Sylvestris L. and/or Malva Neglecta Wallr., Follium and Malva Sylvestris L., Flos; European Medicines Agency: London, UK, 2018.
- Farhan, H.; Rammal, H.; Hijazi, A. Chemical composition, in vitro cytotoxicity and anti-free radical properties of six extracts from Lebanese Trigonella berythea boiss. Pak. J. Pharm. Sci. 2013, 26, 1157–1163. [Google Scholar]
- Raghda Rayssan, S.; Muayad, S. Cytotoxicity Assessment of Malva Sylvestris Crude Extract. Int. J. Pharm. Sci. Res. 2019, 11, 70–74. [Google Scholar]
- Ali, M.; Abul Farah, M.; Al-Hemaid, F.; Abou-Tarboush, F. In vitro cytotoxicity screening of wild plant extracts from Saudi Arabia on human breast adenocarcinoma cells. Genet. Mol. Res. 2014, 13, 3981–3990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donaldson, J.; Cates, R. Screening for anticancer agents from Sonoran desert plants: A chemical ecology approach. Pharm. Biol. 2014, 42, 478–487. [Google Scholar] [CrossRef]
- American Cancer Society. What Is Acute Myeloid Leukemia (AML). Available online: https://www.cancer.org/cancer/acute-myeloid-leukemia/about/what-is-aml.html (accessed on 3 April 2019).
- Shysh, A.C. The incidence of acute myeloid leukemia in Calgary, Alberta, Canada: A retrospective cohort study. BMC Public Health 2018, 18, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cancer Research UK. Chemotherapy for Acute Myeloid Leukaemia (AML). Available online: https://www.cancerresearchuk.org/about-cancer/acute-myeloid-leukaemia-aml/treating-aml/chemotherapy/chemotherapy-for-aml (accessed on 3 April 2019).
- Atanasov, A. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [Green Version]
- Rates, S. Plants as source of drugs. Toxicon 2001, 39, 603–613. [Google Scholar] [CrossRef]
- National Research Council (US). Committee for the Update of the Guide for the Care and Use of Laboratory Animals. In Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar]
- Zeeni, N.; Daher, C.; Fromentin, G.; Tome, D.; Darcel, N.; Chaumontet, C. A cafeteria diet modifies the response to chronic variable stress in rats. Stress 2013, 16, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Najar, M.; Fayyad-Kazan, H.; Faour, W.H.; Merimi, M.; Sokal, E.M.; Lombard, C.A.; Fahmi, H. Immunological modulation following bone marrow-derived mesenchymal stromal cells and Th17 lymphocyte co-cultures. Inflamm. Res. 2019, 68, 203–213. [Google Scholar] [CrossRef]
- Soleimani, M.; Nadri, S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat. Protoc. 2009, 4, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Haykal, T.; Nasr, P.; Hodroj, M.H.; Taleb, R.I.; Sarkis, R.; Moujabber, M.N.E.; Rizk, S. Annona cherimola Seed Extract Activates Extrinsic and Intrinsic Apoptotic Pathways in Leukemic Cells. Toxins 2019, 11, 506. [Google Scholar] [CrossRef] [Green Version]
- Zibara, K.; Hamdan, R.; Dib, L.; Sindet-Pedersen, S.; Kharfan-Dabaja, M.; Bazarbachi, A.; El-Sabban, M. Acellular Bone Marrow Extracts Significantly Enhance Engraftment Levels of Human Hematopoietic Stem Cells in Mouse Xeno-Transplantation Models. PLoS ONE 2012, 7, e40140. [Google Scholar] [CrossRef] [PubMed]
- Nisrine Machaka-Houri. Available online: http://www.nisrinemachaka.com/ (accessed on 24 April 2019).
- Aung, T.N.; Qu, Z.; Kortschak, R.D.; Adelson, D.L. Understanding the effectiveness of natural compound mixtures in cancer through their molecular mode of action. Int. J. Mol. Sci. 2017, 18, 656. [Google Scholar] [CrossRef]
- Howitz, K.T.; Sinclair, D.A. Xenohormesis: Sensing the chemical cues of other species. Cell 2008, 133, 387–391. [Google Scholar] [CrossRef] [Green Version]
- Solowey, E.; Lichtenstein, M.; Sallon, S.; Paavilainen, H.; Solowey, E.; Lorberboum-Galski, H. Evaluating medicinal plants for anticancer activity. Sci. World J. 2014, 2014, 721402. [Google Scholar] [CrossRef] [Green Version]
- Fattoruso, S.I.; Di Lauro, L.; Conti, F.; Amodio, A.; Lopez, M. Target selectivity of anticancer drugs. Clin. Ter. 2008, 3, 189–206. [Google Scholar]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A target for Anti-cancer therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luparello, C.; Asaro, D.M.L.; Cruciata, I.; Hassell-Hart, S.; Sansook, S.; Spencer, J.; Caradonna, F. Cytotoxic Activity of the Histone Deacetylase 3-Selective Inhibitor Pojamide on MDA-MB-231 Triple-Negative Breast Cancer Cells. Int. J. Mol. Sci. 2019, 20, 804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skidmore, C.J.; Davies, M.I.; Goodwin, P.M.; Halldorsson, H.; Lewis, P.J.; Shall, S.; Zia’ee, A.A. The Involvement of Poly(ADP-ribose) Polymerase in the Degradation of NAD Caused by Radiation and N-Methyl-N-Nitrosourea. Eur. J. Biochem. 1979, 101, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Smulson, M.E.; Pang, D.; Jung, M.; Dimtchev, A.; Chasovskikh, S.; Spoonde, A.; Simbulan-Rosenthal, C.; Rosenthal, D.; Yakovlev, A.; Dritschilo, A. Irreversible binding of poly(ADP)ribose polymerase cleavage product to DNA ends revealed by atomic force microscopy: Possible role in apoptosis. Cancer Res. 1998, 58, 3495–3498. [Google Scholar] [PubMed]
- Chen, Q.; Xu, H.; Xu, A.; Ross, T.; Bowler, E.; Hu, Y.; Lesnefsky, E.J. Inhibition of Bcl-2 Sensitizes Mitochondrial Permeability Transition Pore (MPTP) Opening in Ischemia-Damaged Mitochondria. PLoS ONE 2015, 10, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edlich, F.; Banerjee, S.; Suzuki, M.; Cleland., M.M.; Arnoult, D.; Wang, C.; Neutzner, A.; Tjandra, N.; Youle, R.J. Bcl-xL retrotranslocates Bax from the mitochondria into the cytosol. Cell 2011, 145, 104–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosulich, S.C.; Worrall, V.; Hedge, P.J.; Green, S.; Clarke, P.R. Regulation of apoptosis by BH3 domains in a cell-free system. Curr. Biol. 1997, 7, 913–920. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, C.; Pandey, S. Exploiting Mitochondrial Vulnerabilities to Trigger Apoptosis Selectively in Cancer Cells. Cancers 2019, 11, 916. [Google Scholar] [CrossRef] [Green Version]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, H.; Ma, X.F.; Zhou, X.; Gan, L.; Liu, Y.Y.; Wang, Z.H. Isoliquiritigenin enhances radiosensitivity of HepG2 cells via disturbance of redox status. Cell Biochem. Biophys. 2013, 65, 433–444. [Google Scholar] [CrossRef]
- Galati, G.; Lin, A.; Sultan, A.M.; O’Brien, P.J. Cellular and in vivo hepatotoxicity caused by green tea phenolic acids and catechins. Free Radic. Biol. Med. 2006, 40, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Hardman, W.E. (n-3) Fatty Acids and Cancer Therapy. J. Nutr. 2004, 134, 3427–3430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Eliseo, D.; Velotti, F. Omega-3 Fatty Acids and Cancer Cell Cytotoxicity: Implications for Multi-Targeted Cancer Therapy. J. Clin. Med. 2016, 5, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahipal, S.V.; Subhashini, J.; Reddy, M.C.; Reddy, M.M.; Anilkumar, K.; Roy, K.R.; Reddy, G.V.; Reddanna, P. Effect of 15-lipoxygenase metabolites, 15-(S)-HPETE and 15-(S)-HETE on chronic myelogenous leukemia cell line K-562: Reactive oxygen species (ROS) mediate caspase-dependent apoptosis. Biochem. Pharmacol. 2007, 74, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Komiya, T.; Kyohkon, M.; Ohwaki, S.; Eto, J.; Katsuzaki, H.; Imai, K.; Kataoka, T.; Yoshioka, K.; Ishii, Y.; Hibasami., H. Phytol induces programmed cell death in human lymphoid leukemia Molt 4B cells. Int. J. Mol. Med. 1999, 4, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, P.S.; Mehta, A.; Verma, R.S. Synergistic interaction of β-caryophyllene with aromadendrene oxide-2 and phytol induces apoptosis on skin epidermoid cancer cells. Phytomedicine 2018, 47, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Akbas, S.H.; Timur, M.; Ozben, T. The Effect of Quercetin on Topotecan Cytotoxicity in MCF-7 and MDA-MB 231 Human Breast Cancer Cells. J. Surg. Res. 2005, 125, 49–55. [Google Scholar] [CrossRef]
- Bakheet, S.A. Assessment of anti-cytogenotoxic effects of quercetin in animals treated with topotecan. Oxid. Med. Cell. Longev. 2011, 2011, 824597. [Google Scholar] [CrossRef] [Green Version]
- Goto, T.; Teraminami, A.; Lee, J.Y.; Ohyama, K.; Funakoshi, K.; Kim, Y.I.; Hirai, S.; Uemura, T.; Yu, R.; Takahashi, N.; et al. Tiliroside, a glycosidic flavonoid, ameliorates obesity-induced metabolic disorders via activation of adiponectin signaling followed by enhancement of fatty acid oxidation in liver and skeletal muscle in obese-diabetic mice. J. Nutr. Biochem. 2012, 23, 768–776. [Google Scholar] [CrossRef]
- Esteves-Souza, A.; Silva, T.; Alves, C.; Carvalho, M.; Braz-Filho, R.; Echevarria, A. Cytotoxic activities against Ehrlich carcinoma and human K562 leukaemia of alkaloids and flavonoid from two Solanum Species. J. Brazil. Chem. Soc. 2002, 13, 838–842. [Google Scholar] [CrossRef]
- Dai, M.; Wikantyasning, E.; Wahyuni, A.; Kusumawati, I.; Saifudin, A.; Suhendi, A. Antiproliferative properties of tiliroside from Guazuma ulmifolia lamk on T47D and MCF7 cancer cell lines. Natl. J. Physiol. Pharm. Pharmacol. 2016, 6, 627. [Google Scholar] [CrossRef] [Green Version]
- Endrini, S.; Rahmat, A.; Ismail, P.; Taufiq-Yap, Y. Cytotoxic effect of γ-Sitosterol from kejibeling (Strobilanthes crispus) and its mechanism of action towards c-myc gene expresiion and apoptotic pathway. Med. J. Indones. 2014, 23, 203–208. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Li, X.F.; Kang, K.H.; Ryu, B.; Kim, S.K. Stigmasterol isolated from marine microalgae Navicula incerta induces apoptosis in human hepatoma HepG2 cells. BMB Rep. 2014, 47, 433–438. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Yuan, D.; Yan, R.; Meng, L.; Zhang, Y.; Zhu, K. Stigmasterol exhibits potent antitumor effects in human gastric cancer cells mediated via inhibition of cell migration, cell cycle arrest, mitochondrial mediated apoptosis and inhibition of JAK/STAT signaling pathway. J. BUON. 2018, 23, 1420–1425. [Google Scholar]







| Peak | RT | Compound | %MMLE |
|---|---|---|---|
| 1 | 5.751 | Unidentified A | 0.2141 |
| 2 | 28.7101 | Hexadecanoic acid methyl ester | 3.4385 |
| 3 | 35.0314 | Unidentified B | 1.4208 |
| 4 | 35.9573 | (E,E)-9,11-Octadecadienoic acid methyl ester | 2.7188 |
| 5 | 36.3974 | Methyl linolenate | 31.7912 |
| 6 | 36.8717 | Phytol | 19.3447 |
| 7 | 37.3804 | Octadecanoic acid methyl ester | 1.3903 |
| 8 | 38.7407 | Unidentified C | 0.124 |
| 9 | 38.7864 | 7,10,13-Hexadecatrienoic acid methyl ester | 0.0463 |
| 10 | 38.8093 | Unidentified C | 0.0408 |
| 11 | 51.7034 | 2,2′-methylenebis [6-(1,1-dimethylethyl)-4-methyl-phenol | 1.6741 |
| 12 | 53.607 | Unidentified D | 1.47 |
| 13 | 56.3158 | Unidentified E | 1.4302 |
| 14 | 56.4415 | (Z,Z,Z)-9-(3-hexenylidenecyclopropylidene)-,2-hydroxy-1-(hydroxymethyl)nonanoic acid, ethyl ester | 8.0675 |
| 15 | 56.584 | Unidentified F | 1.5 |
| 16 | 57.4074 | Unidentified G | 0.2354 |
| 17 | 57.505 | Unidentified H | 0.3 |
| 18 | 57.8875 | Cyclotetracosane | 0.6203 |
| 19 | 58.322 | Unidentified I | 0.31 |
| 20 | 59.5393 | α-Tocopherol | 0.3324 |
| 21 | 60.168 | Unidentified J | 0.3748 |
| 22 | 60.2195 | Unidentified K | 0.9207 |
| 23 | 60.3509 | 3-β-Ergost-5-en-3-ol | 1.5331 |
| 24 | 60.6538 | Stigmasterol | 5.3751 |
| 25 | 61.2311 | γ-Sitosterol | 13.2396 |
| 26 | 61.4254 | 3-methoxy-19-Norpregna-1,3,5(10)-trien-17-ol | 1.2401 |
| 27 | 64.94 | Unidentified L | 0.39 |
| RT | Compound | Area Max |
|---|---|---|
| 2.609 | DL-phenylalanine | 1.21 × 106 |
| 5.583 | DL-tryptophan | 1.10 × 106 |
| 23.857 | Tiliroside | 4.25 × 105 |
| 10.893 | 5-[(6,7,8-trimethoxy-4-quinazolinyl)amino]pentyl nitrate | 3.12 × 105 |
| 35.512 | 13-hydroperoxyoctadecadienoic acid | 1.95 × 105 |
| 14.915 | N-acetyl-L-phenylalanine | 1.12 × 105 |
| 18.865 | 3-amino-2-pyrazinecarboxylate | 1.04 × 105 |
| 18.598 | quercitrin | 7.35 × 104 |
| 13.603 | N-(4-{methyl[(1-methyl-1H-pyrazol-4-yl)methyl]sulfamoyl}phenyl)acetamide | 7.21 × 104 |
| 26.441 | Decyl hydrogen sulfate | 6.97 × 104 |
| 14.775 | Suberic acid | 6.73 × 104 |
| 19.007 | 9-hydroxynonanoic acid | 6.10 × 104 |
| 16.791 | L-acetyltryptophan | 5.12 × 104 |
| 14.082 | 5-(benzyloxy)-2-piperazinopyrimidine | 5.05 × 104 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Khoury, M.; Haykal, T.; Hodroj, M.H.; Najem, S.A.; Sarkis, R.; Taleb, R.I.; Rizk, S. Malva pseudolavatera Leaf Extract Promotes ROS Induction Leading to Apoptosis in Acute Myeloid Leukemia Cells In Vitro. Cancers 2020, 12, 435. https://doi.org/10.3390/cancers12020435
El Khoury M, Haykal T, Hodroj MH, Najem SA, Sarkis R, Taleb RI, Rizk S. Malva pseudolavatera Leaf Extract Promotes ROS Induction Leading to Apoptosis in Acute Myeloid Leukemia Cells In Vitro. Cancers. 2020; 12(2):435. https://doi.org/10.3390/cancers12020435
Chicago/Turabian StyleEl Khoury, Marianne, Tony Haykal, Mohammad H. Hodroj, Sonia Abou Najem, Rita Sarkis, Robin I. Taleb, and Sandra Rizk. 2020. "Malva pseudolavatera Leaf Extract Promotes ROS Induction Leading to Apoptosis in Acute Myeloid Leukemia Cells In Vitro" Cancers 12, no. 2: 435. https://doi.org/10.3390/cancers12020435