Is There an Interplay between Immune Checkpoint Inhibitors, Thromboprophylactic Treatments and Thromboembolic Events? Mechanisms and Impact in Non-Small Cell Lung Cancer Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Identification of TE Events and Anticoagulant and Antiplatelet Treatment
2.3. Study Objectives
2.4. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Clinical Characteristics and Risk Factors of TE Events
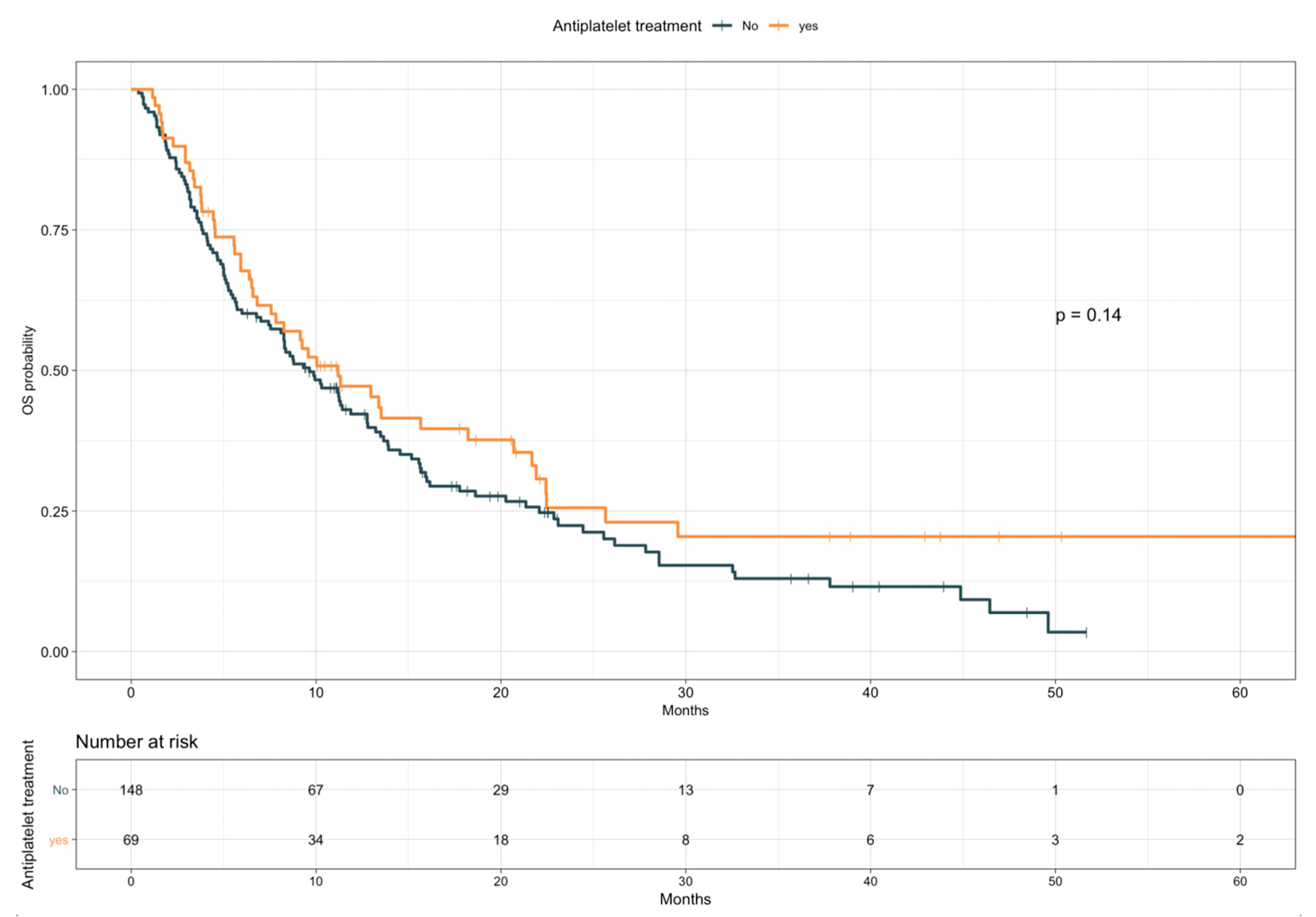
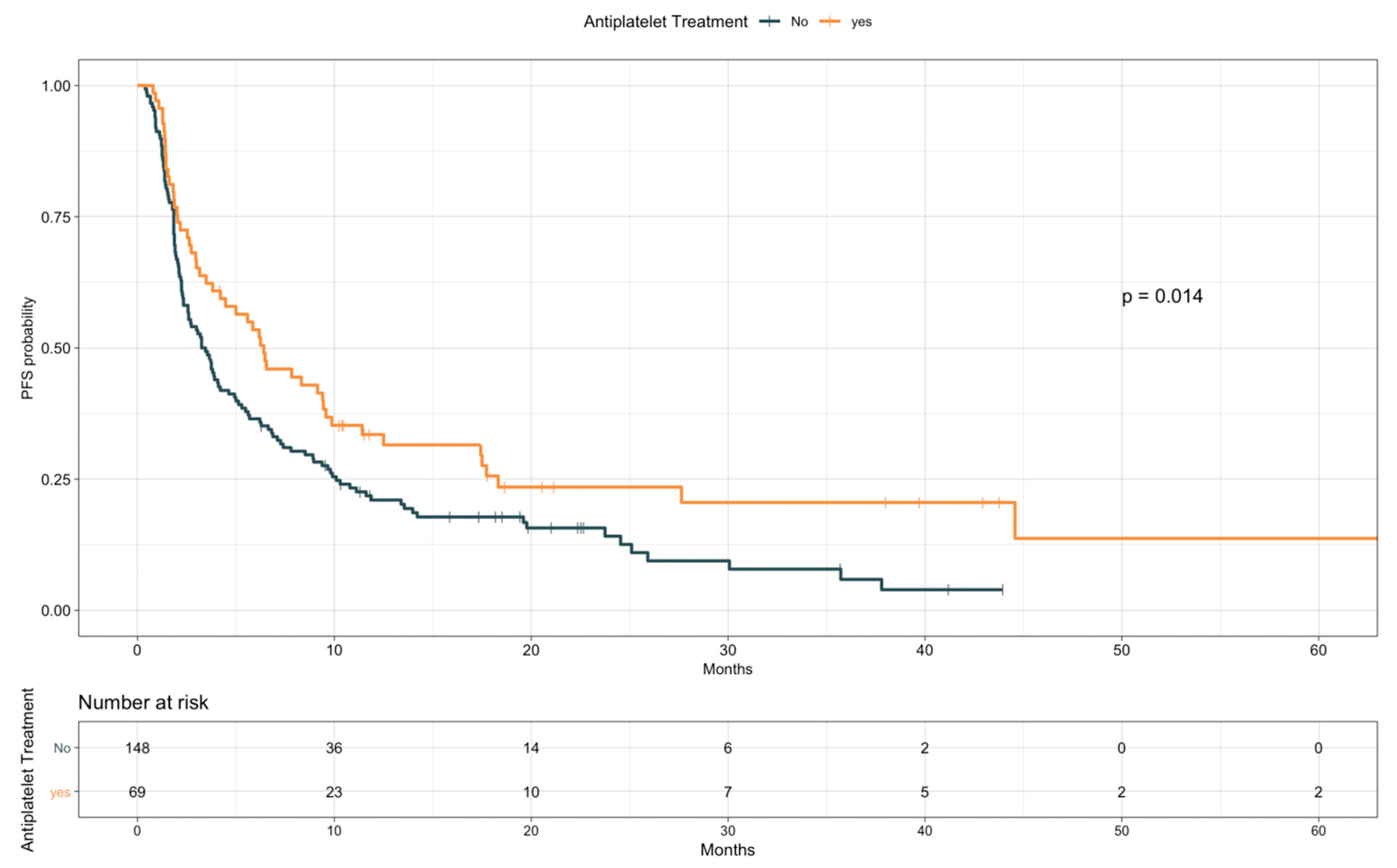
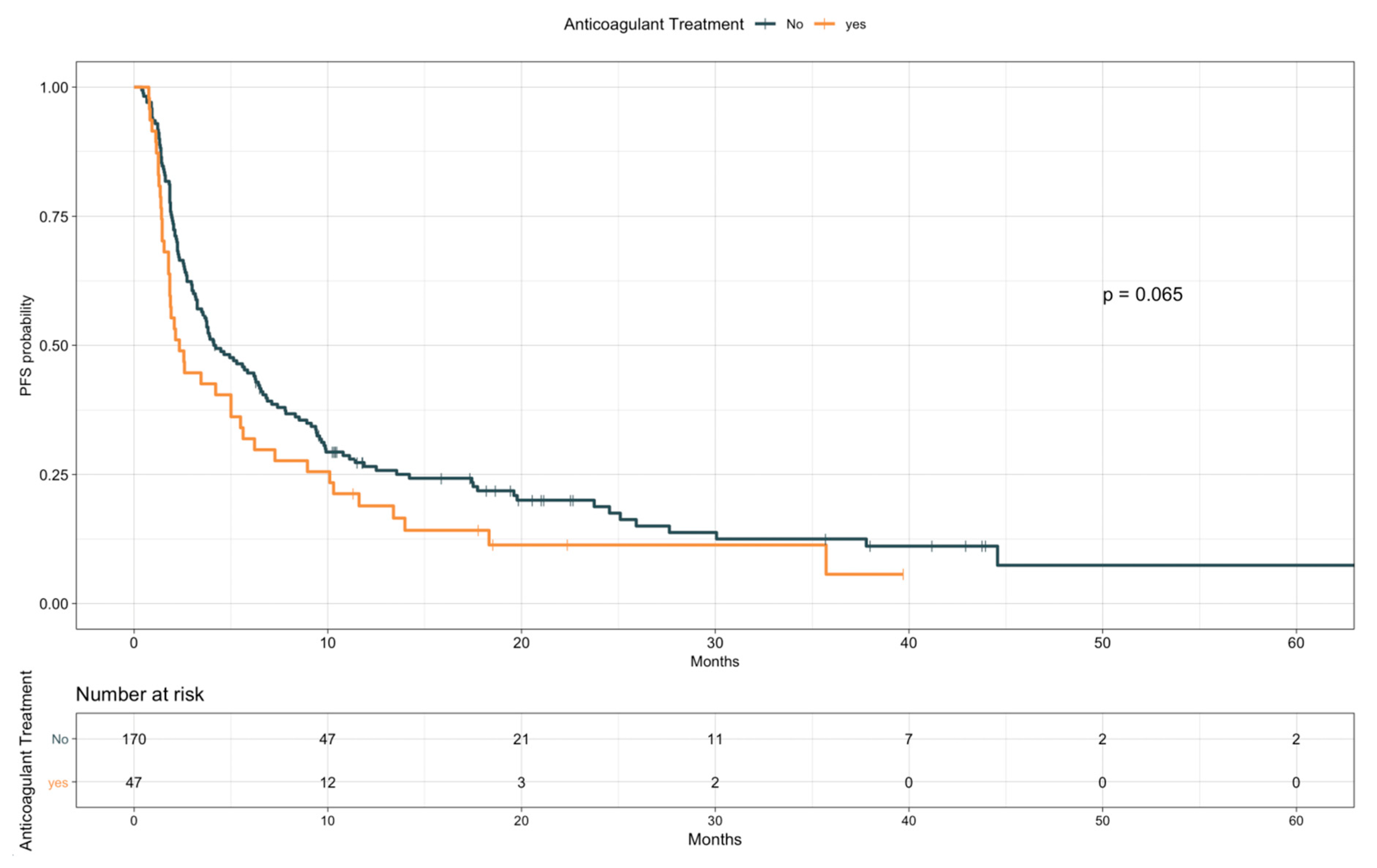
3.3. Impact of TE Events and Anticoagulant and Antiplatelet Treatments on Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations and Acronyms
| ASA | Aspirin |
| BMI | Body mass index |
| CI | Confidence interval |
| COX | Cyclooxygenase |
| COXi | COX Inhibitors |
| CT | Computerized tomography |
| CTLA-4 | Cytotoxic T-lymphocyte antigen 4 |
| ECOG PS | Eastern Cooperative Oncology Group Performance Status |
| HR | Hazards ratio |
| ICIs | Immune checkpoint inhibitors |
| irAEs | Immune-related adverse events |
| LDH | Lactate dehydrogenase |
| LIPI | Lung immune prognostic index |
| LMWH | Low molecular weight heparin |
| mPGES1 | Human prostaglandin E synthase 1 |
| NLR | Neutrophil to lymphocyte ratio |
| NSCLC | Non-small cell lung cancer |
| OS | Overall Survival |
| PD-1/PD-L1 | Programmed death-1/programmed death-ligand 1 |
| PFS | Progression-free survival |
| PGE2 | Prostaglandin E2 |
| PLR | Platelet to lymphocyte ratio |
| ROC | Receiver operating characteristic |
| TE | Thromboembolic |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Connolly, G.C.; Dalal, M.; Lin, J.; Khorana, A.A. Incidence and predictors of venous thromboembolism (VTE) among ambulatory patients with lung cancer. Lung Cancer 2012, 78, 253–258. [Google Scholar] [CrossRef]
- Lyman, G.H.; Eckert, L.; Wang, Y.; Wang, H.; Cohen, A. Venous thromboembolism risk in patients with cancer receiving chemotherapy: A real-world analysis. Oncologist 2013, 18, 1321–1329. [Google Scholar] [CrossRef] [Green Version]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef]
- Garassino, M.C.; Cho, B.C.; Kim, J.H.; Mazieres, J.; Vansteenkiste, J.; Lena, H.; Corral Jaime, J.; Gray, J.E.; Powderly, J.; Chouaid, C.; et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): An open-label, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 521–536. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crino, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [Green Version]
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Perez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Reck, M.; Rodriguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csoszi, T.; Fulop, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [Green Version]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, S.; Yang, F.; Qi, X.; Wang, X.; Guan, X.; Shen, C.; Duma, N.; Vera Aguilera, J.; Chintakuntlawar, A.; et al. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-analysis. JAMA Oncol. 2019. [Google Scholar] [CrossRef]
- Lyon, A.R.; Yousaf, N.; Battisti, N.M.L.; Moslehi, J.; Larkin, J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018, 19, e447–e458. [Google Scholar] [CrossRef]
- Bu, D.X.; Tarrio, M.; Maganto-Garcia, E.; Stavrakis, G.; Tajima, G.; Lederer, J.; Jarolim, P.; Freeman, G.J.; Sharpe, A.H.; Lichtman, A.H. Impairment of the programmed cell death-1 pathway increases atherosclerotic lesion development and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1100–1107. [Google Scholar] [CrossRef] [Green Version]
- Cochain, C.; Chaudhari, S.M.; Koch, M.; Wiendl, H.; Eckstein, H.H.; Zernecke, A. Programmed cell death-1 deficiency exacerbates T cell activation and atherogenesis despite expansion of regulatory T cells in atherosclerosis-prone mice. PLoS ONE 2014, 9, e93280. [Google Scholar] [CrossRef] [Green Version]
- Navi, B.B.; Reiner, A.S.; Kamel, H.; Iadecola, C.; Okin, P.M.; Tagawa, S.T.; Panageas, K.S.; DeAngelis, L.M. Arterial thromboembolic events preceding the diagnosis of cancer in older persons. Blood 2019, 133, 781–789. [Google Scholar] [CrossRef]
- Niers, T.M.; Klerk, C.P.; DiNisio, M.; Van Noorden, C.J.; Buller, H.R.; Reitsma, P.H.; Richel, D.J. Mechanisms of heparin induced anti-cancer activity in experimental cancer models. Crit. Rev. Oncol. Hematol. 2007, 61, 195–207. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.L.; Ma, K.X.; Qu, J.M. Efficacy and safety of adjunctive anticoagulation in patients with lung cancer without indication for anticoagulants: A systematic review and meta-analysis. Thorax 2013, 68, 442–450. [Google Scholar] [CrossRef] [Green Version]
- Macbeth, F.; Noble, S.; Evans, J.; Ahmed, S.; Cohen, D.; Hood, K.; Knoyle, D.; Linnane, S.; Longo, M.; Moore, B.; et al. Randomized Phase III Trial of Standard Therapy Plus Low Molecular Weight Heparin in Patients With Lung Cancer: FRAGMATIC Trial. J. Clin. Oncol. 2016, 34, 488–494. [Google Scholar] [CrossRef]
- Ek, L.; Gezelius, E.; Bergman, B.; Bendahl, P.O.; Anderson, H.; Sundberg, J.; Wallberg, M.; Falkmer, U.; Verma, S.; Belting, M.; et al. Randomized phase III trial of low-molecular-weight heparin enoxaparin in addition to standard treatment in small-cell lung cancer: The RASTEN trial. Ann. Oncol. 2018, 29, 398–404. [Google Scholar] [CrossRef]
- Zelenay, S.; van der Veen, A.G.; Bottcher, J.P.; Snelgrove, K.J.; Rogers, N.; Acton, S.E.; Chakravarty, P.; Girotti, M.R.; Marais, R.; Quezada, S.A.; et al. Cyclooxygenase-Dependent Tumor Growth through Evasion of Immunity. Cell 2015, 162, 1257–1270. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Roszik, J.; Cho, S.N.; Ogata, D.; Milton, D.R.; Peng, W.; Menter, D.G.; Ekmekcioglu, S.; Grimm, E.A. The COX2 Effector Microsomal PGE2 Synthase 1 is a Regulator of Immunosuppression in Cutaneous Melanoma. Clin. Cancer Res. 2019, 25, 1650–1663. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wenes, M.; Romero, P.; Huang, S.C.; Fendt, S.M.; Ho, P.C. Navigating metabolic pathways to enhance antitumour immunity and immunotherapy. Nat. Rev. Clin. Oncol. 2019. [Google Scholar] [CrossRef]
- Botti, G.; Fratangelo, F.; Cerrone, M.; Liguori, G.; Cantile, M.; Anniciello, A.M.; Scala, S.; D’Alterio, C.; Trimarco, C.; Ianaro, A.; et al. COX-2 expression positively correlates with PD-L1 expression in human melanoma cells. J. Transl. Med. 2017, 15, 46. [Google Scholar] [CrossRef] [Green Version]
- Hamada, T.; Cao, Y.; Qian, Z.R.; Masugi, Y.; Nowak, J.A.; Yang, J.; Song, M.; Mima, K.; Kosumi, K.; Liu, L.; et al. Aspirin Use and Colorectal Cancer Survival According to Tumor CD274 (Programmed Cell Death 1 Ligand 1) Expression Status. J. Clin. Oncol. 2017, 35, 1836–1844. [Google Scholar] [CrossRef]
- Edelman, M.J.; Wang, X.; Hodgson, L.; Cheney, R.T.; Baggstrom, M.Q.; Thomas, S.P.; Gajra, A.; Bertino, E.; Reckamp, K.L.; Molina, J.; et al. Phase III Randomized, Placebo-Controlled, Double-Blind Trial of Celecoxib in Addition to Standard Chemotherapy for Advanced Non-Small-Cell Lung Cancer With Cyclooxygenase-2 Overexpression: CALGB 30801 (Alliance). J. Clin. Oncol. 2017, 35, 2184–2192. [Google Scholar] [CrossRef]
- Groen, H.J.; Sietsma, H.; Vincent, A.; Hochstenbag, M.M.; van Putten, J.W.; van den Berg, A.; Dalesio, O.; Biesma, B.; Smit, H.J.; Termeer, A.; et al. Randomized, placebo-controlled phase III study of docetaxel plus carboplatin with celecoxib and cyclooxygenase-2 expression as a biomarker for patients with advanced non-small-cell lung cancer: The NVALT-4 study. J. Clin. Oncol. 2011, 29, 4320–4326. [Google Scholar] [CrossRef]
- Edelman, M.J.; Tan, M.T.; Fidler, M.J.; Sanborn, R.E.; Otterson, G.; Sequist, L.V.; Evans, T.L.; Schneider, B.J.; Keresztes, R.; Rogers, J.S.; et al. Randomized, double-blind, placebo-controlled, multicenter phase II study of the efficacy and safety of apricoxib in combination with either docetaxel or pemetrexed in patients with biomarker-selected non-small-cell lung cancer. J. Clin. Oncol. 2015, 33, 189–194. [Google Scholar] [CrossRef]
- Wang, S.-J.; Khullar, K.; Yegya-Raman, N.; Kim, S.; Silk, A.W.; Malhotra, J.; Gentile, M.A.; Mehnert, J.M.; Jabbour, S.K. Cyclooxygenase inhibitor use during checkpoint blockade immunotherapy and effect on time to progression for metastatic melanoma patients. J. Clin. Oncol. 2019, 37. [Google Scholar] [CrossRef]
- Yoshimatsu, K.; Altorki, N.K.; Golijanin, D.; Zhang, F.; Jakobsson, P.J.; Dannenberg, A.J.; Subbaramaiah, K. Inducible prostaglandin E synthase is overexpressed in non-small cell lung cancer. Clin. Cancer Res. 2001, 7, 2669–2674. [Google Scholar]
- Khorana, A.A.; Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008, 111, 4902–4907. [Google Scholar] [CrossRef] [Green Version]
- Grilz, E.; Posch, F.; Konigsbrugge, O.; Schwarzinger, I.; Lang, I.M.; Marosi, C.; Pabinger, I.; Ay, C. Association of Platelet-to-Lymphocyte Ratio and Neutrophil-to-Lymphocyte Ratio with the Risk of Thromboembolism and Mortality in Patients with Cancer. Thromb. Haemost. 2018, 118, 1875–1884. [Google Scholar] [CrossRef]
- Conner, S.C.; Trinquart, L. Survivorship Bias in Analyses of Immune Checkpoint Inhibitor Trials. JAMA Oncol. 2019. [Google Scholar] [CrossRef]
- Blom, J.W.; Osanto, S.; Rosendaal, F.R. The risk of a venous thrombotic event in lung cancer patients: Higher risk for adenocarcinoma than squamous cell carcinoma. J. Thromb. Haemost. 2004, 2, 1760–1765. [Google Scholar] [CrossRef] [Green Version]
- Walker, A.J.; Baldwin, D.R.; Card, T.R.; Powell, H.A.; Hubbard, R.B.; Grainge, M.J. Risk of venous thromboembolism in people with lung cancer: A cohort study using linked UK healthcare data. Br. J. Cancer 2017, 116, e1. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, M.; Pichon, E.; Carmier, D.; Bouquet, E.; Pageot, C.; Bejan-Angoulvant, T.; Campana, M.; Vermes, E.; Marchand-Adam, S. Coronary Toxicities of Anti-PD-1 and Anti-PD-L1 Immunotherapies: A Case Report and Review of the Literature and International Registries. Target. Oncol. 2018, 13, 509–515. [Google Scholar] [CrossRef]
- Tomita, Y.; Sueta, D.; Kakiuchi, Y.; Saeki, S.; Saruwatari, K.; Sakata, S.; Jodai, T.; Migiyama, Y.; Akaike, K.; Hirosako, S.; et al. Acute coronary syndrome as a possible immune-related adverse event in a lung cancer patient achieving a complete response to anti-PD-1 immune checkpoint antibody. Ann. Oncol. 2017, 28, 2893–2895. [Google Scholar] [CrossRef]
- Boutros, C.; Scoazec, J.Y.; Mateus, C.; Routier, E.; Roy, S.; Robert, C. Arterial thrombosis and anti-PD-1 blockade. Eur. J. Cancer 2018, 91, 164–166. [Google Scholar] [CrossRef]
- Roopkumar, J.; Kim, A.S.; Bicky, T.; Hobbs, B.P.; Khorana, A.A. Venous Thromboembolism in Cancer Patients Receiving Immunotherapy. Blood 2018, 132, 2510. [Google Scholar] [CrossRef]
- Chew, H.K.; Wun, T.; Harvey, D.; Zhou, H.; White, R.H. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch. Int. Med. 2006, 166, 458–464. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Kang, D.; Yang, D.; Tang, Y. Activation of PGE2/EP2 and PGE2/EP4 signaling pathways positively regulate the level of PD-1 in infiltrating CD8+ T cells in patients with lung cancer. Oncol. Lett. 2018, 15, 552–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verso, M.; Agnelli, G.; Barni, S.; Gasparini, G.; LaBianca, R. A modified Khorana risk assessment score for venous thromboembolism in cancer patients receiving chemotherapy: The Protecht score. Int. Emerg. Med. 2012, 7, 291–292. [Google Scholar] [CrossRef] [PubMed]
- Agnelli, G.; Gussoni, G.; Bianchini, C.; Verso, M.; Mandala, M.; Cavanna, L.; Barni, S.; Labianca, R.; Buzzi, F.; Scambia, G.; et al. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: A randomised, placebo-controlled, double-blind study. Lancet Oncol. 2009, 10, 943–949. [Google Scholar] [CrossRef]
- Carrier, M.; Abou-Nassar, K.; Mallick, R.; Tagalakis, V.; Shivakumar, S.; Schattner, A.; Kuruvilla, P.; Hill, D.; Spadafora, S.; Marquis, K.; et al. Apixaban to Prevent Venous Thromboembolism in Patients with Cancer. N. Engl. J. Med. 2019, 380, 711–719. [Google Scholar] [CrossRef]
- Khorana, A.A.; Soff, G.A.; Kakkar, A.K.; Vadhan-Raj, S.; Riess, H.; Wun, T.; Streiff, M.B.; Garcia, D.A.; Liebman, H.A.; Belani, C.P.; et al. Rivaroxaban for Thromboprophylaxis in High-Risk Ambulatory Patients with Cancer. N. Engl. J. Med. 2019, 380, 720–728. [Google Scholar] [CrossRef]
- Sharma, S.; Yang, S.C.; Zhu, L.; Reckamp, K.; Gardner, B.; Baratelli, F.; Huang, M.; Batra, R.K.; Dubinett, S.M. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res. 2005, 65, 5211–5220. [Google Scholar] [CrossRef] [Green Version]
- Veltman, J.D.; Lambers, M.E.; van Nimwegen, M.; Hendriks, R.W.; Hoogsteden, H.C.; Aerts, J.G.; Hegmans, J.P. COX-2 inhibition improves immunotherapy and is associated with decreased numbers of myeloid-derived suppressor cells in mesothelioma. Celecoxib influences MDSC function. BMC Cancer 2010, 10, 464. [Google Scholar] [CrossRef] [Green Version]
- Hennequart, M.; Pilotte, L.; Cane, S.; Hoffmann, D.; Stroobant, V.; Plaen, E.; Van den Eynde, B.J. Constitutive IDO1 Expression in Human Tumors Is Driven by Cyclooxygenase-2 and Mediates Intrinsic Immune Resistance. Cancer Immunol. Res. 2017, 5, 695–709. [Google Scholar] [CrossRef] [Green Version]
- Prima, V.; Kaliberova, L.N.; Kaliberov, S.; Curiel, D.T.; Kusmartsev, S. COX2/mPGES1/PGE2 pathway regulates PD-L1 expression in tumor-associated macrophages and myeloid-derived suppressor cells. Proc. Natl. Acad. Sci. USA 2017, 114, 1117–1122. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, K.; Okita, R.; Saisho, S.; Maeda, A.; Nojima, Y.; Nakata, M. Prognostic value of Cox-2 and PD-L1 expression and its relationship with tumor-infiltrating lymphocytes in resected lung adenocarcinoma. Cancer Manag. Res. 2017, 9, 741–750. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.; Liu, Y.; Wang, C.; Zheng, H.; Chen, Y.; Liu, W.; Chen, X.; Zhang, J.; Chen, H.; Yang, Y.; et al. Inhibition of COX-2 and EGFR by Melafolone Improves Anti-PD-1 Therapy through Vascular Normalization and PD-L1 Downregulation in Lung Cancer. J. Pharmacol. Exp. Ther. 2019, 368, 401–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, K.; Okita, R.; Saisho, S.; Maeda, A.I.; Nojima, Y.; Nakata, M. Impact of COX2 Inhibitor for Regulation of PD-L1 Expression in Non-small Cell Lung Cancer. Anticancer Res. 2018, 38, 4637–4644. [Google Scholar] [CrossRef] [PubMed]
- Veglia, F.; Tyurin, V.A.; Blasi, M.; De Leo, A.; Kossenkov, A.V.; Donthireddy, L.; To, T.K.J.; Schug, Z.; Basu, S.; Wang, F.; et al. Fatty acid transport protein 2 reprograms neutrophils in cancer. Nature 2019, 569, 73–78. [Google Scholar] [CrossRef]
- Ng, T.L.; Smith, D.E.; Mushtaq, R.; Patil, T.; Dimou, A.; Yang, S.; Liu, Q.; Li, X.; Zhou, C.; Jones, R.T.; et al. ROS1 Gene Rearrangements Are Associated With an Elevated Risk of Peridiagnosis Thromboembolic Events. J. Thorac. Oncol. 2019, 14, 596–605. [Google Scholar] [CrossRef] [Green Version]
- Dou, F.; Li, H.; Zhu, M.; Liang, L.; Zhang, Y.; Yi, J.; Zhang, Y. Association between oncogenic status and risk of venous thromboembolism in patients with non-small cell lung cancer. Respir. Res. 2018, 19, 88. [Google Scholar] [CrossRef] [Green Version]
- Zugazagoitia, J.; Biosca, M.; Oliveira, J.; Olmedo, M.E.; Domine, M.; Nadal, E.; Ruffinelli, J.C.; Munoz, N.; Luna, A.M.; Hernandez, B.; et al. Incidence, predictors and prognostic significance of thromboembolic disease in patients with advanced ALK-rearranged non-small cell lung cancer. Eur. Respir. J. 2018, 51. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, L.; Rodriguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gumus, M.; Mazieres, J.; Hermes, B.; Cay Senler, F.; Csoszi, T.; Fulop, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef]
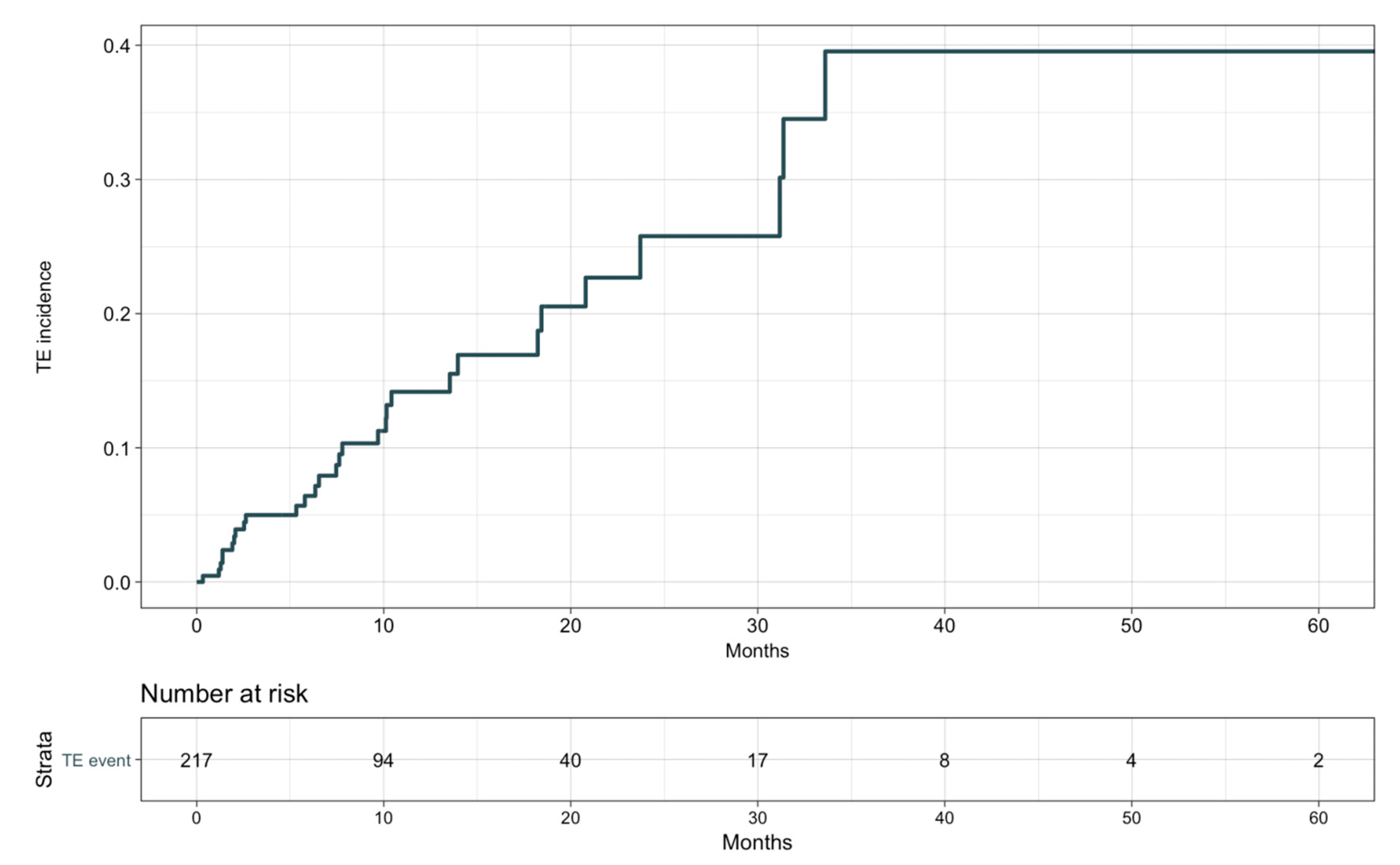

| Characteristic | Overall n = 217 | Without TE n = 187 | With TE n = 30 |
|---|---|---|---|
| Sex, Male | 136 (62.7) | 115 (61.5) | 21 (70.0) |
| Age, median (range), y | 70 (32–90) | 70 (32–90) | 70 (56–83) |
| ≤65 | 77 (35.5) | 66 (35.3) | 11 (36.7) |
| >65 | 140 (64.5) | 121 (64.7) | 19 (63.3) |
| ECOG PS | |||
| 0–1 | 198 (91.2) | 168 (89.8) | 30 (100.0) |
| ≥2 | 19 (8.8) | 19 (10.2) | \ |
| Smoking * | |||
| Never or former | 154 (71.0) | 138 (76.7) | 16 (57.1) |
| Current | 54 (24.9) | 42 (23.3) | 12 (42.9) |
| Pathologic subtype | |||
| Non-squamous | 168 (77.4) | 146 (78.1) | 22 (73.3) |
| Squamous | 49 (22.6) | 41 (21.9) | 8 (26.7) |
| Molecular status x | |||
| EGFR mutation | 11 (5.1) | 10 (6.3) | 1 (3.7) |
| ALK translocation | 2 (0.9) | 1 (0.7) | 1 (3.8) |
| PD-L1 status # | |||
| ≤50% | 89 (41.0) | 79 (42.2) | 10 (33.3) |
| >50% | 48 (22.1) | 35 (18.8) | 13 (43.3) |
| Not assessed | 80 (36.9) | 73 (39.0) | 7 (23.4) |
| Previous Tx with TKI | 18 (8.3) | 15 (8.0) | 3 (10.0) |
| Disease stage | |||
| Locally advanced | 10 (4.6) | 8 (4.3) | 2 (6.7) |
| Metastatic | 207 (95.4) | 179 (95.7) | 28 (93.3) |
| Number of disease sites ** | |||
| ≤2 | 98 (45.2) | 80 (42.8) | 18 (60.0) |
| >2 | 109 (50.2) | 99 (52.9) | 10 (33.3) |
| Brain metastases | 44 (20.3) | 39 (20.9) | 5 (16.7) |
| Liver metastases | 44 (20.3) | 39 (20.9) | 5 (16.7) |
| Comorbidities | |||
| Arterial hypertension | 44 (20.3) | 38 (20.3) | 6 (20.0) |
| COPD | 25 (11.5) | 27 (11.8) | 3 (10.0) |
| Diabetes mellitus | 21 (9.7) | 18 (9.6) | 3 (10.0) |
| Previous ACS | 18 (8.3) | 13 (7.0) | 5 (16.7) |
| Previous stroke | 9 (4.1) | 6 (3.2) | 3 (10.0) |
| Atrial fibrillation | 10 (4.6) | 10 (5.4) | \ |
| Previous venous TE events | 37 (17.1) | 31 (16.6) | 6 (20.0) |
| BMI xx | |||
| >25 | 88 (40.6) | 71 (47.3) | 17 (68.0) |
| >30 | 41 (18.9) | 34 (18.2) | 7 (23.3) |
| Antiplatelet treatment | 69 (31.8) | 56 (29.9) | 13 (43.3) |
| ASA based treatment | 61 (28.1) | 49 (26.2) | 12 (40.0) |
| Anticoagulant treatment | 47 (21.7) | 42 (22.5) | 5 (16.7) |
| Therapeutic dosage | 8 (3.7) | 7 (3.7) | 1 (3.3) |
| Characteristic | Overall n = 217 | Without TE n = 187 | With TE n = 30 |
|---|---|---|---|
| Line of Treatment | |||
| <2 | 55 (25.4) | 51 (27.3) | 4 (13.3) |
| ≥2 | 162 (74.6) | 136 (72.7) | 26 (86.7) |
| Drug Class | |||
| Anti-PD-1 | 151 (69.6) | 133 (71.1) | 18 (60.0) |
| Anti-PD-L1 | 58 (26.7) | 46 (24.6) | 12 (40.0) |
| Anti PD-L1 + Anti CTLA-4 | 8 (3.7) | 8 (4.3) | \ |
| No. of administered cycles, median (IQR) | 7 (3–19) | 6 (3–16) | 20 (9–31) |
| Treatment duration, median (IQR, months) | 3.6 (1.5–10.3) | 2.9 (1.4–9.0) | 9.4 (5.4–21.7) |
| Best Response | |||
| Complete Response | 5 (2.3) | 4 (2.1) | 1 (3.3) |
| Partial Response | 36 (16.6) | 26 (13.9) | 10 (33.3) |
| Stable Disease | 78 (35.9) | 64 (34.2) | 14 (46.7) |
| Progressive Disease | 98 (45.2) | 93 (49.7) | 5 (16.7) |
| Objective Response | 41 (18.9) | 30 (16.0) | 11 (36.7) |
| Disease Control | 119 (54.8) | 94 (50.3) | 25 (83.3) |
| Adverse Events ≥2 (other than TE) | |||
| Anemia | 6 (2.8) | 4 (2.1) | 2 (6.7) |
| Thrombocytopenia | 2 (0.9) | 2 (1.1) | \ |
| Fatigue | 10 (4.6) | 9 (4.8) | 1 (3.3) |
| Liver toxicity | 10 (4.6) | 8 (4.3) | 2 (6.7) |
| Arthralgia | 4 (1.8) | 4 (2.1) | \ |
| Colitis/diarrhea | 7 (3.2) | 7 (3.7) | \ |
| Hyper/hypothyroidism | 9 (4.1) | 7 (3.7) | 2 (6.7) |
| Pneumonitis | 9 (4.1) | 8 (4.3) | 1 (3.3) |
| Pruritus or skin rash | 8 (3.7) | 6 (3.2) | 2 (6.7) |
| Variables | Subgroups | HR | 95% CI | p |
|---|---|---|---|---|
| Sex | Male vs. female | 1.73 | 0.79–3.79 | 0.17 |
| Age | >65 vs. ≤65 | 0.97 | 0.46–2.03 | 0.93 |
| Smoking | Current vs. never/former | 2.48 | 1.17–5.26 | 0.02 |
| ECOG PS * | ≥1 vs. 0 | 1.12 | 0.54–2.35 | 0.76 |
| PD-L1 status | >50% vs. <50% | 2.16 | 0.94–4.95 | 0.07 |
| Pathologic subtype | Squamous vs. non-squamous | 1.52 | 0.68–3.43 | 0.31 |
| Number of disease sites | >2 vs. ≤2 | 0.52 | 0.24–1.12 | 0.09 |
| Brain metastases | Yes vs. no | 0.74 | 0.28–1.93 | 0.53 |
| Liver metastases | Yes vs. no | 0.74 | 0.28–1.93 | 0.53 |
| BMI | >25 vs. ≤25 | 1.98 | 0.85–4.59 | 0.11 |
| >30 vs. ≤30 | 1.18 | 0.49–2.82 | 0.71 | |
| Previous venous TEs | Yes vs. no | 1.12 | 0.46–2.75 | 0.80 |
| Khorana score | 2 vs. 1 | 1.37 | 0.58–3.20 | 0.47 |
| 3 vs. 1 | 1.75 | 0.64–4.78 | 0.28 | |
| Antiplatelet treatment | Yes vs. no | 1.57 | 0.76–3.25 | 0.22 |
| Anticoagulant treatment | Yes vs. no | 0.86 | 0.33–2.25 | 0.75 |
| Drug class | Anti-PD-1 vs. anti-PD-L1 (+/−anti CTLA4) | 1.23 | 0.59–2.55 | 0.59 |
| Line of treatment | ≥2 vs. <2 | 2.34 | 0.81–6.72 | 0.11 |
| LDH | >480 vs. ≤480 U/L | 2.01 | 0.85–4.73 | 0.11 |
| NLR | >3.2 vs. ≤3.2 | 0.80 | 0.38–1.66 | 0.54 |
| PLR | >181 vs. ≤181 | 0.42 | 0.19–0.92 | 0.03 |
| Variables | Subgroups | HR | 95% CI | p |
|---|---|---|---|---|
| Smoking | Current vs. never/former | 3.61 | 1.52–8.60 | 0.004 |
| PD-L1 status | >50% vs. <50% | 2.55 | 1.05–6.19 | 0.038 |
| PLR | > 181 vs. ≤181 | 0.53 | 0.21–1.33 | 0.178 |
| Variables | Subgroups | HR | 95% CI | p |
|---|---|---|---|---|
| TE events | Yes vs. no, time independent | 0.59 | 0.37–0.93 | 0.023 |
| Yes vs. no, time dependent | 1.70 | 1.06–2.72 | 0.029 | |
| Anticoagulant treatment | Yes vs. no | 1.21 | 0.84–1.73 | 0.31 |
| Antiplatelet treatment | Yes vs. no | 0.78 | 0.55–1.09 | 0.14 |
| Sex | Male vs. female | 1.26 | 0.92–1.73 | 0.16 |
| Age | >65 vs. ≤65 | 1.06 | 0.77–1.45 | 0.74 |
| Smoking | Current vs. never/former | 0.89 | 0.62–1.28 | 0.53 |
| ECOG PS | ≥2 vs. 0–1 | 3.71 | 2.21–6.25 | <0.0001 |
| PD-L1 status | >50% vs. ≤50% | 0.48 | 0.30–0.76 | 0.0016 |
| Number of disease sites | >2 vs. ≤2 | 1.05 | 0.77–1.43 | 0.74 |
| Brain metastases | Yes vs. no | 1.04 | 0.71–1.51 | 0.85 |
| Liver metastases | Yes vs. no | 1.38 | 0.95–1.98 | 0.09 |
| Line of treatment | ≥2 vs. <2 | 1.63 | 1.09–2.43 | 0.018 |
| BMI | >25 vs. ≤25 | 0.92 | 0.65–1.30 | 0.64 |
| >30 vs. ≤30 | 1.20 | 0.82–1.77 | 0.35 | |
| LIPI | High vs. low | 3.44 | 2.11–5.62 | <0.0001 |
| Intermediate vs. low | 1.89 | 1.29–2.75 | ||
| PLR | 311.18 vs. 145.73 | 1.17 | 1.05–1.31 | 0.005 |
| Variables | Subgroups | HR | 95% CI | p |
|---|---|---|---|---|
| TE events | Yes vs. no, time dependent | 2.93 | 1.59–5.42 | 0.0006 |
| ECOG PS | ≥2 vs. 0–1 | 3.29 | 1.50–7.21 | 0.0029 |
| PD-L1 status | >50% vs. ≤50% | 0.36 | 0.21–0.64 | 0.0004 |
| Line of treatment | ≥2 vs. <2 | 1.64 | 0.93–2.91 | 0.0889 |
| LIPI | High vs. low | 3.14 | 1.58–6.27 | 0.0001 |
| Intermediate vs. low | 3.14 | 1.82–5.40 |
| Variables | Subgroups | HR | 95% CI | p |
|---|---|---|---|---|
| Anticoagulant treatment | Yes vs. no | 1.38 | 0.98–1.96 | 0.065 |
| Antiplatelet treatment | Yes vs. no | 0.67 | 0.48–0.92 | 0.014 |
| Sex | Male vs. female | 1.08 | 0.80–1.46 | 0.62 |
| Age | >65 vs. ≤65 | 0.91 | 0.67–1.23 | 0.54 |
| Smoking | Current vs. never/former | 0.79 | 0.56–1.11 | 0.18 |
| ECOG PS | ≥2 vs. 0–1 | 2.55 | 1.57–4.13 | 0.0001 |
| PD-L1 status | >50% vs. ≤50% | 0.40 | 0.26–0.61 | <0.0001 |
| Number of disease sites | >2 vs. ≤2 | 1.00 | 0.75–1.35 | 0.99 |
| Brain metastases | Yes vs. no | 1.05 | 0.73–1.50 | 0.80 |
| Liver metastases | Yes vs. no | 1.44 | 1.02–2.05 | 0.041 |
| Line of Treatment | ≥2 vs. <2 | 1.78 | 1.23–2.57 | 0.002 |
| BMI | >25 vs. ≤25 | 0.78 | 0.56–1.09 | 0.15 |
| >30 vs. ≤30 | 1.12 | 0.77–1.63 | 0.56 | |
| LIPI | High vs. low | 2.80 | 1.76–4.47 | 0.0001 |
| Intermediate vs. low | 1.59 | 1.11–2.26 | ||
| PLR | 311.18 vs. 145.73 | 1.35 | 1.18–1.55 | <0.0001 |
| Variables | Subgroups | HR | 95% CI | p |
|---|---|---|---|---|
| Antiplatelet treatment | Yes vs. no | 0.96 | 0.59–1.57 | 0.88 |
| Anticoagulant treatment | Yes vs. No | 1.38 | 0.82–2.32 | 0.22 |
| ECOG PS | ≥2 vs. 0–1 | 2.01 | 0.94–4.32 | 0.073 |
| Liver metastases | Yes vs. no | 1.10 | 0.64–1.89 | 0.73 |
| PD-L1 status | >50% vs. ≤50% | 0.41 | 0.25–0.67 | 0.0004 |
| Line of treatment | ≥2 vs. <2 | 1.79 | 1.07–2.98 | 0.025 |
| LIPI | High vs. low | 2.74 | 1.41–5.31 | 0.0039 |
| Intermediate vs. low | 2.04 | 1.25–3.32 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nichetti, F.; Ligorio, F.; Zattarin, E.; Signorelli, D.; Prelaj, A.; Proto, C.; Galli, G.; Marra, A.; Apollonio, G.; Porcu, L.; et al. Is There an Interplay between Immune Checkpoint Inhibitors, Thromboprophylactic Treatments and Thromboembolic Events? Mechanisms and Impact in Non-Small Cell Lung Cancer Patients. Cancers 2020, 12, 67. https://doi.org/10.3390/cancers12010067
Nichetti F, Ligorio F, Zattarin E, Signorelli D, Prelaj A, Proto C, Galli G, Marra A, Apollonio G, Porcu L, et al. Is There an Interplay between Immune Checkpoint Inhibitors, Thromboprophylactic Treatments and Thromboembolic Events? Mechanisms and Impact in Non-Small Cell Lung Cancer Patients. Cancers. 2020; 12(1):67. https://doi.org/10.3390/cancers12010067
Chicago/Turabian StyleNichetti, Federico, Francesca Ligorio, Emma Zattarin, Diego Signorelli, Arsela Prelaj, Claudia Proto, Giulia Galli, Antonio Marra, Giulia Apollonio, Luca Porcu, and et al. 2020. "Is There an Interplay between Immune Checkpoint Inhibitors, Thromboprophylactic Treatments and Thromboembolic Events? Mechanisms and Impact in Non-Small Cell Lung Cancer Patients" Cancers 12, no. 1: 67. https://doi.org/10.3390/cancers12010067






