The Tumor Suppressor Roles of MYBBP1A, a Major Contributor to Metabolism Plasticity and Stemness
Abstract
:1. Introduction
2. Tumor Suppressor Roles of MYBBP1A
2.1. MYB Binding Protein 1A
2.2. Regulation of MYBBP1A
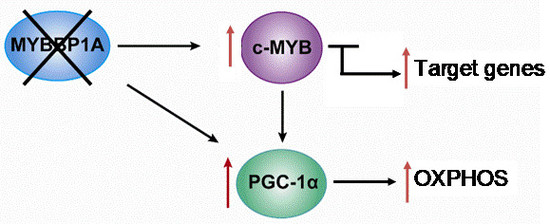
2.3. MYBBP1A as a Regulator of Transcription Factors
2.4. MYBBP1A and Epigenetic Regulation
2.5. Role of MYBBP1A in Cell Cycle and Mitosis
2.6. Role of MYBBP1A in Senescence
2.7. Role of MYBBP1A in the Regulation of Intracellular Energy Status
2.8. Role of MYBBP1A in the Synthesis of Ribosomes
2.9. MYBBP1A and Cancer
2.10. VHL, the Third Player
3. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Tavner, F.J.; Simpson, R.; Tashiro, S.; Favier, D.; Jenkins, N.A.; Gilbert, D.J.; Copeland, N.G.; Macmillan, E.M.; Lutwyche, J.; Keough, R.A.; et al. Molecular cloning reveals that the p160 Myb-binding protein is a novel, predominantly nucleolar protein which may play a role in transactivation by Myb. Mol. Cell Biol. 1998, 18, 989–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumazawa, T.; Nishimura, K.; Kuroda, T.; Ono, W.; Yamaguchi, C.; Katagiri, N.; Tsuchiya, M.; Masumoto, H.; Nakajima, Y.; Murayama, A.; et al. Novel nucleolar pathway connecting intracellular energy status with p53 activation. J. Biol. Chem. 2011, 286, 20861–20869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamauchi, T.; Keough, R.A.; Gonda, T.J.; Ishii, S. Ribosomal stress induces processing of Mybbp1a and its translocation from the nucleolus to the nucleoplasm. Genes Cells 2008, 13, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Kawasaki, Y.; Hiraga, S.; Tawaramoto, M.; Nakashima, N.; Sugino, A. The fifth essential DNA polymerase phi in Saccharomyces cerevisiae is localized to the nucleolus and plays an important role in synthesis of rRNA. Proc. Natl. Acad. Sci. USA 2002, 99, 9133–9138. [Google Scholar] [CrossRef] [Green Version]
- Mori, S.; Bernardi, R.; Laurent, A.; Resnati, M.; Crippa, A.; Gabrieli, A.; Keough, R.; Gonda, T.J.; Blasi, F. Myb-binding protein 1A (MYBBP1A) is essential for early embryonic development, controls cell cycle and mitosis, and acts as a tumor suppressor. PLoS ONE 2012, 7, e39723. [Google Scholar] [CrossRef] [Green Version]
- Lai, Y.; Qiao, M.; Song, M.; Weintraub, S.T.; Shiio, Y. Quantitative proteomics identifies the Myb-binding protein p160 as a novel target of the von Hippel-Lindau tumor suppressor. PLoS ONE 2011, 6, e16975. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, S.M.; Rhodes, L.; Blanco, I.; Chung, W.K.; Eng, C.; Maher, E.R.; Richard, S.; Giles, R.H. Von hippel-lindau disease: Genetics and role of genetic counseling in a multiple neoplasia syndrome. J. Clin. Oncol. 2016, 34, 2172–2181. [Google Scholar] [CrossRef]
- Shenoy, N.; Pagliaro, L. Sequential pathogenesis of metastatic VHL mutant clear cell renal cell carcinoma: Putting it together with a translational perspective. Ann. Oncol. 2016, 27, 1685–1695. [Google Scholar] [CrossRef]
- Oriente, F.; Fernandez Diaz, L.C.; Miele, C.; Iovino, S.; Mori, S.; Diaz, V.M.; Troncone, G.; Cassese, A.; Formisano, P.; Blasi, F.; et al. Prep1 deficiency induces protection from diabetes and increased insulin sensitivity through a p160-mediated mechanism. Mol. Cell Biol. 2008, 28, 5634–5645. [Google Scholar] [CrossRef] [Green Version]
- Kanzleiter, T.; Rath, M.; Penkov, D.; Puchkov, D.; Schulz, N.; Blasi, F.; Schurmann, A. Pknox1/Prep1 regulates mitochondrial oxidative phosphorylation components in skeletal muscle. Mol. Cell Biol. 2014, 34, 290–298. [Google Scholar] [CrossRef] [Green Version]
- Olsen, J.V.; Blagoev, B.; Gnad, F.; Macek, B.; Kumar, C.; Mortensen, P.; Mann, M. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell 2006, 127, 635–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nousiainen, M.; Sillje, H.H.; Sauer, G.; Nigg, E.A.; Korner, R. Phosphoproteome analysis of the human mitotic spindle. Proc. Natl. Acad. Sci. USA 2006, 103, 5391–5396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantin, G.T.; Yi, W.; Lu, B.; Park, S.K.; Xu, T.; Lee, J.D.; Yates, J.R., 3rd. Combining protein-based IMAC, peptide-based IMAC, and MudPIT for efficient phosphoproteomic analysis. J. Proteome Res. 2008, 7, 1346–1351. [Google Scholar] [CrossRef] [PubMed]
- Imami, K.; Sugiyama, N.; Kyono, Y.; Tomita, M.; Ishihama, Y. Automated phosphoproteome analysis for cultured cancer cells by two-dimensional nanoLC-MS using a calcined titania/C18 biphasic column. Anal. Sci. 2008, 24, 161–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keough, R.A.; Macmillan, E.M.; Lutwyche, J.K.; Gardner, J.M.; Tavner, F.J.; Jans, D.A.; Henderson, B.R.; Gonda, T.J. Myb-binding protein 1a is a nucleocytoplasmic shuttling protein that utilizes CRM1-dependent and independent nuclear export pathways. Exp. Cell Res. 2003, 289, 108–123. [Google Scholar] [CrossRef]
- Perrera, C.; Colombo, R.; Valsasina, B.; Carpinelli, P.; Troiani, S.; Modugno, M.; Gianellini, L.; Cappella, P.; Isacchi, A.; Moll, J.; et al. Identification of Myb-binding protein 1A (MYBBP1A) as a novel substrate for aurora B kinase. J. Biol. Chem. 2010, 285, 11775–11785. [Google Scholar] [CrossRef] [Green Version]
- Ramsay, R.G.; Gonda, T.J. MYB function in normal and cancer cells. Nat. Rev. Cancer 2008, 8, 523–534. [Google Scholar] [CrossRef]
- Zhou, Y.E.; O’Rourke, J.P.; Edwards, J.S.; Ness, S.A. Single molecule analysis of c-myb alternative splicing reveals novel classifiers for precursor B-ALL. PLoS ONE 2011, 6, e22880. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Ness, S.A. Myb proteins: Angels and demons in normal and transformed cells. Front. Biosci. (Landmark Edition) 2011, 16, 1109–1131. [Google Scholar] [CrossRef] [Green Version]
- Fan, M.; Rhee, J.; St-Pierre, J.; Handschin, C.; Puigserver, P.; Lin, J.; Jaeger, S.; Erdjument-Bromage, H.; Tempst, P.; Spiegelman, B.M. Suppression of mitochondrial respiration through recruitment of p160 myb binding protein to PGC-1alpha: Modulation by p38 MAPK. Genes Dev. 2004, 18, 278–289. [Google Scholar] [CrossRef] [Green Version]
- Diaz, V.M.; Mori, S.; Longobardi, E.; Menendez, G.; Ferrai, C.; Keough, R.A.; Bachi, A.; Blasi, F. p160 Myb-binding protein interacts with Prep1 and inhibits its transcriptional activity. Mol. Cell Biol. 2007, 27, 7981–7990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owen, H.R.; Elser, M.; Cheung, E.; Gersbach, M.; Kraus, W.L.; Hottiger, M.O. MYBBP1a is a novel repressor of NF-kappaB. J. Mol. Biol. 2007, 366, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Onishi, Y.; Oishi, K.; Miyazaki, K.; Fukamizu, A.; Ishida, N. Molecular characterization of Mybbp1a as a co-repressor on the Period2 promoter. Nucleic Acids Res. 2009, 37, 1115–1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, L.C.; Okino, S.T.; Gonda, T.J.; Whitlock, J.P., Jr. Myb-binding protein 1a augments AhR-dependent gene expression. J. Biol. Chem. 2002, 277, 22515–22519. [Google Scholar] [CrossRef] [Green Version]
- Ho, L.; Ronan, J.L.; Wu, J.; Staahl, B.T.; Chen, L.; Kuo, A.; Lessard, J.; Nesvizhskii, A.I.; Ranish, J.; Crabtree, G.R. An embryonic stem cell chromatin remodeling complex, esBAF, is essential for embryonic stem cell self-renewal and pluripotency. Proc. Natl. Acad. Sci. USA 2009, 106, 5181–5186. [Google Scholar] [CrossRef] [Green Version]
- Ho, L.; Jothi, R.; Ronan, J.L.; Cui, K.; Zhao, K.; Crabtree, G.R. An embryonic stem cell chromatin remodeling complex, esBAF, is an essential component of the core pluripotency transcriptional network. Proc. Natl. Acad. Sci. USA 2009, 106, 5187–5191. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.C.; Liu, H.; Chen, S.L.; Wang, T.H.; Hsieh, C.L.; Huang, Y.; Chen, S.J.; Chen, H.C.; Yung, B.Y.; Chin-Ming Tan, B. Epigenetic silencing of myogenic gene program by Myb-binding protein 1a suppresses myogenesis. EMBO J. 2012, 31, 1739–1751. [Google Scholar] [CrossRef]
- Kumazawa, T.; Nishimura, K.; Katagiri, N.; Hashimoto, S.; Hayashi, Y.; Kimura, K. Gradual reduction in rRNA transcription triggers p53 acetylation and apoptosis via MYBBP1A. Sci. Rep. 2015, 5, 10854. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, T.; Murayama, A.; Katagiri, N.; Ohta, Y.M.; Fujita, E.; Masumoto, H.; Ema, M.; Takahashi, S.; Kimura, K.; Yanagisawa, J. RNA content in the nucleolus alters p53 acetylation via MYBBP1A. EMBO J. 2011, 30, 1054–1066. [Google Scholar] [CrossRef] [Green Version]
- Holmberg Olausson, K.; Nister, M.; Lindstrom, M.S. p53 -Dependent and -Independent Nucleolar Stress Responses. Cells 2012, 1, 774–798. [Google Scholar] [CrossRef] [Green Version]
- Ono, W.; Hayashi, Y.; Yokoyama, W.; Kuroda, T.; Kishimoto, H.; Ito, I.; Kimura, K.; Akaogi, K.; Waku, T.; Yanagisawa, J. The nucleolar protein Myb-binding protein 1A (MYBBP1A) enhances p53 tetramerization and acetylation in response to nucleolar disruption. J. Biol. Chem. 2014, 289, 4928–4940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ono, W.; Akaogi, K.; Waku, T.; Kuroda, T.; Yokoyama, W.; Hayashi, Y.; Kimura, K.; Kishimoto, H.; Yanagisawa, J. Nucleolar protein, Myb-binding protein 1A, specifically binds to nonacetylated p53 and efficiently promotes transcriptional activation. Biochem. Biophys. Res. Commun. 2013, 434, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, M.; Katagiri, N.; Kuroda, T.; Kishimoto, H.; Nishimura, K.; Kumazawa, T.; Iwasaki, N.; Kimura, K.; Yanagisawa, J. Critical role of the nucleolus in activation of the p53-dependent postmitotic checkpoint. Biochem. Biophys. Res. Commun. 2011, 407, 378–382. [Google Scholar] [CrossRef] [PubMed]
- George, B.; Horn, D.; Bayo, P.; Zaoui, K.; Flechtenmacher, C.; Grabe, N.; Plinkert, P.; Krizhanovsky, V.; Hess, J. Regulation and function of Myb-binding protein 1A (MYBBP1A) in cellular senescence and pathogenesis of head and neck cancer. Cancer Lett. 2015, 358, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.A. Is reliance on mitochondrial respiration a “chink in the armor” of therapy-resistant cancer? Cancer Cell 2014, 26, 788–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanli, T.; Steinberg, G.R.; Singh, G.; Tsakiridis, T. AMP-activated protein kinase (AMPK) beyond metabolism: A novel genomic stress sensor participating in the DNA damage response pathway. Cancer Biol. Ther. 2014, 15, 156–169. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.; Wang, J.; Zhao, M.; Xie, T.X.; Tanaka, N.; Sano, D.; Patel, A.A.; Ward, A.M.; Sandulache, V.C.; Jasser, S.A.; et al. Gain-of-function mutant p53 promotes cell growth and cancer cell metabolism via inhibition of AMPK activation. Mol. Cell 2014, 54, 960–974. [Google Scholar] [CrossRef] [Green Version]
- Felipe-Abrio, B.; Verdugo-Sivianes, E.M.; Carnero, A. c-MYB- and PGC1a-dependent metabolic switch induced by MYBBP1A loss in renal cancer. Mol. Oncol. 2019, 13, 1519–1533. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.H.; Luo, C.; Vazquez, F.; Puigserver, P. Targeting mitochondrial oxidative metabolism in melanoma causes metabolic compensation through glucose and glutamine utilization. Cancer Res. 2014, 74, 3535–3545. [Google Scholar] [CrossRef] [Green Version]
- Scarpulla, R.C. Nucleus-encoded regulators of mitochondrial function: Integration of respiratory chain expression, nutrient sensing and metabolic stress. Biochim. Biophys. Acta 2012, 1819, 1088–1097. [Google Scholar] [CrossRef] [Green Version]
- Scarpulla, R.C. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim. Biophys. Acta 2011, 1813, 1269–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ventura-Clapier, R.; Garnier, A.; Veksler, V. Transcriptional control of mitochondrial biogenesis: The central role of PGC-1alpha. Cardiovasc. Res. 2008, 79, 208–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puigserver, P.; Rhee, J.; Donovan, J.; Walkey, C.J.; Yoon, J.C.; Oriente, F.; Kitamura, Y.; Altomonte, J.; Dong, H.; Accili, D.; et al. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature 2003, 423, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Puigserver, P.; Spiegelman, B.M. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): Transcriptional coactivator and metabolic regulator. Endocr. Rev. 2003, 24, 78–90. [Google Scholar] [CrossRef] [Green Version]
- Tan, Z.; Luo, X.; Xiao, L.; Tang, M.; Bode, A.M.; Dong, Z.; Cao, Y. The role of PGC1alpha in cancer metabolism and its therapeutic implications. Mol. Cancer Ther. 2016, 15, 774–782. [Google Scholar] [CrossRef] [Green Version]
- Vazquez, F.; Lim, J.H.; Chim, H.; Bhalla, K.; Girnun, G.; Pierce, K.; Clish, C.B.; Granter, S.R.; Widlund, H.R.; Spiegelman, B.M.; et al. PGC1alpha expression defines a subset of human melanoma tumors with increased mitochondrial capacity and resistance to oxidative stress. Cancer Cell 2013, 23, 287–301. [Google Scholar] [CrossRef] [Green Version]
- Sancho, P.; Burgos-Ramos, E.; Tavera, A.; Bou Kheir, T.; Jagust, P.; Schoenhals, M.; Barneda, D.; Sellers, K.; Campos-Olivas, R.; Grana, O.; et al. MYC/PGC-1alpha balance determines the metabolic phenotype and plasticity of pancreatic cancer stem cells. Cell Metab. 2015, 22, 590–605. [Google Scholar] [CrossRef] [Green Version]
- Dang, C.V.; Kim, J.W.; Gao, P.; Yustein, J. The interplay between MYC and HIF in cancer. Nat. Rev. Cancer 2008, 8, 51–56. [Google Scholar] [CrossRef]
- Li, F.; Wang, Y.; Zeller, K.I.; Potter, J.J.; Wonsey, D.R.; O’Donnell, K.A.; Kim, J.W.; Yustein, J.T.; Lee, L.A.; Dang, C.V. Myc stimulates nuclearly encoded mitochondrial genes and mitochondrial biogenesis. Mol. Cell Biol. 2005, 25, 6225–6234. [Google Scholar] [CrossRef] [Green Version]
- Wise, D.R.; DeBerardinis, R.J.; Mancuso, A.; Sayed, N.; Zhang, X.Y.; Pfeiffer, H.K.; Nissim, I.; Daikhin, E.; Yudkoff, M.; McMahon, S.B.; et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl. Acad. Sci. USA 2008, 105, 18782–18787. [Google Scholar] [CrossRef] [Green Version]
- Felipe-Abrio, B.; Verdugo-Sivianes, E.M.; Saez, C.; Carnero, A. Loss of MYBBP1A induces cancer stem cell activity in renal cancer. Cancers 2019, 11, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonenberg, N.; Hinnebusch, A.G. Regulation of translation initiation in eukaryotes: Mechanisms and biological targets. Cell 2009, 136, 731–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, L.C.; Cook, R.S.; Chen, J. mTORC1 and mTORC2 in cancer and the tumor microenvironment. Oncogene 2017, 36, 2191–2201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hochstatter, J.; Holzel, M.; Rohrmoser, M.; Schermelleh, L.; Leonhardt, H.; Keough, R.; Gonda, T.J.; Imhof, A.; Eick, D.; Langst, G.; et al. Myb-binding protein 1a (Mybbp1a) regulates levels and processing of pre-ribosomal RNA. J. Biol. Chem. 2012, 287, 24365–24377. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.C.; Yang, C.C.; Hsieh, C.L.; Chou, Y.H.; Zhong, C.Z.; Yung, B.Y.; Liu, H. Epigeneitc silencing of ribosomal RNA genes by Mybbp1a. J. Biomed. Sci. 2012, 19, 57. [Google Scholar] [CrossRef] [Green Version]
- Hardie, D.G. The AMP-activated protein kinase pathway--new players upstream and downstream. J. Cell Sci. 2004, 117, 5479–5487. [Google Scholar] [CrossRef] [Green Version]
- Hardie, D.G. AMP-activated protein kinase: The guardian of cardiac energy status. J. Clin. Investig. 2004, 114, 465–468. [Google Scholar] [CrossRef]
- Keough, R.; Woollatt, E.; Crawford, J.; Sutherland, G.R.; Plummer, S.; Casey, G.; Gonda, T.J. Molecular cloning and chromosomal mapping of the human homologue of MYB binding protein (P160) 1A (MYBBP1A) to 17p13.3. Genomics 1999, 62, 483–489. [Google Scholar] [CrossRef]
- Weng, X.; Wu, J.; Lv, Z.; Peng, C.; Chen, J.; Zhang, C.; He, B.; Tong, R.; Hu, W.; Ding, C.; et al. Targeting Mybbp1a suppresses HCC progression via inhibiting IGF1/AKT pathway by CpG islands hypo-methylation dependent promotion of IGFBP5. EBioMedicine 2019, 44, 225–236. [Google Scholar] [CrossRef]
- Acuna Sanhueza, G.A.; Faller, L.; George, B.; Koffler, J.; Misetic, V.; Flechtenmacher, C.; Dyckhoff, G.; Plinkert, P.P.; Angel, P.; Simon, C.; et al. Opposing function of MYBBP1A in proliferation and migration of head and neck squamous cell carcinoma cells. BMC Cancer 2012, 12, 72. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Carnero, A.; Garcia-Mayea, Y.; Mir, C.; Lorente, J.; Rubio, I.T.; ME, L.L. The cancer stem-cell signaling network and resistance to therapy. Cancer Treat Rev. 2016, 49, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Carnero, A.; Lleonart, M. The hypoxic microenvironment: A determinant of cancer stem cell evolution. Bioessays 2016, 38, S65–S74. [Google Scholar] [CrossRef] [PubMed]
- Akaogi, K.; Ono, W.; Hayashi, Y.; Kishimoto, H.; Yanagisawa, J. MYBBP1A suppresses breast cancer tumorigenesis by enhancing the p53 dependent anoikis. BMC Cancer 2013, 13, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karim, M.F.; Yoshizawa, T.; Sato, Y.; Sawa, T.; Tomizawa, K.; Akaike, T.; Yamagata, K. Inhibition of H3K18 deacetylation of Sirt7 by Myb-binding protein 1a (Mybbp1a). Biochem. Biophys. Res. Commun. 2013, 441, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Nahalkova, J. The protein-interaction network with functional roles in tumorigenesis, neurodegeneration, and aging. Mol. Cell Biochem. 2016, 423, 187–196. [Google Scholar] [CrossRef]
- Nahalkova, J. Novel protein-protein interactions of TPPII, p53, and SIRT7. Mol. Cell Biochem. 2015, 409, 13–22. [Google Scholar] [CrossRef]
- Nahalkova, J.; Tomkinson, B. TPPII, MYBBP1A and CDK2 form a protein-protein interaction network. Arch. Biochem. Biophys. 2014, 564, 128–135. [Google Scholar] [CrossRef]
- Favier, D.; Gonda, T.J. Detection of proteins that bind to the leucine zipper motif of c-Myb. Oncogene 1994, 9, 305–311. [Google Scholar]
- Quintana, A.M.; Zhou, Y.E.; Pena, J.J.; O’Rourke, J.P.; Ness, S.A. Dramatic repositioning of c-Myb to different promoters during the cell cycle observed by combining cell sorting with chromatin immunoprecipitation. PLoS ONE 2011, 6, e17362. [Google Scholar] [CrossRef]
- Quintana, A.M.; Liu, F.; O’Rourke, J.P.; Ness, S.A. Identification and regulation of c-Myb target genes in MCF-7 cells. BMC Cancer 2011, 11, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidney, L.E.; Branch, M.J.; Dunphy, S.E.; Dua, H.S.; Hopkinson, A. Concise review: Evidence for CD34 as a common marker for diverse progenitors. Stem. Cells 2014, 32, 1380–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheasley, D.; Pereira, L.; Lightowler, S.; Vincan, E.; Malaterre, J.; Ramsay, R.G. Myb controls intestinal stem cell genes and self-renewal. Stem. Cells 2011, 29, 2042–2050. [Google Scholar] [CrossRef] [PubMed]
- Chambers, I.; Silva, J.; Colby, D.; Nichols, J.; Nijmeijer, B.; Robertson, M.; Vrana, J.; Jones, K.; Grotewold, L.; Smith, A. Nanog safeguards pluripotency and mediates germline development. Nature 2007, 450, 1230–1234. [Google Scholar] [CrossRef]
- Chambers, I.; Colby, D.; Robertson, M.; Nichols, J.; Lee, S.; Tweedie, S.; Smith, A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 2003, 113, 643–655. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Sui, Y.; Ni, J.; Yang, T. Insights into the Nanog gene: A propeller for stemness in primitive stem cells. Int. J. Biol. Sci. 2016, 12, 1372–1381. [Google Scholar] [CrossRef] [Green Version]
- Gassenmaier, M.; Chen, D.; Buchner, A.; Henkel, L.; Schiemann, M.; Mack, B.; Schendel, D.J.; Zimmermann, W.; Pohla, H. CXC chemokine receptor 4 is essential for maintenance of renal cell carcinoma-initiating cells and predicts metastasis. Stem. Cells 2013, 31, 1467–1476. [Google Scholar] [CrossRef]
- Cheng, B.; Yang, G.; Jiang, R.; Cheng, Y.; Yang, H.; Pei, L.; Qiu, X. Cancer stem cell markers predict a poor prognosis in renal cell carcinoma: A meta-analysis. Oncotarget 2016, 7, 65862–65875. [Google Scholar] [CrossRef]
- Peired, A.J.; Sisti, A.; Romagnani, P. Renal cancer stem cells: characterization and targeted therapies. Stem. Cells Int. 2016, 2016, 8342625. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.X.; Shang, Z.; Gu, J.; He, L. Renal targeting delivery systems. Future Med. Chem. 2019, 11, 2237–2240. [Google Scholar] [CrossRef]
- Okumura, F.; Uematsu, K.; Byrne, S.D.; Hirano, M.; Joo-Okumura, A.; Nishikimi, A.; Shuin, T.; Fukui, Y.; Nakatsukasa, K.; Kamura, T. Parallel Regulation of von Hippel-Lindau Disease by pVHL-Mediated Degradation of B-Myb and Hypoxia-Inducible Factor alpha. Mol. Cell Biol. 2016, 36, 1803–1817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mello, T.; Simeone, I.; Galli, A. Mito-nuclear communication in hepatocellular carcinoma metabolic rewiring. Cells 2019, 8, 417. [Google Scholar] [CrossRef] [PubMed] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Felipe-Abrio, B.; Carnero, A. The Tumor Suppressor Roles of MYBBP1A, a Major Contributor to Metabolism Plasticity and Stemness. Cancers 2020, 12, 254. https://doi.org/10.3390/cancers12010254
Felipe-Abrio B, Carnero A. The Tumor Suppressor Roles of MYBBP1A, a Major Contributor to Metabolism Plasticity and Stemness. Cancers. 2020; 12(1):254. https://doi.org/10.3390/cancers12010254
Chicago/Turabian StyleFelipe-Abrio, Blanca, and Amancio Carnero. 2020. "The Tumor Suppressor Roles of MYBBP1A, a Major Contributor to Metabolism Plasticity and Stemness" Cancers 12, no. 1: 254. https://doi.org/10.3390/cancers12010254