Acute Myeloid and Lymphoblastic Leukemia Cell Interactions with Endothelial Selectins: Critical Role of PSGL-1, CD44 and CD43
Abstract
1. Introduction
2. Results
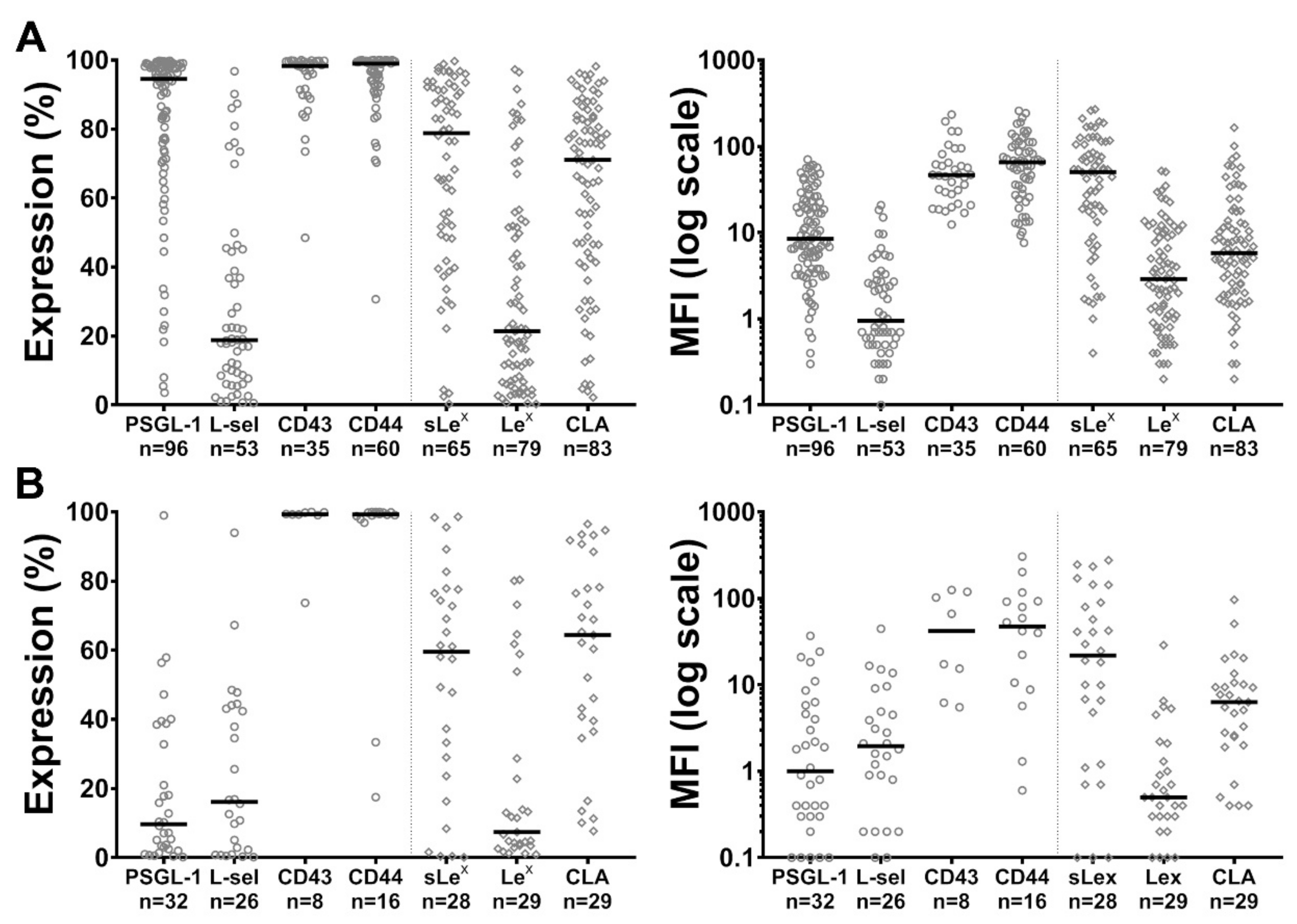
2.1. Expression of PSGL-1, L-Selectin, CD43, CD44 and of sLex, Lex and CLA Carbohydrate Determinants by Blast Cells
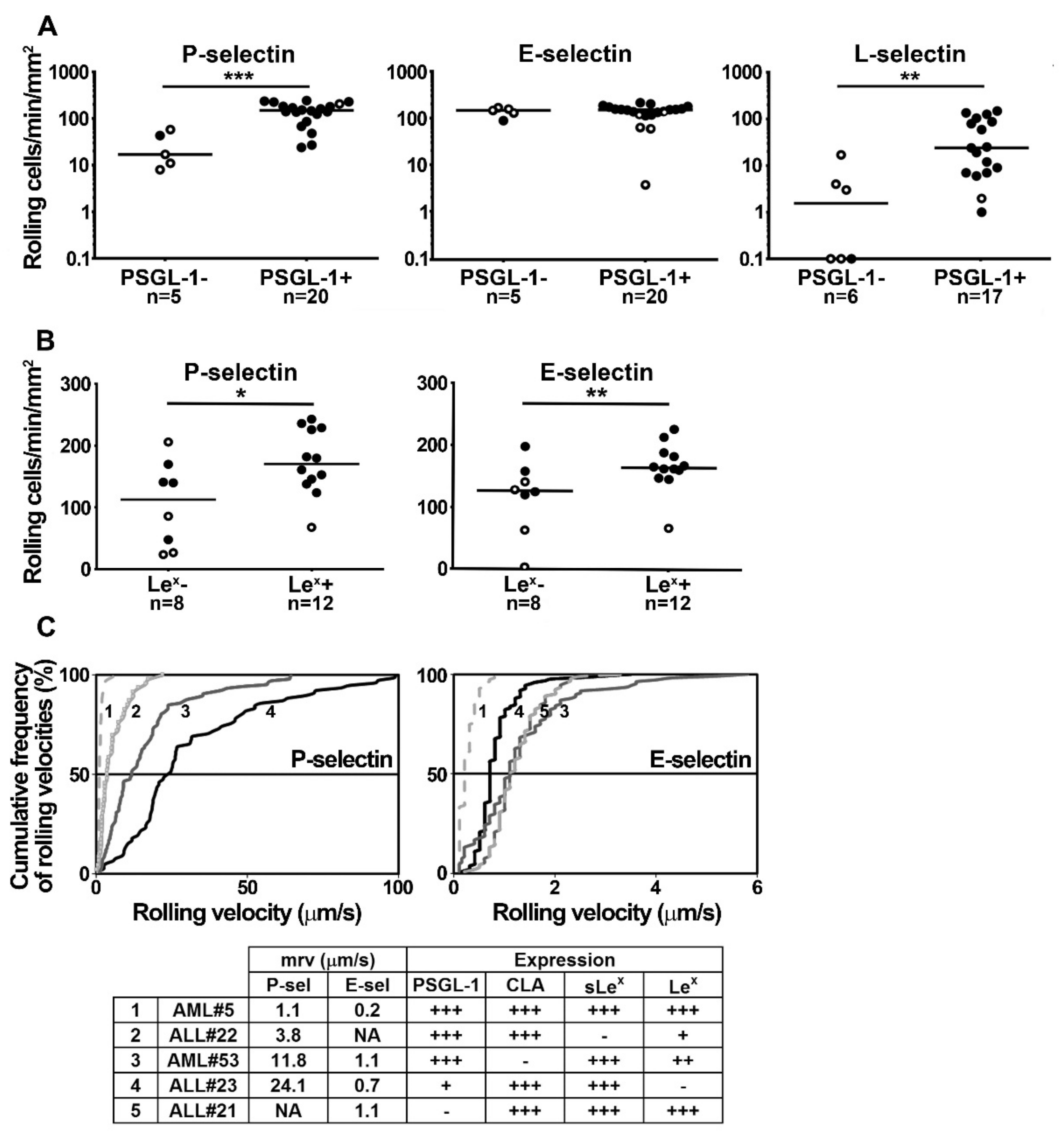
2.2. PSGL-1, Lex, sLex and CLA Determinants Play a Major Role in Supporting Blast Rolling on Selectins
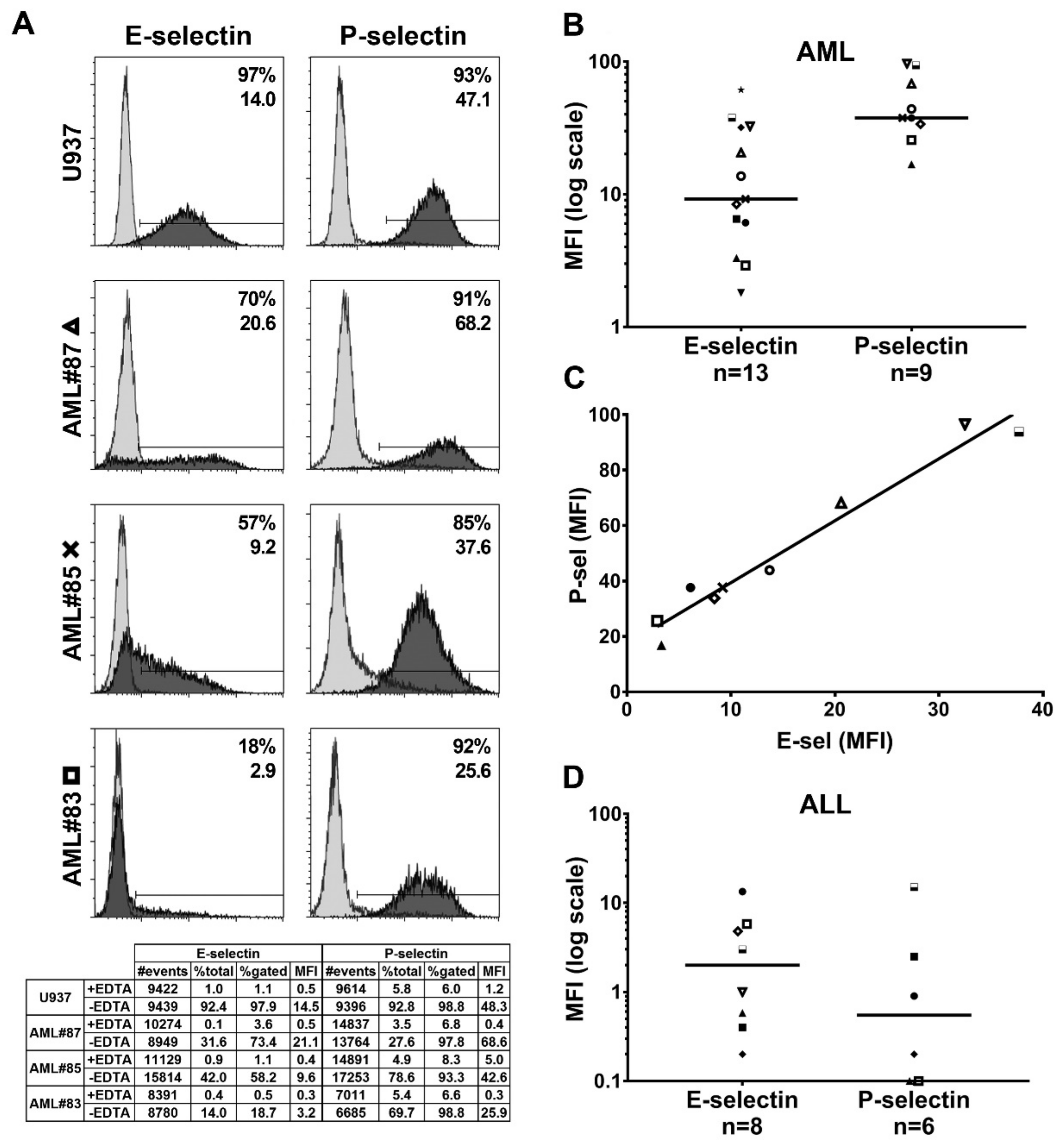
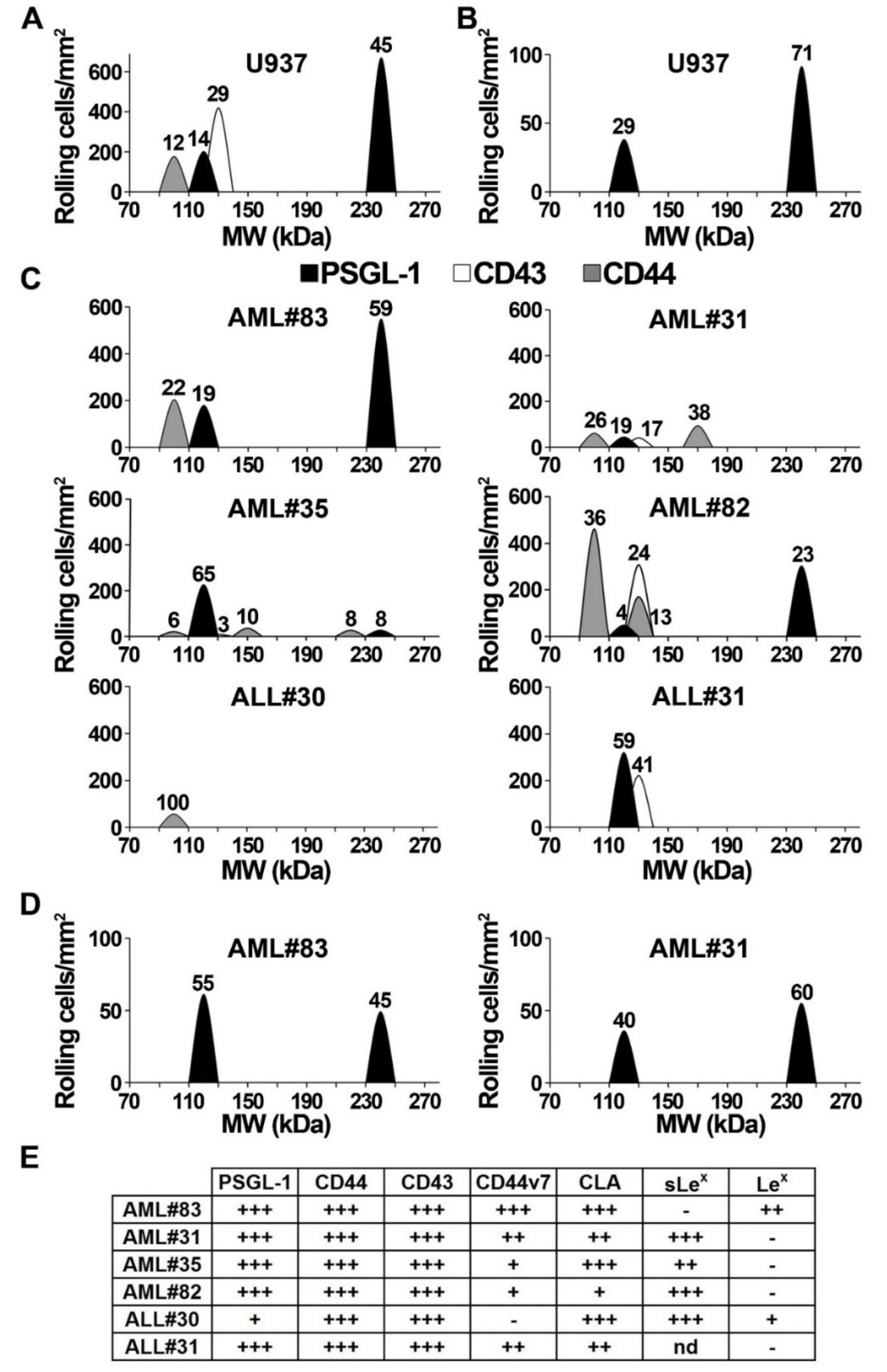
2.3. Functional Endothelial Selectin Ligands are Expressed by Human AML and ALL Blast Cells
2.4. Blast Rolling on L- and P-Selectin is Mediated by PSGL-1 and Other Ligands
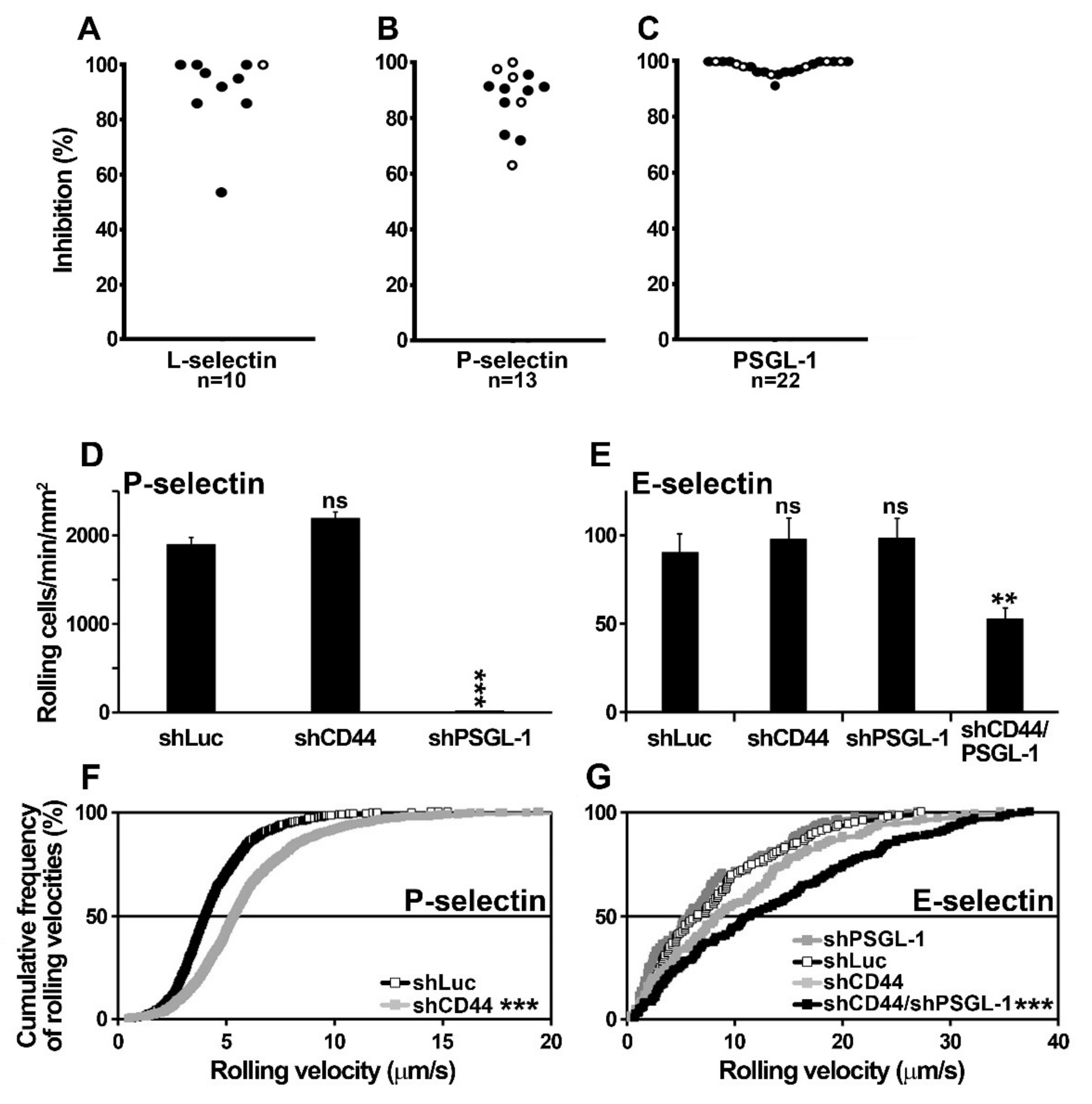
2.5. U937 Cell Rolling on Endothelial Selectins is PSGL-1- and CD44-Dependent
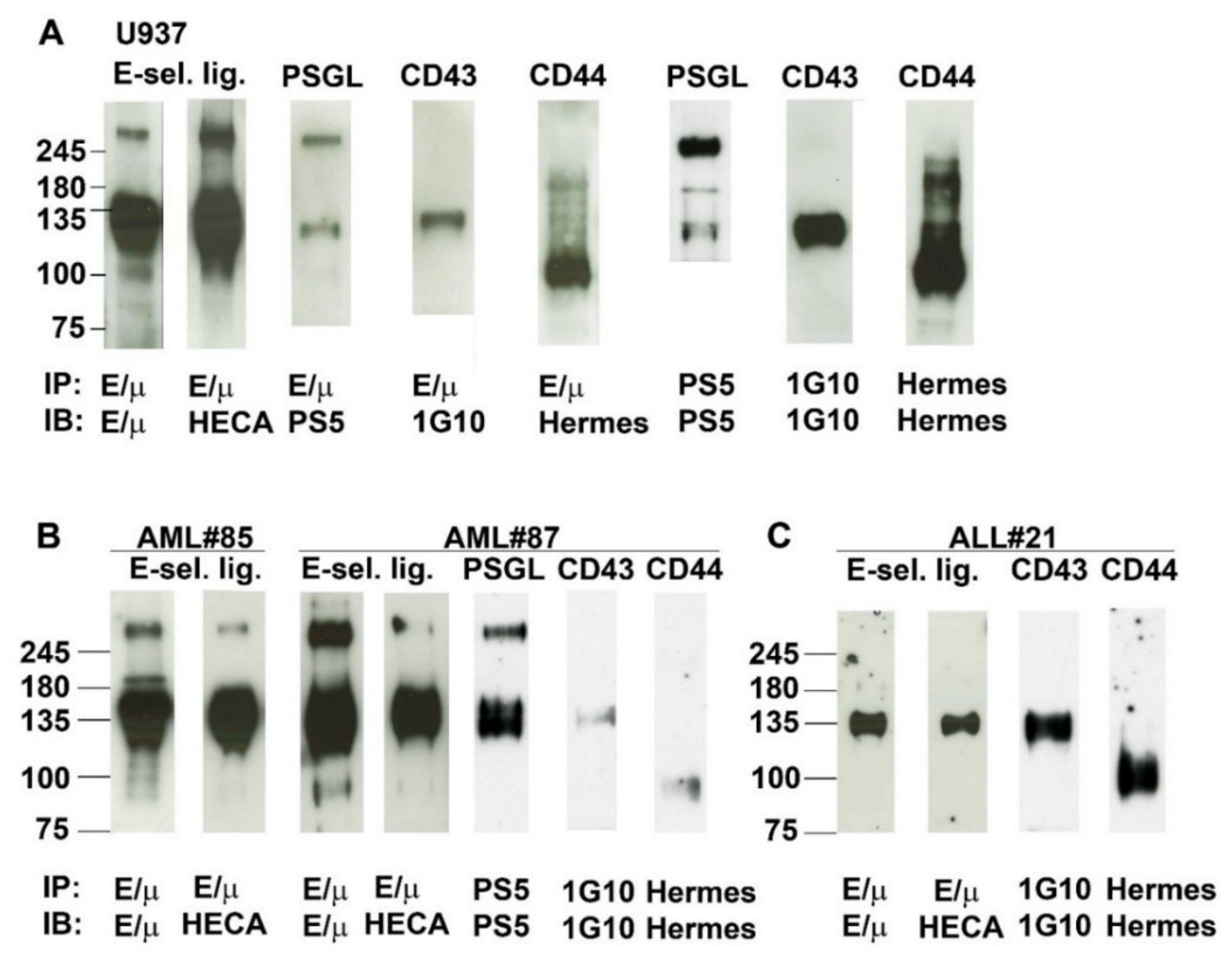
2.6. Analysis of E-Selectin Ligands Expressed by Human AML and ALL Blast Cells
2.7. Contribution of PSGL-1, CD44, and CD43 in Supporting E-Selectin-Dependent Rolling
3. Discussion
4. Materials and Methods
4.1. Antibodies and Recombinant Selectins
4.2. Patients and Cells
4.3. Immunophenotypic Analyzes By Flow Cytometry
4.4. Inhibition of PSGL-1 and/or CD44 Expression by Short Hairpin RNA (shRNA)
4.5. Immunoprecipitation Studies and Western Blot Analysis
4.6. Flow Adhesion Assays
4.7. Blot Rolling Assays
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zarbock, A.; Ley, K.; McEver, R.P.; Hidalgo, A. Leukocyte ligands for endothelial selectins: Specialized glycoconjugates that mediate rolling and signaling under flow. Blood 2011, 118, 6743–6751. [Google Scholar] [CrossRef] [PubMed]
- Katayama, Y.; Hidalgo, A.; Furie, B.C.; Vestweber, D.; Furie, B.; Frenette, P.S. PSGL-1 participates in E-selectin-mediated progenitor homing to bone marrow: Evidence for cooperation between E-selectin ligands and alpha4 integrin. Blood 2003, 102, 2060–2067. [Google Scholar] [CrossRef] [PubMed]
- Borsig, L. Selectins in cancer immunity. Glycobiology 2018, 28, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Mondal, N.; Greco, T.M.; Wei, Y.; Spadazzi, C.; Lin, S.C.; Zheng, H.; Cheung, C.; Magnani, J.L.; Lin, S.H.; et al. Bone vascular niche E-selectin induces mesenchymal-epithelial transition and Wnt activation in cancer cells to promote bone metastasis. Nat. Cell Biol. 2019, 21, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Tinoco, R.; Carrette, F.; Barraza, M.L.; Otero, D.C.; Magana, J.; Bosenberg, M.W.; Swain, S.L.; Bradley, L.M. PSGL-1 Is an Immune Checkpoint Regulator that Promotes T Cell Exhaustion. Immunity 2016, 44, 1190–1203. [Google Scholar] [CrossRef]
- Furie, B.; Furie, B.C. Role of platelet P-selectin and microparticle PSGL-1 in thrombus formation. Trends Mol. Med. 2004, 10, 171–178. [Google Scholar] [CrossRef]
- Windisch, R.; Pirschtat, N.; Kellner, C.; Chen-Wichmann, L.; Lausen, J.; Humpe, A.; Krause, D.S.; Wichmann, C. Oncogenic Deregulation of Cell Adhesion Molecules in Leukemia. Cancers 2019, 11, 311. [Google Scholar] [CrossRef]
- Calvi, L.M.; Link, D.C. The hematopoietic stem cell niche in homeostasis and disease. Blood 2015, 126, 2443–2451. [Google Scholar] [CrossRef]
- Kumar, R.; Godavarthy, P.S.; Krause, D.S. The bone marrow microenvironment in health and disease at a glance. J. Cell Sci. 2018, 131. [Google Scholar] [CrossRef]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef]
- Zarbock, A.; Lowell, C.A.; Ley, K. Spleen tyrosine kinase Syk is necessary for E-selectin-induced alpha(L)beta(2) integrin-mediated rolling on intercellular adhesion molecule-1. Immunity 2007, 26, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Yago, T.; Shao, B.; Miner, J.J.; Yao, L.; Klopocki, A.G.; Maeda, K.; Coggeshall, K.M.; McEver, R.P. E-selectin engages PSGL-1 and CD44 through a common signaling pathway to induce integrin alphaLbeta2-mediated slow leukocyte rolling. Blood 2010, 116, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Winkler, I.G.; Barbier, V.; Nowlan, B.; Jacobsen, R.N.; Forristal, C.E.; Patton, J.T.; Magnani, J.L.; Levesque, J.P. Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat. Med. 2012, 18, 1651–1657. [Google Scholar] [CrossRef] [PubMed]
- Spertini, O.; Cordey, A.-S.; Monai, N.; Giuffrè, L.; Schapira, M. P-selectin glycoprotein ligand-1 (PSGL-1) is a ligand for L-selectin on neutrophils, monocytes and CD34+ hematopoietic progenitor cells. J. Cell Biol. 1996, 135, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Bernimoulin, M.P.; Zeng, X.L.; Abbal, C.; Giraud, S.; Martinez, M.; Michielin, O.; Schapira, M.; Spertini, O. Molecular basis of leukocyte rolling on PSGL-1: Predominant role of core-2 O-glycans and of tyrosine sulfate residue 51. J. Biol. Chem. 2003, 278, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Tauxe, C.; Xie, X.; Joffraud, M.; Martinez, M.; Schapira, M.; Spertini, O. P-selectin Glycoprotein Ligand-1 Decameric Repeats Regulate Selectin-dependent Rolling under Flow Conditions. J. Biol. Chem. 2008, 283, 28536–28545. [Google Scholar] [CrossRef]
- Hidalgo, A.; Peired, A.J.; Wild, M.K.; Vestweber, D.; Frenette, P.S. Complete identification of E-selectin ligands on neutrophils reveals distinct functions of PSGL-1, ESL-1, and CD44. Immunity 2007, 26, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Merzaban, J.S.; Burdick, M.M.; Gadhoum, S.Z.; Dagia, N.M.; Chu, J.T.; Fuhlbrigge, R.C.; Sackstein, R. Analysis of glycoprotein E-selectin ligands on human and mouse marrow cells enriched for hematopoietic stem/progenitor cells. Blood 2011, 118, 1774–1783. [Google Scholar] [CrossRef]
- Silva, M.; Videira, P.A.; Sackstein, R. E-Selectin Ligands in the Human Mononuclear Phagocyte System: Implications for Infection, Inflammation, and Immunotherapy. Front. Immunol. 2017, 8, 1878. [Google Scholar] [CrossRef]
- Matsumoto, M.; Shigeta, A.; Furukawa, Y.; Tanaka, T.; Miyasaka, M.; Hirata, T. CD43 Collaborates with P-Selectin Glycoprotein Ligand-1 to Mediate E-Selectin-Dependent T Cell Migration into Inflamed Skin. J. Immunol. 2007, 178, 2499–2506. [Google Scholar] [CrossRef]
- Stucki, A.; Rivier, A.-S.; Gikic, M.; Monai, N.; Schapira, M.; Spertini, O. Endothelial Cell Activation by Myeloblasts: Molecular Mechanisms of Leukostasis and Leukemic Cell Dissemination. Blood 2001, 97, 2121–2129. [Google Scholar] [CrossRef]
- Spertini, O.; Callegari, P.; Cordey, A.-S.; Hauert, J.; Joggi, J.; von Fliedner, V.; Schapira, M. High levels of the shed form of L-selectin (sL-selectin) are present in patients with acute leukemia and inhibit blast cell adhesion to activated endothelium. Blood 1994, 84, 1249–1256. [Google Scholar]
- Krause, D.S.; Lazarides, K.; von Andrian, U.H.; Van Etten, R.A. Requirement for CD44 in homing and engraftment of BCR-ABL-expressing leukemic stem cells. Nat. Med. 2006, 12, 1175–1180. [Google Scholar] [CrossRef]
- Krause, D.S.; Lazarides, K.; Lewis, J.B.; von Andrian, U.H.; Van Etten, R.A. Selectins and their ligands are required for homing and engraftment of BCR-ABL1+ leukemic stem cells in the bone marrow niche. Blood 2014, 123, 1361–1371. [Google Scholar] [CrossRef]
- Godavarthy, P.S.; Kumar, R.; Herkt, S.C.; Pereira, R.S.; Hayduk, N.; Weissenberger, E.S.; Aggoune, D.; Manavski, Y.; Lucas, T.; Pan, K.T.; et al. The vascular bone marrow niche influences outcome in chronic myeloid leukemia via the E-selectin-SCL/TAL1-CD44 axis. Haematologica 2019. [Google Scholar] [CrossRef]
- Jin, L.; Hope, K.J.; Zhai, Q.; Smadja-Joffe, F.; Dick, J.E. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat. Med. 2006, 12, 1167–1174. [Google Scholar] [CrossRef]
- Winkler, I.G.; Barbier, V.; Ward, M.; Tallack, M.; Lowe, J.; Davies, J.; Erbani, J.; Magnani, J.L.; Levesque, J.-P. Vascular E-selectin protects leukemia cells from chemotherapy by directly activating pro-survival NF-kB signaling: Therapeutic blockade of E-selectin dampens NF-kB activation. Blood 2016, 128, 2823. [Google Scholar]
- DeAngelo, D.J.; Jonas, B.A.; Liesveld, J.L.; Bixby, D.L.; Advani, A.S.; Marlton, P.; O’Dwyer, M.; Magnani, J.L.; Thackray, H.M.; Becker, P.S. GMI-1271 Improves Efficacy and Safety of Chemotherapy in R/R and Newly Diagnosed Older Patients with AML: Results of a Phase 1/2 Study. Blood 2018, 130, 894. [Google Scholar]
- Dohner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Buchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef]
- Sperandio, M.; Smith, M.L.; Forlow, S.B.; Olson, T.S.; Xia, L.; McEver, R.P.; Ley, K. P-selectin glycoprotein ligand-1 mediates L-selectin-dependent leukocyte rolling in venules. J. Exp. Med. 2003, 197, 1355–1363. [Google Scholar] [CrossRef]
- Walcheck, B.; Moore, K.L.; Mcever, R.P.; Kishimoto, T.K. Neutrophil-neutrophil interactions under hydrodynamic shear stress involve L-selectin and PSGL-1. A mechanism that amplifies initial leukocyte accumulation of P-selectin in vitro. J. Clin. Invest. 1996, 98, 1081–1087. [Google Scholar] [CrossRef]
- Homeister, J.W.; Thall, A.D.; Petryniak, B.; Maly, P.; Rogers, C.E.; Smith, P.L.; Kelly, R.J.; Gersten, K.M.; Askari, S.W.; Cheng, G.; et al. The alpha(1,3)fucosyltransferases FucT-IV and FucT-VII exert collaborative control over selectin-dependent leukocyte recruitment and lymphocyte homing. Immunity 2001, 15, 115–126. [Google Scholar] [CrossRef]
- Martinez, M.; Joffraud, M.; Giraud, S.; Baisse, B.; Bernimoulin, M.P.; Schapira, M.; Spertini, O. Regulation of PSGL-1 Interactions with L-selectin, P-selectin, and E-selectin: Role of Human Fucosyltransferase-IV and -VII. J. Biol. Chem. 2005, 280, 5378–5390. [Google Scholar] [CrossRef]
- Spertini, C.; Baisse, B.; Spertini, O. Ezrin-radixin-moesin-binding sequence of PSGL-1 glycoprotein regulates leukocyte rolling on selectins and activation of extracellular signal-regulated kinases. J. Biol. Chem. 2012, 287, 10693–10702. [Google Scholar] [CrossRef]
- Cheung, L.S.; Raman, P.S.; Balzer, E.M.; Wirtz, D.; Konstantopoulos, K. Biophysics of selectin-ligand interactions in inflammation and cancer. Phys. Biol. 2011, 8, 015013. [Google Scholar] [CrossRef]
- Ohmori, K.; Fukui, F.; Kiso, M.; Imai, T.; Yoshie, O.; Hasegawa, H.; Matsushima, K.; Kannagi, R. Identification of cutaneous lymphocyte-associated antigen as sialyl 6-sulfo Lewis X, a selectin ligand expressed on a subset of skin-homing helper memory T cells. Blood 2006, 107, 3197–3204. [Google Scholar] [CrossRef]
- Varki, A. Selectin ligands: Will the real ones please stand up? J. Clin. Invest. 1997, 99, 158–162. [Google Scholar] [CrossRef]
- Baumhueter, S.; Singer, M.S.; Henzel, W.; Hemmerich, S.; Renz, M.; Rosen, S.D.; Lasky, L.A. Binding of L-selectin to the vascular sialomucin CD34. Science 1993, 262, 436–438. [Google Scholar] [CrossRef]
- AbuSamra, D.B.; Aleisa, F.A.; Al-Amoodi, A.S.; Jalal Ahmed, H.M.; Chin, C.J.; Abuelela, A.F.; Bergam, P.; Sougrat, R.; Merzaban, J.S. Not just a marker: CD34 on human hematopoietic stem/progenitor cells dominates vascular selectin binding along with CD44. Blood Adv. 2017, 1, 2799–2816. [Google Scholar] [CrossRef]
- Becker, P.S. Dependence of acute myeloid leukemia on adhesion within the bone marrow microenvironment. Sci. World J. 2012, 2012, 856467. [Google Scholar] [CrossRef]
- Sipkins, D.A.; Wei, X.; Wu, J.W.; Runnels, J.M.; Cote, D.; Means, T.K.; Luster, A.D.; Scadden, D.T.; Lin, C.P. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature 2005, 435, 969–973. [Google Scholar] [CrossRef]
- Ding, L.; Ley, T.J.; Larson, D.E.; Miller, C.A.; Koboldt, D.C.; Welch, J.S.; Ritchey, J.K.; Young, M.A.; Lamprecht, T.; McLellan, M.D.; et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature 2012, 481, 506–510. [Google Scholar] [CrossRef]
- Maly, P.; Thall, A.; Petryniak, B.; Rogers, C.E.; Smith, P.L.; Marks, R.M.; Kelly, R.J.; Gersten, K.M.; Cheng, G.; Saunders, T.L.; et al. The alpha(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell 1996, 86, 643–653. [Google Scholar] [CrossRef]
- Bengtson, P.; Lundblad, A.; Larson, G.; Pahlsson, P. Polymorphonuclear leukocytes from individuals carrying the G329A mutation in the alpha 1,3-fucosyltransferase VII gene (FUT7) roll on E- and P-selectins. J. Immunol. 2002, 169, 3940–3946. [Google Scholar] [CrossRef]
- Picker, L.J.; Kishimoto, T.K.; Smith, C.W.; Warnock, R.A.; Butcher, E.C. ELAM-1 is an adhesion molecule for skin-homing T cells. Nature 1991, 349, 796–799. [Google Scholar] [CrossRef]
- Fuhlbrigge, R.C.; Kieffer, J.D.; Armerding, D.; Kupper, T.S. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature 1997, 389, 978–981. [Google Scholar] [CrossRef]
- Dimitroff, C.J.; Lee, J.Y.; Schor, K.S.; Sandmaier, B.M.; Sackstein, R. Differential L-selectin binding activities of human hematopoietic cell L-selectin ligands, HCELL and PSGL-1. J. Biol. Chem. 2001, 276, 47623–47631. [Google Scholar] [CrossRef]
- Kieffer, J.D.; Fuhlbrigge, R.C.; Armerding, D.; Robert, C.; Ferenczi, K.; Camphausen, R.T.; Kupper, T.S. Neutrophils, monocytes, and dendritic cells express the same specialized form of PSGL-1 as do skin-homing memory T cells: Cutaneous lymphocyte antigen. Biochem. Biophys. Res. Commun. 2001, 285, 577–587. [Google Scholar] [CrossRef]
- Mitsuoka, C.; Sawada-Kasugai, M.; Ando-Furui, K.; Izawa, M.; Nakanishi, H.; Nakamura, S.; Ishida, H.; Kiso, M.; Kannagi, R. Identification of a major carbohydrate capping group of the L-selectin ligand on high endothelial venules in human lymph nodes as 6-sulfo sialyl Lewis X. J. Biol. Chem. 1998, 273, 11225–11233. [Google Scholar] [CrossRef]
- Spertini, O.; Kansas, G.S.; Reimann, K.A.; Mackay, C.R.; Tedder, T.F. Function and evolutionary conservation of distinct epitopes on the leukocyte adhesion molecule-1 (TQ-1, Leu-8) that regulate leukocyte migration. J. Immunol. 1991, 147, 942–949. [Google Scholar]
- Benett, J.M.; Catovsky, D.; Daniel, M.T.; Flandrin, G.; Galton, D.A.G.; Gralnick, H.R.; Sultan, C. Proposals for the classification of the acute leukemias. Br. J. Haematol. 1976, 33, 451–458. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J.; Vardiman, J.W. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; IARC Press: Lyon, France, 2008. [Google Scholar]
- Bene, M.C.; Nebe, T.; Bettelheim, P.; Buldini, B.; Bumbea, H.; Kern, W.; Lacombe, F.; Lemez, P.; Marinov, I.; Matutes, E.; et al. Immunophenotyping of acute leukemia and lymphoproliferative disorders: A consensus proposal of the European LeukemiaNet Work Package 10. Leukemia 2011, 25, 567–574. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, J.; Qian, J.; Qiu, P.; Hanabuchi, S.; Lu, Y.; Wang, Z.; Liu, Z.; Li, H.; He, J.; et al. PSGL-1/selectin and ICAM-1/CD18 interactions are involved in macrophage-induced drug resistance in myeloma. Leukemia 2013, 27, 702–710. [Google Scholar] [CrossRef]
- Godar, S.; Ince, T.A.; Bell, G.W.; Feldser, D.; Donaher, J.L.; Bergh, J.; Liu, A.; Miu, K.; Watnick, R.S.; Reinhardt, F.; et al. Growth-inhibitory and tumor- suppressive functions of p53 depend on its repression of CD44 expression. Cell 2008, 134, 62–73. [Google Scholar] [CrossRef]
- Naldini, L.; Blomer, U.; Gallay, P.; Ory, D.; Mulligan, R.; Gage, F.H.; Verma, I.M.; Trono, D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 1996, 272, 263–267. [Google Scholar] [CrossRef]
- Baisse, B.; Spertini, C.; Galisson, F.; Smirnova, T.; Spertini, O. The function of P-selectin glycoprotein ligand-1 is conserved from ancestral fishes to mammals. J. Leukoc. Biol. 2019. [Google Scholar] [CrossRef]
- Fuhlbrigge, R.C.; King, S.L.; Dimitroff, C.J.; Kupper, T.S.; Sackstein, R. Direct real-time observation of E- and P-selectin-mediated rolling on cutaneous lymphocyte-associated antigen immobilized on Western blots. J. Immunol. 2002, 168, 5645–5651. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spertini, C.; Baïsse, B.; Bellone, M.; Gikic, M.; Smirnova, T.; Spertini, O. Acute Myeloid and Lymphoblastic Leukemia Cell Interactions with Endothelial Selectins: Critical Role of PSGL-1, CD44 and CD43. Cancers 2019, 11, 1253. https://doi.org/10.3390/cancers11091253
Spertini C, Baïsse B, Bellone M, Gikic M, Smirnova T, Spertini O. Acute Myeloid and Lymphoblastic Leukemia Cell Interactions with Endothelial Selectins: Critical Role of PSGL-1, CD44 and CD43. Cancers. 2019; 11(9):1253. https://doi.org/10.3390/cancers11091253
Chicago/Turabian StyleSpertini, Caroline, Bénédicte Baïsse, Marta Bellone, Milica Gikic, Tatiana Smirnova, and Olivier Spertini. 2019. "Acute Myeloid and Lymphoblastic Leukemia Cell Interactions with Endothelial Selectins: Critical Role of PSGL-1, CD44 and CD43" Cancers 11, no. 9: 1253. https://doi.org/10.3390/cancers11091253
APA StyleSpertini, C., Baïsse, B., Bellone, M., Gikic, M., Smirnova, T., & Spertini, O. (2019). Acute Myeloid and Lymphoblastic Leukemia Cell Interactions with Endothelial Selectins: Critical Role of PSGL-1, CD44 and CD43. Cancers, 11(9), 1253. https://doi.org/10.3390/cancers11091253




